Abstract
Objective
Permanent biventricular pacing benefits patients with heart failure and interventricular conduction delay, but the importance of pacing with and without optimization in patients at risk of low cardiac output after heart surgery is unknown. We hypothesized that pacing parameters independently affect cardiac output. Accordingly, we analyzed aortic flow measured with an electromagnetic flowmeter in patients at risk of low cardiac output, during an ongoing randomized clinical trial of biventricular pacing (n=11) vs. standard of care (n=9).
Methods
A sub-study was conducted in all 20 patients, in both groups, with stable pacing after coronary artery bypass grafting and/or valve surgery. Ejection fraction averaged 33±15%, QRS duration 116±19 msec. Effects were measured within one hour of the conclusion of cardiopulmonary bypass. Atrioventricular delay (7 settings) and interventricular delay (9 settings) were optimized in random sequence.
Results
Optimization of atrioventricular delay (171±8 msec), at an interventricular delay of 0 msec, increased flow 14% vs. the worst setting (111±11 msec, p < 0.001) and 7% vs. nominal atrioventricular delay (120 msec, p < 0.001). Interventricular delay optimization increased flow 10% vs. the worst setting (p < 0.001) and 5% vs. nominal interventricular delay (0 msec, p < 0.001). Optimized pacing increased cardiac output 13% vs. atrial pacing at matched heart rate (5.5±0.5 vs. 4.9±0.6 L/min; p = 0.003) and 10% vs. sinus rhythm (5.0±0.6 L/min; p = 0.019).
Conclusions
Temporary biventricular pacing increases intraoperative cardiac output in patients with left ventricular dysfunction undergoing cardiac surgery. Atrioventricular and interventricular delay optimization maximizes this benefit.
Introduction
Biventricular pacing (BiVP) is an established therapy for congestive heart failure (CHF), and it is currently the standard of care for select patients with advanced CHF associated with left ventricular (LV) dysfunction and intraventricular conduction delay (IVCD).1 Permanent BiVP improves LV dimension and function and decreases morbidity and mortality, although it is associated with a nonresponse rate of up to 30%.2-5 While the long-term benefits of BiVP are typically not appreciated until several months after implantation, hemodynamic effects of changes to pacing parameters are reflected acutely by metrics such as stroke volume, ventricular dyssynchrony, and the maximal first derivative of pressure (dP/dtmax).6,7 These properties have facilitated the study of the optimization of programmable BiVP parameters, such as atrioventricular (AV) delay (AVD) and interventricular delay (VVD), to maximize the hemodynamic benefit of BiVP and to reduce its nonresponse rate. 8-10
The acute hemodynamic effects of BiVP also enable the study of temporary BiVP as a treatment for low output states after cardiac surgery. Low LV ejection fraction (LVEF) is an independent risk factor for poor outcomes following cardiac surgery.11 BiVP improves hemodynamics without increasing myocardial oxygen consumption,8 and, therefore, it is particularly appealing as a potential therapy in cardiac surgery patients. Prior studies have assessed temporary perioperative BiVP in heterogeneous groups of patients with varying results.12-24 Moreover, the role of optimization of temporary BiVP parameters in the perioperative setting is unclear.25,26
The BiVP After Cardiac Surgery (BiPACS) trial is a randomized clinical trial to study the effect of optimized temporary BiVP on cardiac output (CO) in postoperative cardiac surgery patients with preoperative LV systolic dysfunction and an IVCD. Patients undergo BiVP optimization at multiple time points in the intraoperative and postoperative periods and are randomized to continuous optimized BiVP vs. standard of care. The hypothesis underlying the BiPACS trial is that CO will increase 15% in patients undergoing temporary BiVP. In this sub-study of the BiPACS trial, we hypothesized that optimization of pacing parameters would increase CO. Accordingly, we assessed the contribution of AVD and VVD optimization to the effect of BiVP optimization in the intraoperative period, following separation from cardiopulmonary bypass (CPB), and evaluated the effect of optimized BiVP on CO as compared to atrial pacing (AAI) and to sinus rhythm with no pacing (NSR).
Methods
BiPACS Study Population
The study protocol was approved by the Columbia University Medical Center Institutional Review Board. Adult patients undergoing elective open-heart surgery on CPB were screened for eligibility to enroll in the BiPACS trial. All patients gave written, informed consent. Recruitment was done prior to the day of surgery by qualified and trained study coordinators and investigators on the study team with permission of the Attending surgeon. Inclusion criteria included: preoperative CHF, LVEF ≤40% and QRS duration ≥100 msec, or patients undergoing combined mitral and aortic valve surgery. LVEF and QRS criteria were liberalized from values of 35% and 120 msec, respectively, in the original protocol. Exclusion criteria included: atrial fibrillation, second or third degree AV block, congenital heart disease, intracardiac shunts, or heart rate >120 beats per minute (bpm) after separation from CPB. Preoperative data obtained by chart review included: LVEF, as measured by echocardiogram or left ventriculogram; heart rhythm, QRS duration, and intraventricular blocks from electrocardiogram (EKG) tracings; the type of surgery performed; and demographic characteristics. The BiPACS trial is ongoing, and endpoints will not be examined until 212 patients have been randomized.
