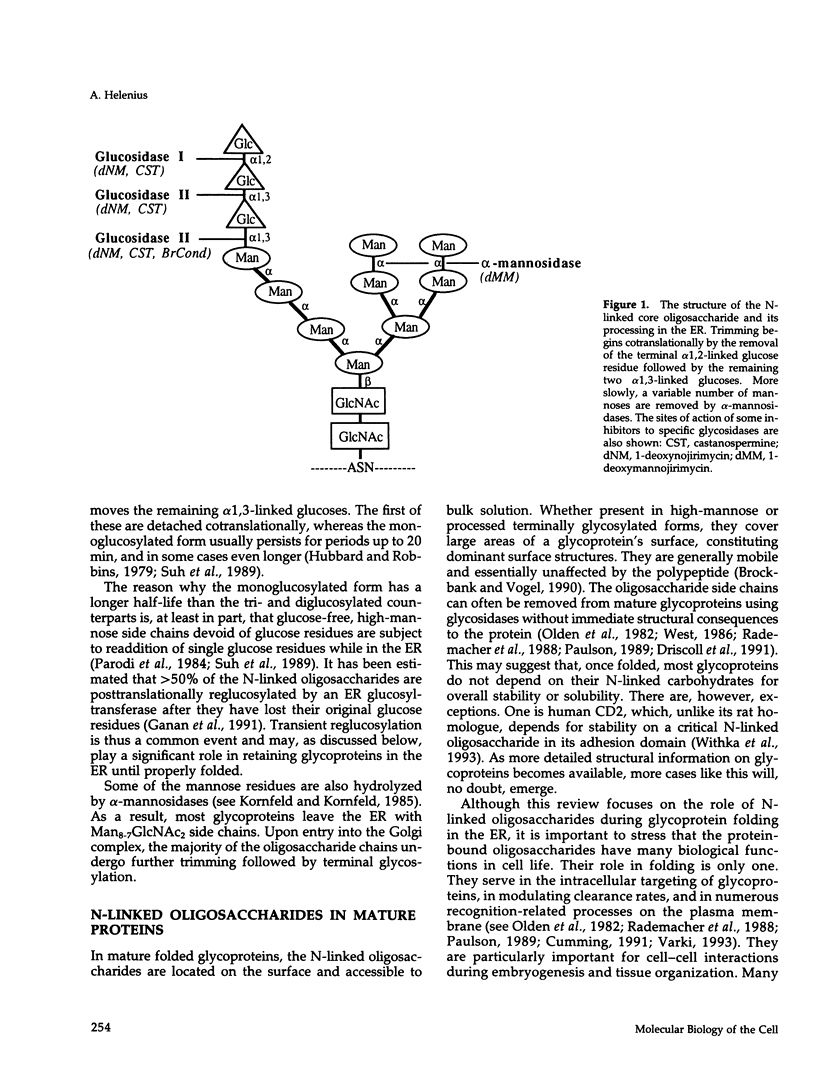
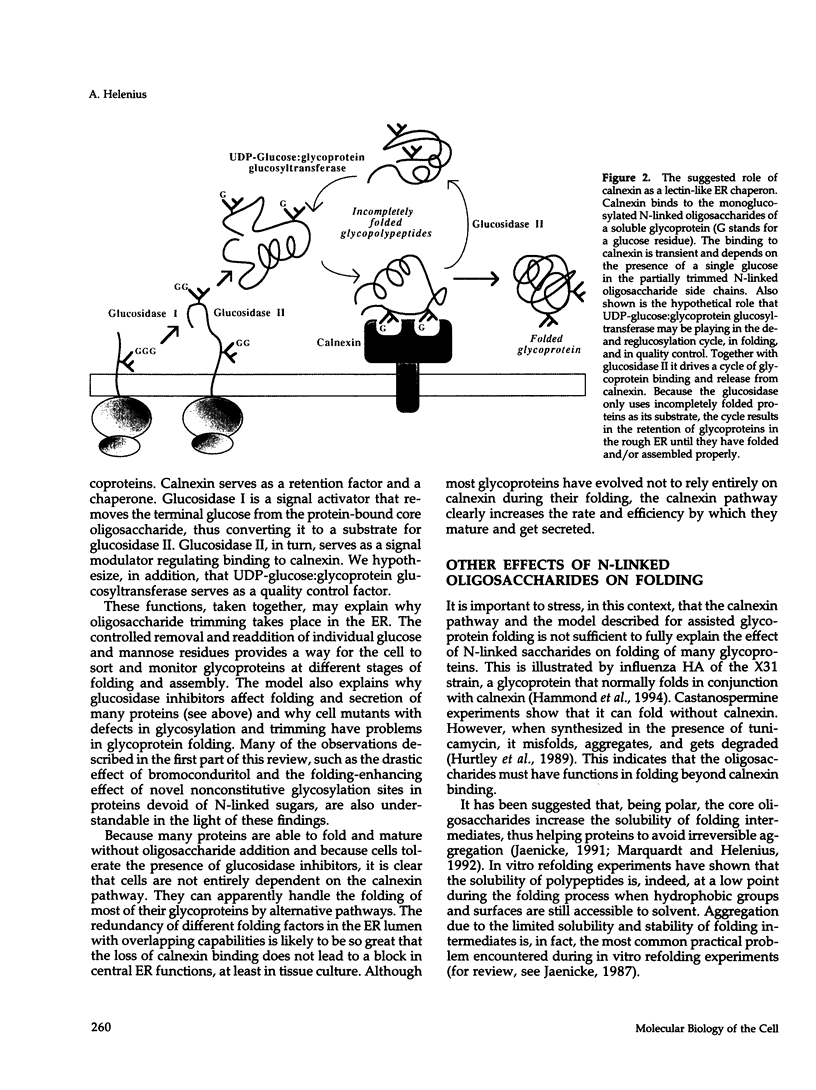
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahluwalia N., Bergeron J. J., Wada I., Degen E., Williams D. B. The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J Biol Chem. 1992 May 25;267(15):10914–10918. [PubMed] [Google Scholar]
- Arakaki R. F., Hedo J. A., Collier E., Gorden P. Effects of castanospermine and 1-deoxynojirimycin on insulin receptor biogenesis. Evidence for a role of glucose removal from core oligosaccharides. J Biol Chem. 1987 Aug 25;262(24):11886–11892. [PubMed] [Google Scholar]
- Atkinson P. H., Lee J. T. Co-translational excision of alpha-glucose and alpha-mannose in nascent vesicular stomatitis virus G protein. J Cell Biol. 1984 Jun;98(6):2245–2249. doi: 10.1083/jcb.98.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J. V., Tlusty A., McDowell W., Legler G., Schwarz R. T. The mannosidase inhibitors 1-deoxymannojirimycin and swainsonine have no effect on the biosynthesis and infectivity of Rous sarcoma virus. Virology. 1985 May;143(1):342–346. doi: 10.1016/0042-6822(85)90122-9. [DOI] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature. 1992 Mar 19;356(6366):260–262. doi: 10.1038/356260a0. [DOI] [PubMed] [Google Scholar]
- Brada D., Dubach U. C. Isolation of a homogeneous glucosidase II from pig kidney microsomes. Eur J Biochem. 1984 May 15;141(1):149–156. doi: 10.1111/j.1432-1033.1984.tb08169.x. [DOI] [PubMed] [Google Scholar]
- Brockbank R. L., Vogel H. J. Structure of the oligosaccharide of hen phosvitin as determined by two-dimensional 1H NMR of the intact glycoprotein. Biochemistry. 1990 Jun 12;29(23):5574–5583. doi: 10.1021/bi00475a023. [DOI] [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984 Mar;3(3):551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins D. L., Schlesinger S. Physical properties of the glycoprotein of vesicular stomatitis virus measured by intrinsic fluorescence and aggregation. Biochemistry. 1982 Jul 6;21(14):3518–3524. doi: 10.1021/bi00257a040. [DOI] [PubMed] [Google Scholar]
- Crowe A. J., Hayman M. J. Altered glycosylation of the env-sea oncoprotein inhibits intracellular transport and transformation. Cell Growth Differ. 1993 May;4(5):403–410. [PubMed] [Google Scholar]
- Cumming D. A. Glycosylation of recombinant protein therapeutics: control and functional implications. Glycobiology. 1991 Mar;1(2):115–130. doi: 10.1093/glycob/1.2.115. [DOI] [PubMed] [Google Scholar]
- Datema R., Romero P. A., Legler G., Schwarz R. T. Inhibition of formation of complex oligosaccharides by the glucosidase inhibitor bromoconduritol. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6787–6791. doi: 10.1073/pnas.79.22.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V., Hochstenbach F., Rajagopalan S., Brenner M. B. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin). J Biol Chem. 1993 May 5;268(13):9585–9592. [PubMed] [Google Scholar]