Study Design and Optimization Protocol
To avoid imbalances that may occur using simple randomization, patients in the BiPACS trial are randomized to the two treatment groups at the end of Phase I, using randomly permuted blocks of four, six, and eight. A treatment allocation ratio of one to one was used; each group will be of equal size. The Phase 1 testing described here occurs in all patients, prior to randomization. Optimization of AVD, ventricular pacing site, and VVD are tested in random sequence. Randomization and testing sequences are determined by forms in sealed envelopes that are not opened until needed. These forms were prepared prior to the enrollment of the first patient. Before separation from CPB, temporary epicardial pacing leads were sewn to the right atrial (RA) appendage, anterior right ventricle (RV), and two randomized sites, out of six possible sites, on the LV. One of the LV leads (LV1) was placed at the basal LV at either the obtuse margin, circumflex, or posterior regions; the second LV lead (LV2) was placed at either the mid infero-medial, mid infero-lateral or apical LV. Data from BiVP using LV1 were analyzed in this study. The leads were attached to a Medtronic InSync III permanent biventricular pacemaker (Medtronic, Inc, Minneapolis, MN), mounted in an external housing unit, and their sensing and pacing functions were tested and confirmed. An appropriately sized electromagnetic flow probe (Carolina Medical Electronics, East Bend, NC) was placed on the ascending aorta. After separation from CPB and establishment of stable inotrope and vasopressor dosing, the BiVP optimization protocol was initiated. The pacing rate was set at 90 bpm, or at 10 bpm above the patient's intrinsic heart rate if greater than 90 bpm to ensure atrial capture, up to a maximum of 120 bpm. These heart rates were selected empirically. A wider range of heart rates is studied in phase 3 of the BiPACS trial, including cardiac resynchronization therapy at the intrinsic heart rate.
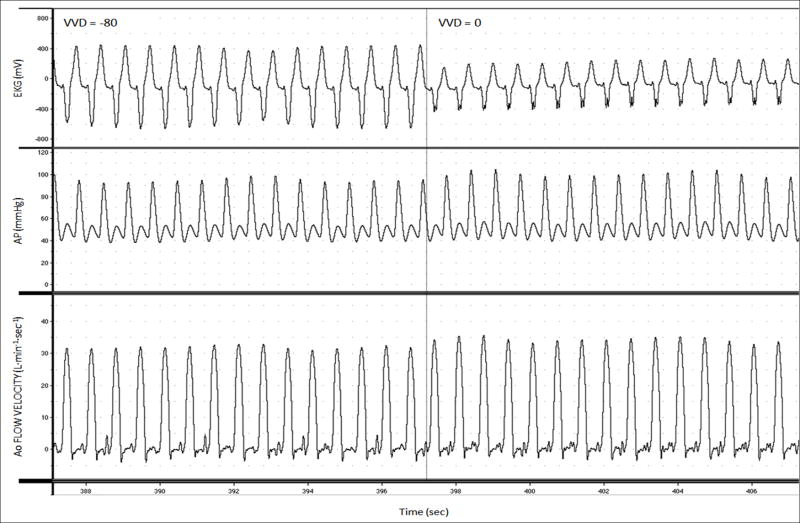
Real-time aortic volume flow, EKG, and arterial pressure signals were collected with an analog-to-digital converter (PowerLab, ADInstruments, Inc., Milford, MA) and recorded on a personal computer (iMac, Apple Computer, Inc, Cupertino, CA) (Figure 5). CO was measured by integrating aortic volume flow tracings over one respiratory cycle using MacLab software (ADInstruments, Inc, Milford, MA) and custom designed routines in Matlab (The MathWorks, Inc, Natick, MA).
Figure 5.
Intraoperative recordings from a representative patient displaying changes in electrocardiogram (EKG), arterial pressure (AP), and aortic flow velocity during biventricular pacing (BiVP) optimization.
BiVP optimization was performed by optimization of AVD, followed by VVD. All pacing settings during optimization were conducted over 10-second intervals and were tested twice. The use of a rapid optimization protocol measuring changes in cardiac mechanics over brief intervals has been described previously.14, 27 AVD optimization was performed during sequential RA-BiVP, with a VVD of 0 msec. AVD was varied in 30-msec increments, ranging from 90-270 msec, in randomized order. AVDs that were longer than the patient's intrinsic paced AVD were not tested. The AVD yielding the highest CO was selected as the optimum AVD. AVD optimization data from a representative patient is shown in Figure 1. VVD optimization was then performed, using the optimum AVD, by varying VVD by 20-msec increments, ranging from -80 msec (LV first) to +80 msec (RV first), in randomized order. CO as a function of VVD was plotted, and the VVD yielding the highest CO was selected as the optimum VVD (Figure 2), thereby defining the optimum BiVP parameters for the patient. Optimized BiVP was then compared to RA pacing (AAI mode), at the same heart rate, and to NSR with no pacing in randomized order, over 30-second intervals. The aortic flow probe was then removed and the temporary pacing leads were externalized for further BiVP optimization in subsequent phases of the BiPACS trial.
Figure 1.
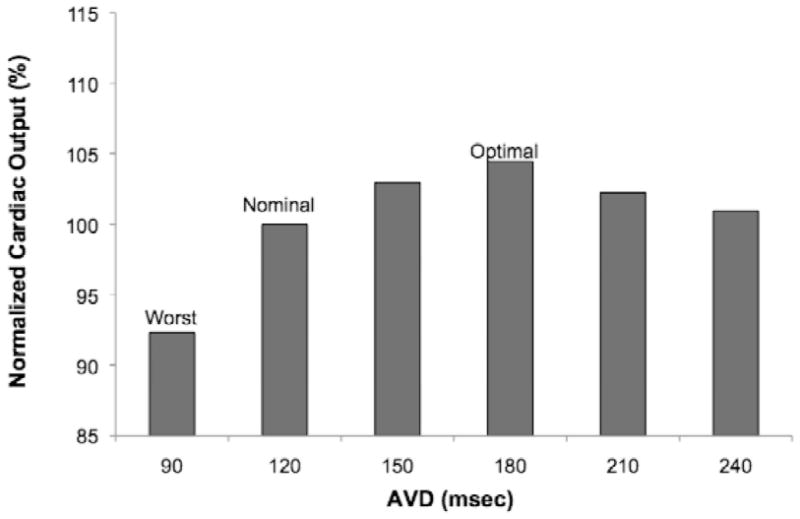
Cardiac output (CO) vs. atrioventricular delay (AVD) from a representative patient during AVD optimization.
Figure 2.
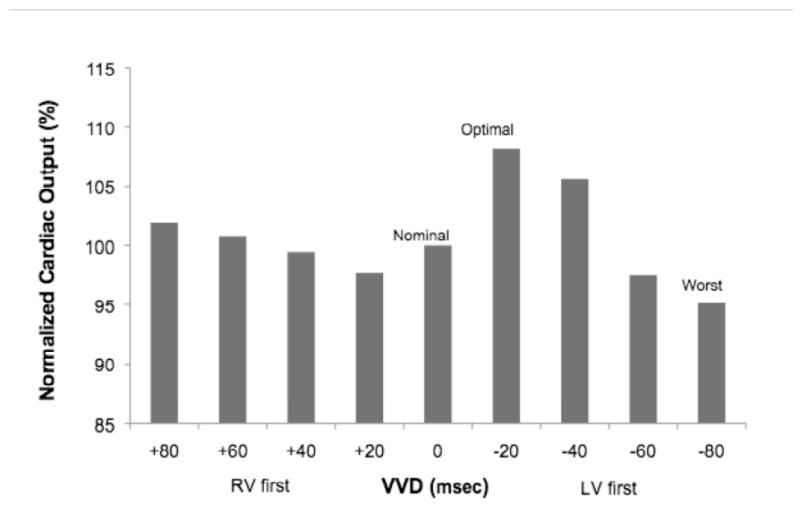
CO vs. interventricular delay (VVD) from a representative patient during VVD optimization.
Statistical Analysis
For AVD and VVD optimization data and for the comparison among optimized BiVP, AAI, and NSR, descriptive statistics were calculated for each group. Differences among multiple groups (3) were tested using blocked, one-way Analysis of Variation (ANOVA). Post hoc comparisons to assess pairwise differences between groups were performed using the Tukey test adjusted for multiple comparisons. Differences between two groups were tested using a two-way paired Student's t-test. P values of <0.05 were considered significant. Statistical analysis was performed using SAS 9.1 (SAS Institute, Inc., Cary, NC).
Results
Patient flow for the BiPACS trial and this sub-study covers recruitment from 4/1/07 to 6/2/09. The number of patients screened was 2,261, and 60 were enrolled. Thirty-three patients received the intended testing in Phase 1 and were subsequently randomized to BiVP (Experimental Group) or standard of care (Control Group). There were no adverse events during Phase 1. The number of these patients for whom the primary endpoint was measured was 13 in the BiVP group and 13 in the standard of care group. The study has not been completed, however, so no analysis of primary and secondary outcomes has been done, and the present study only describes results for Phase 1. Accordingly, the data after randomization and the period of follow-up are not relevant and are not summarized.
Among the 33 patients who received Phase I testing in the BiPACS trial, 13 were eliminated from this sub-study because of frequent ventricular ectopy, an intra-aortic balloon pump, or noisy aortic flow tracings. Ultimately, of the 20 patients included in this sub-study, there were 13 without second or third degree AV block who completed comparison of optimized BiVP, AAI, and NSR. Baseline clinical characteristics are shown in Table O-1.
Table O-1.
Baseline Characteristics (ON LINE ONLY)
| Number of Patients: | |
|---|---|
| Total: | 20 |
| Optimization analysis | 20 |
| Optimized BiVP vs. AAI vs. sinus rhythm | 13 |
| Age (years ± SD) | 67.6 ± 12.2 |
| Ejection Fraction (% ± SD) | 33.4 ± 15.4 |
| QRS duration (ms ± SD) | 115.7 ± 19.1 |
| Male gender (%) | 75 |
| Type of Surgery: | |
| CABG/AVR, CABG/MVR, CABG/AVR/MVR | 9 |
| AVR/MVR | 5 |
| CABG | 3 |
| AVR | 3 |
BiVP, biventricular pacing; AAI, atrial pacing; SD, Standard Deviation; CABG, coronary artery bypass grafting; AVR, aortic valve replacement; MVR, mitral valve replacement or repair.