- Degen E., Cohen-Doyle M. F., Williams D. B. Efficient dissociation of the p88 chaperone from major histocompatibility complex class I molecules requires both beta 2-microglobulin and peptide. J Exp Med. 1992 Jun 1;175(6):1653–1661. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen E., Williams D. B. Participation of a novel 88-kD protein in the biogenesis of murine class I histocompatibility molecules. J Cell Biol. 1991 Mar;112(6):1099–1115. doi: 10.1083/jcb.112.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll P. C., Cyster J. G., Campbell I. D., Williams A. F. Structure of domain 1 of rat T lymphocyte CD2 antigen. Nature. 1991 Oct 24;353(6346):762–765. doi: 10.1038/353762a0. [DOI] [PubMed] [Google Scholar]
- Dubé S., Fisher J. W., Powell J. S. Glycosylation at specific sites of erythropoietin is essential for biosynthesis, secretion, and biological function. J Biol Chem. 1988 Nov 25;263(33):17516–17521. [PubMed] [Google Scholar]
- Duronio V., Jacobs S., Romero P. A., Herscovics A. Effects of inhibitors of N-linked oligosaccharide processing on the biosynthesis and function of insulin and insulin-like growth factor-I receptors. J Biol Chem. 1988 Apr 15;263(11):5436–5445. [PubMed] [Google Scholar]
- Edwards E. H., Sprague E. A., Kelley J. L., Kerbacher J. J., Schwartz C. J., Elbein A. D. Castanospermine inhibits the function of the low-density lipoprotein receptor. Biochemistry. 1989 Sep 19;28(19):7679–7687. doi: 10.1021/bi00445a024. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Glycosidase inhibitors as antiviral and/or antitumor agents. Semin Cell Biol. 1991 Oct;2(5):309–317. [PubMed] [Google Scholar]
- Elbein A. D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991 Dec;5(15):3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Legler G., Tlusty A., McDowell W., Schwarz R. The effect of deoxymannojirimycin on the processing of the influenza viral glycoproteins. Arch Biochem Biophys. 1984 Dec;235(2):579–588. doi: 10.1016/0003-9861(84)90232-7. [DOI] [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Freedman R. B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989 Jun 30;57(7):1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Gallagher P. J., Henneberry J. M., Sambrook J. F., Gething M. J. Glycosylation requirements for intracellular transport and function of the hemagglutinin of influenza virus. J Virol. 1992 Dec;66(12):7136–7145. doi: 10.1128/jvi.66.12.7136-7145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P., Henneberry J., Wilson I., Sambrook J., Gething M. J. Addition of carbohydrate side chains at novel sites on influenza virus hemagglutinin can modulate the folding, transport, and activity of the molecule. J Cell Biol. 1988 Dec;107(6 Pt 1):2059–2073. doi: 10.1083/jcb.107.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin K., Krishna S., Ponchel F., Frohlich M., Cummings D. E., Carlson R., Wands J. R., Isselbacher K. J., Pillai S., Ozturk M. The major histocompatibility complex class I antigen-binding protein p88 is the product of the calnexin gene. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8452–8456. doi: 10.1073/pnas.89.18.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gañn S., Cazzulo J. J., Parodi A. J. A major proportion of N-glycoproteins are transiently glucosylated in the endoplasmic reticulum. Biochemistry. 1991 Mar 26;30(12):3098–3104. doi: 10.1021/bi00226a017. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Gotz G., Gañn S., Parodi A. J. Glucosylation of glycoproteins in Crithidia fasciculata. Mol Biochem Parasitol. 1991 Apr;45(2):265–273. doi: 10.1016/0166-6851(91)90094-m. [DOI] [PubMed] [Google Scholar]