The majority of patients underwent valvular surgery, including double valve cases, as well as combination valve and coronary artery bypass graft (CABG) surgery. Average demographics were age 67±12 (S.D.) years, male gender 75%. LVEF averaged 33±15%, and average QRS duration was 116±19 msec. Nine patients underwent combined CABG and aortic and/or mitral valve replacement. Five underwent combined aortic and mitral valve surgery, three underwent aortic surgery alone, and three underwent isolated CABG.
Results of AVD and VVD optimization are shown in Figure 3. The intrinsic, paced AVD was >150 msec in all patients and >270 msec in 10 patients. The mean optimum AVD was 171 ± 8 msec vs. 111 ± 11 msec for the mean worst AVD (p < 0.001). The optimum AVD was >150 msec in 10 patients and >120 msec in all but two patients. Comparison of mean CO for the optimized, worst, and nominal (120 msec) AVD settings showed significant differences among groups (p < 0.001). In pairwise comparisons, mean CO was different in both the optimum and worst groups, compared to that of AVD 120 msec. BiVP with the optimum AVD differed from the worst AVD (p < 0.001), with a mean increase in CO of 14% (range: 2-34%). The optimal AVD differed from an AVD of 120 msec (p < 0.001), with a mean increase in CO of 7% (range: 0-34%).
Figure 3.
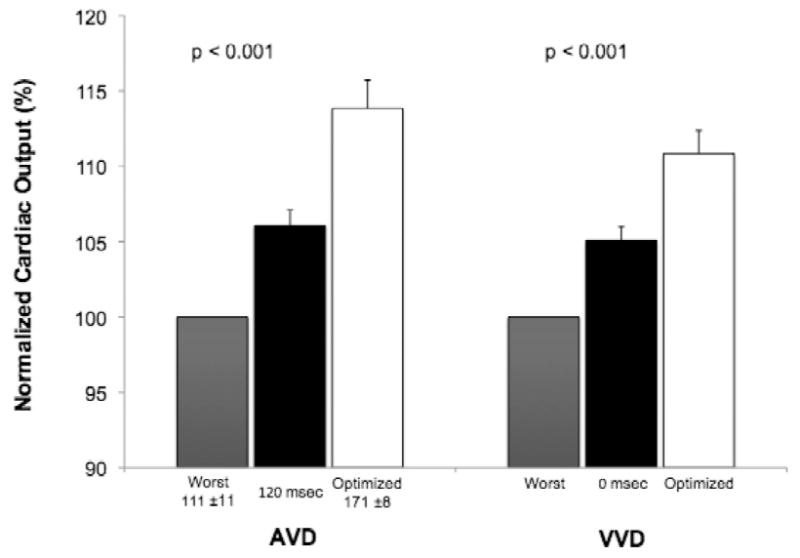
BiVP optimization profiles for AVD and VVD. Mean COs are normalized to a scale of 100, with the worst parameter as the reference group. Error bars depict one standard error of the mean.
VVD optimization after AVD optimization yielded significant differences in CO when comparing the optimum, worst, and nominal (0 msec) VVD settings (p < 0.001) (Figure 3). In pairwise comparisons, both the optimum and worst VVD differed from the nominal VVD. BiVP with the optimum VVD differed from the worst VVD (p < 0.001), increasing CO by 10% (range: 4-29%), and from the nominal VVD (p < 0.001), increasing CO by 5.0% (range: 0-29%).
Distributions of optimal and worst AVDs and VVDs are shown in Figures O-6 and O-7, respectively. An AVD of 90 yielded the lowest CO in the majority of patients. In all but two patients, the optimal AVD was >120 msec, and, in three patients, an AVD of 120 msec was the worst setting. VVD optimization resulted in a pattern of optimum and worst VVD settings, ranging from RV-first to LV-first pacing, likely reflecting heterogeneity in the types of IVCD among patients. In two patients, the nominal VVD yielded the lowest CO.
Figure O-6.
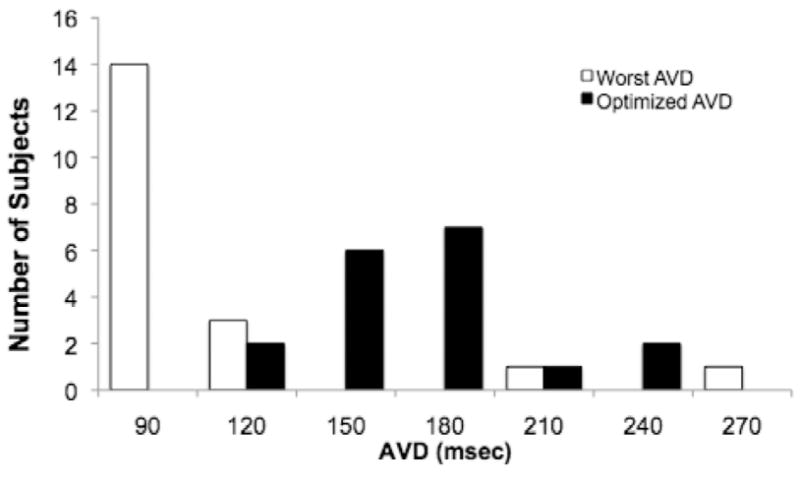
Distribution of optimal and worst AVD settings determined during AVD optimization.