- Grafl R., Lang K., Vogl H., Schmid F. X. The mechanism of folding of pancreatic ribonucleases is independent of the presence of covalently linked carbohydrate. J Biol Chem. 1987 Aug 5;262(22):10624–10629. [PubMed] [Google Scholar]
- Gross V., Andus T., Tran-Thi T. A., Schwarz R. T., Decker K., Heinrich P. C. 1-deoxynojirimycin impairs oligosaccharide processing of alpha 1-proteinase inhibitor and inhibits its secretion in primary cultures of rat hepatocytes. J Biol Chem. 1983 Oct 25;258(20):12203–12209. [PubMed] [Google Scholar]
- Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985 Sep;42(2):489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Hammond C., Braakman I., Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Helenius A. A chaperone with a sweet tooth. Curr Biol. 1993 Dec 1;3(12):884–886. doi: 10.1016/0960-9822(93)90226-e. [DOI] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Cell surface expression of membrane-anchored v-sis gene products: glycosylation is not required for cell surface transport. J Cell Biol. 1986 Dec;103(6 Pt 1):2311–2322. doi: 10.1083/jcb.103.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hawn T. R., Tom T. D., Strand M. Molecular cloning and expression of SmIrV1, a Schistosoma mansoni antigen with similarity to calnexin, calreticulin, and OvRal1. J Biol Chem. 1993 Apr 15;268(11):7692–7698. [PubMed] [Google Scholar]
- Hearing J., Gething M. J., Sambrook J. Addition of truncated oligosaccharides to influenza virus hemagglutinin results in its temperature-conditional cell-surface expression. J Cell Biol. 1989 Feb;108(2):355–365. doi: 10.1083/jcb.108.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing J., Hunter E., Rodgers L., Gething M. J., Sambrook J. Isolation of Chinese hamster ovary cell lines temperature conditional for the cell-surface expression of integral membrane glycoproteins. J Cell Biol. 1989 Feb;108(2):339–353. doi: 10.1083/jcb.108.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Hettkamp H., Legler G., Bause E. Purification by affinity chromatography of glucosidase I, an endoplasmic reticulum hydrolase involved in the processing of asparagine-linked oligosaccharides. Eur J Biochem. 1984 Jul 2;142(1):85–90. doi: 10.1111/j.1432-1033.1984.tb08253.x. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Hochstenbach F., David V., Watkins S., Brenner M. B. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991 Apr 2;30(13):3147–3161. doi: 10.1021/bi00227a001. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Kreibich G., Gilmore R. Oligosaccharyltransferase activity is associated with a protein complex composed of ribophorins I and II and a 48 kd protein. Cell. 1992 Apr 3;69(1):55–65. doi: 10.1016/0092-8674(92)90118-v. [DOI] [PubMed] [Google Scholar]
- Kern G., Kern D., Jaenicke R., Seckler R. Kinetics of folding and association of differently glycosylated variants of invertase from Saccharomyces cerevisiae. Protein Sci. 1993 Nov;2(11):1862–1868. doi: 10.1002/pro.5560021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern G., Schülke N., Schmid F. X., Jaenicke R. Stability, quaternary structure, and folding of internal, external, and core-glycosylated invertase from yeast. Protein Sci. 1992 Jan;1(1):120–131. doi: 10.1002/pro.5560010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D. Architectural editing: determining the fate of newly synthesized membrane proteins. New Biol. 1989 Oct;1(1):3–8. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Landolfi N. F., Rich R. R., Cook R. G. Differential glycosylation requirements for the cell surface expression of class I molecules. J Immunol. 1985 Jan;134(1):423–430. [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem. 1977 Dec 25;252(24):9018–9023. [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Cathepsin D and beta-hexosaminidase synthesized in the presence of 1-deoxynojirimycin accumulate in the endoplasmic reticulum. J Biol Chem. 1984 Aug 25;259(16):10129–10135. [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Glucose removal from N-linked oligosaccharides is required for efficient maturation of certain secretory glycoproteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1984 May;98(5):1720–1729. doi: 10.1083/jcb.98.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Doms R. W., Bole D. G., Helenius A., Rose J. K. Heavy chain binding protein recognizes incompletely disulfide-bonded forms of vesicular stomatitis virus G protein. J Biol Chem. 1990 Apr 25;265(12):6879–6883. [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Influence of new glycosylation sites on expression of the vesicular stomatitis virus G protein at the plasma membrane. J Biol Chem. 1988 Apr 25;263(12):5948–5954. [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- Margolese L., Waneck G. L., Suzuki C. K., Degen E., Flavell R. A., Williams D. B. Identification of the region on the class I histocompatibility molecule that interacts with the molecular chaperone, p88 (calnexin, IP90). J Biol Chem. 1993 Aug 25;268(24):17959–17966. [PubMed] [Google Scholar]
- Marquardt T., Helenius A. Misfolding and aggregation of newly synthesized proteins in the endoplasmic reticulum. J Cell Biol. 1992 May;117(3):505–513. doi: 10.1083/jcb.117.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Boime I. The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J Cell Biol. 1988 Apr;106(4):1049–1059. doi: 10.1083/jcb.106.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell W., Romero P. A., Datema R., Schwarz R. T. Glucose trimming and mannose trimming affect different phases of the maturation of Sindbis virus in infected BHK cells. Virology. 1987 Nov;161(1):37–44. doi: 10.1016/0042-6822(87)90168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J. M., Kornfeld S. Partial purification and characterization of the glucosidases involved in the processing of asparagine-linked oligosaccharides. Arch Biochem Biophys. 1980 Jan;199(1):249–258. doi: 10.1016/0003-9861(80)90278-7. [DOI] [PubMed] [Google Scholar]