Figure O-7.
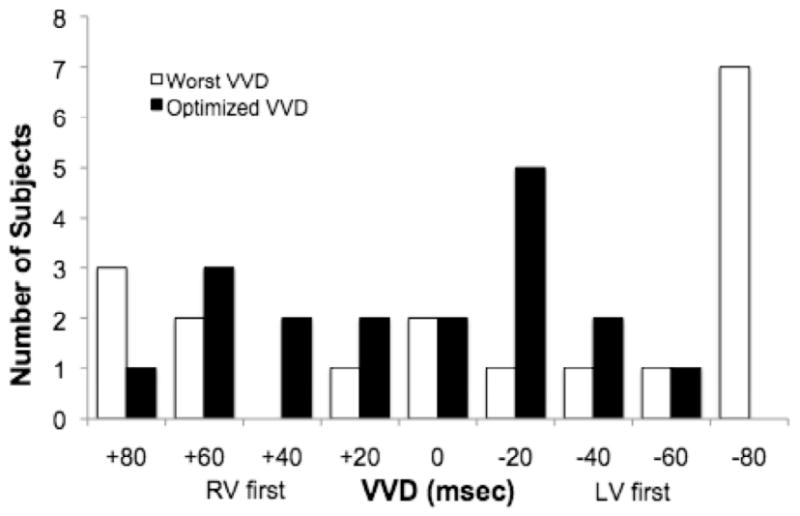
Distribution of optimal and worst VVD settings determined during VVD optimization.
Comparison of optimized BiVP to AAI and NSR is shown in Figure 4. The differences among the three groups were significant (p = 0.003), as were pairwise comparisons between optimized BiVP vs. AAI and NSR (p = 0.003 and 0.019, respectively). Optimized BiVP resulted in an increase in mean CO by 13% vs. AAI at the same heart rate (CO 5.5 ± 0.6 vs. 4.9 ± 0.6 L/min) and by 10% vs. NSR (5.0 ± 0.5 L/min). The paced heart rate was greater in the BiVP and AAI groups (97 ± 3 bpm), compared to the NSR group (80 ± 4 bpm) (p < 0.001).
Figure 4.
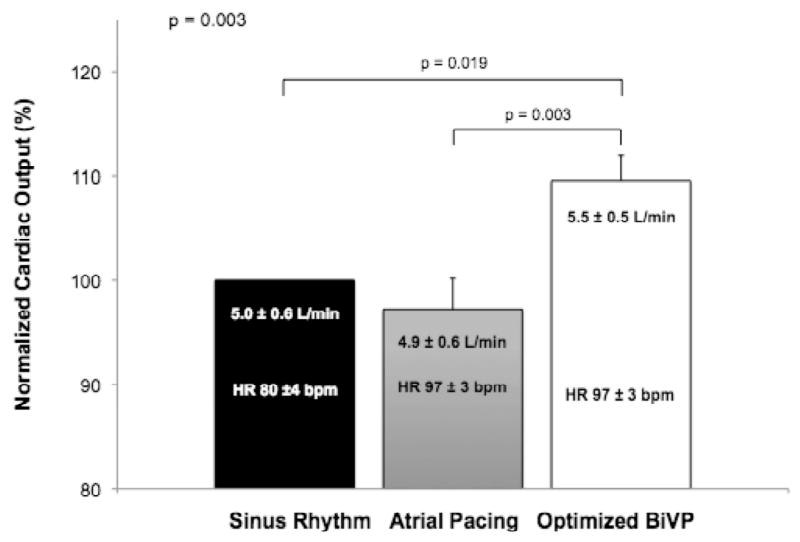
Comparison of CO with optimized BiVP vs. right atrial pacing or sinus rhythm. Error bars depict one standard error of the mean.
Discussion
Defining the best modality and method for permanent BiVP optimization, in order to improve BiVP response rates and heart failure outcomes, is an active area of investigation. To our knowledge, the BiPACS trial is the first randomized clinical trial to assess the role of temporary BiVP optimization at multiple time points in the perioperative cardiac surgery setting. In this sub-study, which focused on intraoperative BiVP in the immediately post-CPB period, we found that BiVP optimization increased CO compared to both AAI and NSR (no pacing), with significant contributions from optimization of both AVD and VVD (Figure 3). The difference in CO between the best and the worst settings was considerable, ranging as high as 34% in an individual patient. Moreover, in five patients, programming a nominal AVD or VVD resulted in the least effective BiVP setting, which supports a rationale for routine optimization in all patients undergoing temporary BiVP.