- Michalak M., Milner R. E., Burns K., Opas M. Calreticulin. Biochem J. 1992 Aug 1;285(Pt 3):681–692. doi: 10.1042/bj2850681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Zhao H., Forman J., Ozato K. Expression and function of a nonglycosylated major histocompatibility class I antigen. J Exp Med. 1986 Apr 1;163(4):856–871. doi: 10.1084/jem.163.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. E., Spiro R. G. Inhibition of glucose trimming by castanospermine results in rapid degradation of unassembled major histocompatibility complex class I molecules. J Biol Chem. 1993 Feb 25;268(6):3809–3812. [PubMed] [Google Scholar]
- Neuberger M. S., Rajewsky K. Switch from hapten-specific immunoglobulin M to immunoglobulin D secretion in a hybrid mouse cell line. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1138–1142. doi: 10.1073/pnas.78.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D. T., Hiebert S. W., Lamb R. A. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol Cell Biol. 1990 May;10(5):1989–2001. doi: 10.1128/mcb.10.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E. J., Manger R., Hakomori S., Herscovics A., Rohrschneider L. R. Transformation by the v-fms oncogene product: role of glycosylational processing and cell surface expression. Mol Cell Biol. 1985 Dec;5(12):3467–3475. doi: 10.1128/mcb.5.12.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Jackson M., Peterson P. A. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989 Aug 25;58(4):707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Noiva R., Lennarz W. J. Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem. 1992 Feb 25;267(6):3553–3556. [PubMed] [Google Scholar]
- Olden K., Parent J. B., White S. L. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982 May 12;650(4):209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993 Aug 26;364(6440):771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Pal R., Hoke G. M., Sarngadharan M. G. Role of oligosaccharides in the processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3384–3388. doi: 10.1073/pnas.86.9.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. T., Hori H., Saul R., Sanford B. A., Molyneux R. J., Elbein A. D. Castanospermine inhibits the processing of the oligosaccharide portion of the influenza viral hemagglutinin. Biochemistry. 1983 Aug 2;22(16):3975–3984. doi: 10.1021/bi00285a038. [DOI] [PubMed] [Google Scholar]
- Parent J. B., Yeo T. K., Yeo K. T., Olden K. Differential effects of 1-deoxynojirimycin on the intracellular transport of secretory glycoproteins of human hepatoma cells in culture. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):21–33. doi: 10.1007/BF00230633. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Mendelzon D. H., Lederkremer G. Z., Martin-Barrientos J. Evidence that transient glucosylation of protein-linked Man9GlcNAc2, Man8GlcNAc2, and Man7GlcNAc2 occurs in rat liver and Phaseolus vulgaris cells. J Biol Chem. 1984 May 25;259(10):6351–6357. [PubMed] [Google Scholar]
- Paulson J. C. Glycoproteins: what are the sugar chains for? Trends Biochem Sci. 1989 Jul;14(7):272–276. doi: 10.1016/0968-0004(89)90062-5. [DOI] [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J Biol Chem. 1986 May 25;261(15):6822–6830. [PubMed] [Google Scholar]
- Peyrieras N., Bause E., Legler G., Vasilov R., Claesson L., Peterson P., Ploegh H. Effects of the glucosidase inhibitors nojirimycin and deoxynojirimycin on the biosynthesis of membrane and secretory glycoproteins. EMBO J. 1983;2(6):823–832. doi: 10.1002/j.1460-2075.1983.tb01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Li J. S. Studies with inhibitors of oligosaccharide processing indicate a functional role for complex sugars in the transport and proteolysis of Friend mink cell focus-inducing murine leukemia virus envelope proteins. Virology. 1984 Jul 15;136(1):196–210. doi: 10.1016/0042-6822(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Pique C., Pham D., Tursz T., Dokhélar M. C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992 Feb;66(2):906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitta A. M., Rose J. K., Machamer C. E. A single-amino-acid substitution eliminates the stringent carbohydrate requirement for intracellular transport of a viral glycoprotein. J Virol. 1989 Sep;63(9):3801–3809. doi: 10.1128/jvi.63.9.3801-3809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Reitman M. L., Trowbridge I. S., Kornfeld S. A lectin-resistant mouse lymphoma cell line is deficient in glucosidase II, a glycoprotein-processing enzyme. J Biol Chem. 1982 Sep 10;257(17):10357–10363. [PubMed] [Google Scholar]
- Repp R., Tamura T., Boschek C. B., Wege H., Schwarz R. T., Niemann H. The effects of processing inhibitors of N-linked oligosaccharides on the intracellular migration of glycoprotein E2 of mouse hepatitis virus and the maturation of coronavirus particles. J Biol Chem. 1985 Dec 15;260(29):15873–15879. doi: 10.1016/S0021-9258(17)36339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo L. J., Kornfeld R. Post-translational protein modification in the endoplasmic reticulum. Demonstration of fatty acylase and deoxymannojirimycin-sensitive alpha-mannosidase activities. J Biol Chem. 1988 Jul 5;263(19):9520–9525. [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Roberts P. C., Garten W., Klenk H. D. Role of conserved glycosylation sites in maturation and transport of influenza A virus hemagglutinin. J Virol. 1993 Jun;67(6):3048–3060. doi: 10.1128/jvi.67.6.3048-3060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. A., Datema R., Schwarz R. T. N-methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation. Virology. 1983 Oct 15;130(1):238–242. doi: 10.1016/0042-6822(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Knutson V. P., Kohanski R. A., Simpson T. L., Lane M. D. Role of glycosylation in the processing of newly translated insulin proreceptor in 3T3-L1 adipocytes. J Biol Chem. 1984 Apr 10;259(7):4566–4575. [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Malfer C., Schlesinger M. J. The formation of vesicular stomatitis virus (San Juan strain) becomes temperature-sensitive when glucose residues are retained on the oligosaccharides of the glycoprotein. J Biol Chem. 1984 Jun 25;259(12):7597–7601. [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Analysis of the oligosaccharides on the HLA-DR and DC1 B cell antigens. J Immunol. 1983 Jan;130(1):274–282. [PubMed] [Google Scholar]