AVD optimization represents a balance between optimizing LV filling and atrial emptying and minimizing diastolic mitral regurgitation. Nominal, “out of the box” AVD settings are typically programmed to short intervals, such as 120 msec, to ensure biventricular capture. However, empiric use of short AVDs may routinely underestimate the optimal AVD.28 Longer intrinsic paced AVDs, in the post-CPB period, allowed for testing of AVDs greater than or equal to 240 msec in the majority of patients. AVD optimization alone resulted in a 14% increase in CO between the best and worst settings and a 7% increase compared to an AVD of 120. In all but two patients, the optimum AVD was longer than 120 msec (Figure O-6). Indeed, in the immediate post-CPB period, factors contributing to impaired diastolic function, such as myocardial ischemia and edema, may necessitate the use of longer AVDs in patients with impaired LV function. In this study, atrio-BiVP was achieved by pacing from the RA. RA pacing alters interatrial delay and the timing of left atrial-LV contraction, which implies that the optimum AVD would differ in RA paced, biatrially paced, and atrial-sensed BiVP modes.29, 30
Modulation of VVD has been shown to reduce ventricular dyssynchrony and improve hemodynamic parameters.9, 10 In the present study, VVD optimization increased CO by 10% and 5% compared to the worst and nominal settings, respectively, underscoring the additional benefit of VVD optimization, even after AVD has been optimized. Whether the sequence in which AVD and VVD are optimized affects the determination of optimized BiVP parameters is uncertain and warrants further study.
Optimized BiVP improved CO by 13% compared to AAI at the same heart rate, indicating that the mechanism of hemodynamic benefit in BiVP was not explained solely by an increased paced rate compared to the patient's intrinsic sinus rate (Figure 4). This finding is consistent with previous studies of temporary BiVP.13, 17
In this study, the mean preoperative LVEF (33.4%) was higher and the mean QRS (115.7 msec) was narrower than in permanent BiVP trials (Table O-1).2-5 Although the criteria for permanent BiVP implantation are established,1 the predictors of acute response to temporary perioperative BiVP have yet to be defined and are an area of ongoing investigation. Cardiac surgery and extracorporeal circulation cause transient myocardial depression and edema and may exacerbate conduction abnormalities. Patients with pre-existing LV dysfunction are among the highest risk cardiac surgery patients and are an appropriate group in which to assess the benefit of temporary BiVP. Recent evidence suggests that ambulatory CHF patients with narrow QRS complexes do not benefit from permanent BiVP, despite exhibiting echocardiographic evidence of dyssynchrony.31 Whether this applies to temporary perioperative BiVP remains to be seen, especially in a heterogeneous population of ischemic and valvular heart surgery patients.
The mechanism by which BiVP acutely reduces dyssynchrony and improves hemodynamics is not fully understood, particularly in perioperative ventricular failure. We have previously described animal models of acute right- and left-sided heart failure and found BiVP to be beneficial in those settings.32-34 The mechanism of action appears to be synchronization of pressure development across the interventricular septum, which allows the less impaired ventricle to assist the failing one.34 These findings provide further rationale for studying temporary BiVP in the perioperative setting and will serve as a guide for future studies examining the mechanism behind the effect demonstrated in this study.
Another area of uncertainty lies in selecting the best metric by which to assess BiVP optimization, including: measures of ventricular dyssynchrony, mitral inflow, stroke volume, and dP/dtmax. The present study optimized BiVP based on CO, which is an important short-term endpoint for end-organ perfusion, particularly in critically ill, postoperative patients with low output states. Further study is needed to evaluate the relationship between hemodynamic responses to temporary BiVP, changes in ventricular synchrony, and patient outcomes.
Outcomes data including morbidity, mortality, ICU length of stay, and hospital costs are important secondary objectives of the BiPACS trial. Though the absolute changes in CO reported here during BiVP are relatively modest, the clinical impact may be important if amplified by a reduced requirement for beta agonists and vasopressors, with secondary improvements in peripheral organ function, fluid requirements, and incidence of arrhythmias.
Accumulating data indicate differences between patients in the BiPACS trial and those undergoing permanent BiVP for chronic heart failure. The optimal BiPACS protocol changes over time, and the effect also changes, with benefits being primarily rate-related on postoperative day number one but primarily related to stroke volume increases in the early post-CPB period. Absolute increases in CO during BiVP also tend to be larger in BiPACS patients with higher preoperative EFs, contradicting observations in chronic heart failure.35 These differences suggest that the effects of BiVP in BiPACS are primarily mediated through effects on reversible myocardial injury rather than chronic dysfunction. These differences provide a rationale for expanding the selection of patients in our trial beyond the current recommendations for permanent BiVP. Digital transesophageal echo data capable of defining changes in regional wall motion abnormalities is an important research goal of the BiPACS trial.
The BiPACS trial is being done under an Investigational Device Exemption from the Food and Drug Administration because, as this is written, there is no biventricular pacemaker approved for temporary pacing in the operating room. Similarly, until our trial is completed, we will not have definitive information regarding optimum LV lead locations. Empirically, temporary BiVP can be implemented by adding a temporary bipolar lead configuration to the lateral basal segment of the LV. These leads can be connected to the output terminals of a standard external temporary pacemaker in conjunction with bipolar temporary RV leads connected to the same terminals. This will result in BIVP with a VVD of zero. AVD can then be optimized empirically, using MAP or aortic flow criteria. Our current observations indicate that the optimum AVD may be as long as 300 msec in the early postoperative period, particularly in patients with atrial latency in excess of 100 msec (Rusanov A, et al., unpublished data).
In conclusion, optimization of temporary BiVP improves CO in patients undergoing cardiac surgery with preoperative evidence of LV systolic dysfunction and an IVCD. Individualized optimization of AVD and VVD each contributes to the overall benefit of optimized BiVP, and optimization should be considered as a routine step in temporary BiVP protocols. Temporary BiVP to treat low output states after cardiac surgery is a promising area of investigation. Further studies with larger numbers of patients will better define patient selection criteria and refine optimization techniques.
Acknowledgments
The authors thank the BiPACS trial patients and investigators and the Columbia University Division of Cardiothoracic Surgery for their participation. Lauren Bedrosian, our study coordinator 2009-2010, and Giselle Brown provided important assistance with manuscript preparation.
Funding Sources: This study was funded by a grant from the National Institutes of Health (RO1 HL080152 to H. M. Spotnitz). Dr. Spotnitz is the George H. Humphreys, II, Professor of Surgery. Dr. Wang is supported by National Institutes of Health Training Grant T32 HL007854.
Footnotes
Disclosures: Presented as an oral presentation at the 2009 American Heart Association Scientific Sessions in November. Published as an abstract: Wang DY, Richmond ME, Quinn TA, Mirani AJ, Rusanov A, Yalamanchi Y, Weinberg AD, Cabreriza SE, Spotnitz HM. Optimized temporary biventricular pacing acutely improves intraoperative cardiac output after weaning from cardiopulmonary bypass (abstract). Circulation 2009;120:S800.
Disclosures: Dr. Spotnitz is on the Scientific Advisory Board of and holds an ownership interest in Biophan Technologies, Inc. The other authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 2.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 6.Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–73. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 7.Nelson GS, Berger RD, Fetics BJ, Talbot M, Spinelli JC, Hare JM, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–9. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 8.Stellbrink C, Breithardt OA, Franke A, Sack S, Bakker P, Auricchio A, et al. Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol. 2001;38:1957–65. doi: 10.1016/s0735-1097(01)01637-0. [DOI] [PubMed] [Google Scholar]
- 9.León AR, Abraham WT, Brozena S, Daubert JP, Fisher WG, Gurley JC, et al. Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol. 2005;46:2298–304. doi: 10.1016/j.jacc.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 10.van Gelder BM, Bracke FA, Meijer A, Lakerveld LJ, Pijls NH. Effect of optimizing the VV interval on left ventricular contractility in cardiac resynchronization therapy. Am J Cardiol. 2004;93:1500–3. doi: 10.1016/j.amjcard.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 11.Yau TM, Fedak PW, Weisel RD, Teng C, Ivanov J. Predictors of operative risk for coronary bypass operations in patients with left ventricular dysfunction. J Thorac Cardiovasc Surg. 1999;118:1006–13. doi: 10.1016/S0022-5223(99)70094-2. [DOI] [PubMed] [Google Scholar]
- 12.Saxon LA, Kerwin WF, Cahalan MK, Kalman JM, Olgin JE, Foster E, et al. Acute effects of intraoperative multisite ventricular pacing on left ventricular function and activation/contraction sequence in patients with depressed ventricular function. J Cardiovasc Electrophysiol. 1998;9:13–21. doi: 10.1111/j.1540-8167.1998.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 13.Weisse U, Isgro F, Werling Ch, Lehmann A, Saggau W. Impact of atrio-biventricular pacing to poor left-ventricular function after CABG. Thorac Cardiovasc Surg. 2002;50:131–5. doi: 10.1055/s-2002-32403. [DOI] [PubMed] [Google Scholar]
- 14.Berberian G, Quinn TA, Kanter JP, Curtis LJ, Cabreriza SE, Weinberg AD, et al. Optimized biventricular pacing in atrioventricular block after cardiac surgery. Ann Thorac Surg. 2005;80:870–5. doi: 10.1016/j.athoracsur.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 15.Dzemali O, Bakhtiary F, Dogan S, Wittlinger T, Moritz A, Kleine P. Perioperative biventricular pacing leads to improvement of hemodynamics in patients with reduced left-ventricular function--interim results. Pacing Clin Electrophysiol. 2006;29:1341–5. doi: 10.1111/j.1540-8159.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt C, Frielingsdorf J, Debrunner M, Tavakoli R, Genoni M, Straumann E, et al. Acute biventricular pacing after cardiac surgery has no influence on regional and global left ventricular systolic function. Europace. 2007;9:432–6. doi: 10.1093/europace/eum042. [DOI] [PubMed] [Google Scholar]
- 17.Pichlmaier M, Bagaev E, Lichtenberg A, Teebken O, Klein G, Niehaus M, et al. Four-chamber pacing in patients with poor ejection fraction but normal QRS durations undergoing open-heart surgery. Pacing Clin Electrophysiol. 2008;31:184–91. doi: 10.1111/j.1540-8159.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 18.Muehlschlegel JD, Peng YG, Lobato EB, Hess PJ, Jr, Martin TD, Klodell CT., Jr Temporary biventricular pacing post-cardiopulmonary bypass in patients with reduced ejection fraction. J Card Surg. 2008;23:324–30. doi: 10.1111/j.1540-8191.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 19.Eberhardt F, Heringlake M, Massalme MS, Dyllus A, Misfeld M, Sievers HH, et al. The effect of biventricular pacing after coronary artery bypass grafting: a prospective randomized trial of different pacing modes in patients with reduced left ventricular function. J Thorac Cardiovasc Surg. 2009;137:1461–7. doi: 10.1016/j.jtcvs.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Foster AH, Gold MR, McLaughlin JS. Acute hemodynamic effects of atrio-biventricular pacing in humans. Ann Thorac Surg. 1995;59:294–300. doi: 10.1016/0003-4975(94)00878-b. [DOI] [PubMed] [Google Scholar]
- 21.Flynn MJ, McComb JM, Dark JH. Temporary left ventricular pacing improves haemodynamic performance in patients requiring epicardial pacing post cardiac surgery. Eur J Cardiothorac Surg. 2005;28(2):250–3. doi: 10.1016/j.ejcts.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Hajiseyedjavadi O, Pasque M, Moon M, Damiano R, Mucha T, Hebert K, et al. Coronary artery bypass grafting and biventricular pacing efficacy: do past trials dictate a change in future practice? J Thorac Cardiovasc Surg. 2006;132:974–5. doi: 10.1016/j.jtcvs.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 23.Evonich RF, Stephens JC, Merhi W, Dukkipati S, Tepe N, Shannon F, et al. The role of temporary biventricular pacing in the cardiac surgical patient with severely reduced left ventricular systolic function. J Thorac Cardiovasc Surg. 2008;136:915–21. doi: 10.1016/j.jtcvs.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 24.Flynn M, Dark JH, McComb JM. Biventricular pacing after cardiac surgery. J Thorac Cardiovasc Surg. 2009;138:259–60. doi: 10.1016/j.jtcvs.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 25.Dekker AL, Phelps B, Dijkman B, van der Nagel T, van der Veen FH, Geskes GG, et al. Epicardial left ventricular lead placement for cardiac resynchronization therapy: optimal pace site selection with pressure-volume loops. J Thorac Cardiovasc Surg. 2004;127:1641–7. doi: 10.1016/j.jtcvs.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Hanke T, Misfeld M, Heringlake M, Schreuder JJ, Wiegand UK, Eberhardt F. The effect of biventricular pacing on cardiac function after weaning from cardiopulmonary bypass in patients with reduced left ventricular function: a pressure-volume loop analysis. J Thorac Cardiovasc Surg. 2009;138:148–56. doi: 10.1016/j.jtcvs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Rabkin DG, Cabreriza SE, Curtis LJ, Mazer SP, Kanter JP, Weinberg AD, et al. Load dependence of cardiac output in biventricular pacing: right ventricular pressure overload in pigs. J Thorac Cardiovasc Surg. 2004;127:1713–22. doi: 10.1016/s0022-5223(03)01319-9. [DOI] [PubMed] [Google Scholar]
- 28.Gold MR, Niazi I, Giudici M, Leman RB, Sturdivant JL, Kim MH, et al. A prospective comparison of AV delay programming methods for hemodynamic optimization during cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2007;18:490–6. doi: 10.1111/j.1540-8167.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 29.Cha YM, Nishimura RA, Hayes DL. Difference in mechanical atrioventricular delay between atrial sensing and atrial pacing modes in patients with hypertrophic and dilated cardiomyopathy: an electrical hemodynamic catheterization study. J Interv Card Electrophysiol. 2002;6:133–40. doi: 10.1023/a:1015311416232. [DOI] [PubMed] [Google Scholar]
- 30.Bernheim A, Ammann P, Sticherling C, Burger P, Schaer B, Brunner-La Rocca HP, et al. Right atrial pacing impairs cardiac function during resynchronization therapy: acute effects of DDD pacing compared to VDD pacing. J Am Coll Cardiol. 2005;45:1482–7. doi: 10.1016/j.jacc.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Beshai JF, Grimm RA, Nagueh SF, Baker JH, 2nd, Beau SL, Greenberg SM, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–71. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 32.Quinn TA, Berberian G, Cabreriza SE, Maskin LJ, Weinberg AD, Holmes JW, et al. Effects of sequential biventricular pacing during acute right ventricular pressure overload. Am J Physiol Heart Circ Physiol. 2006;291:H2380–7. doi: 10.1152/ajpheart.00446.2006. [DOI] [PubMed] [Google Scholar]
- 33.Berberian G, Quinn TA, Cabreriza SE, Garofalo CA, Barrios DM, Weinberg AD, et al. Load dependence of cardiac output in biventricular pacing: left ventricular volume overload in pigs. J Thorac Cardiovasc Surg. 2006;131:666–70. doi: 10.1016/j.jtcvs.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Quinn TA, Cabreriza SE, Richmond ME, Weinberg AD, Holmes JW, Spotnitz HM. Simultaneous variation of ventricular pacing site and timing with biventricular pacing in acute ventricular failure improves function by interventricular assist. Am J Physiol Heart Circ Physiol. 2009;297:H2220–6. doi: 10.1152/ajpheart.00802.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spotnitz MD, Wang DY, Cabreriza S, Rusanov A, Cheng B, Quinn A, et al. Does ejection fraction predict response to biventricular pacing after cardiac surgery (abstract)? ASAIO J. 2010;56:102. [Google Scholar]