- Shipley J. M., Grubb J. H., Sly W. S. The role of glycosylation and phosphorylation in the expression of active human beta-glucuronidase. J Biol Chem. 1993 Jun 5;268(16):12193–12198. [PubMed] [Google Scholar]
- Sidman C. Differing requirements for glycosylation in the secretion of related glycoproteins is determined neither by the producing cell nor by the relative number of oligosaccharide units. J Biol Chem. 1981 Sep 25;256(18):9374–9376. [PubMed] [Google Scholar]
- Sousa M. C., Ferrero-Garcia M. A., Parodi A. J. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992 Jan 14;31(1):97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- Su K., Stoller T., Rocco J., Zemsky J., Green R. Pre-Golgi degradation of yeast prepro-alpha-factor expressed in a mammalian cell. Influence of cell type-specific oligosaccharide processing on intracellular fate. J Biol Chem. 1993 Jul 5;268(19):14301–14309. [PubMed] [Google Scholar]
- Suh K., Bergmann J. E., Gabel C. A. Selective retention of monoglucosylated high mannose oligosaccharides by a class of mutant vesicular stomatitis virus G proteins. J Cell Biol. 1989 Mar;108(3):811–819. doi: 10.1083/jcb.108.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara P. S., Taylor D. L., Kang M. S., Bowlin T. L., Liu P. S., Tyms A. S., Sjoerdsma A. Anti-HIV activity of castanospermine analogues. Lancet. 1989 May 27;1(8648):1206–1206. doi: 10.1016/s0140-6736(89)92787-6. [DOI] [PubMed] [Google Scholar]
- Taylor A. K., Wall R. Selective removal of alpha heavy-chain glycosylation sites causes immunoglobulin A degradation and reduced secretion. Mol Cell Biol. 1988 Oct;8(10):4197–4203. doi: 10.1128/mcb.8.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Sunkara P. S., Liu P. S., Kang M. S., Bowlin T. L., Tyms A. S. 6-0-butanoylcastanospermine (MDL 28,574) inhibits glycoprotein processing and the growth of HIVs. AIDS. 1991 Jun;5(6):693–698. doi: 10.1097/00002030-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Tooze J., Kern H. F., Fuller S. D., Howell K. E. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J Cell Biol. 1989 Jul;109(1):35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta S. E., Bosch M., Parodi A. J. Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry. 1989 Oct 3;28(20):8108–8116. doi: 10.1021/bi00446a022. [DOI] [PubMed] [Google Scholar]
- Trombetta S. E., Gañan S. A., Parodi A. J. The UDP-Glc:glycoprotein glucosyltransferase is a soluble protein of the endoplasmic reticulum. Glycobiology. 1991 Mar;1(2):155–161. doi: 10.1093/glycob/1.2.155. [DOI] [PubMed] [Google Scholar]
- Trombetta S. E., Parodi A. J. Purification to apparent homogeneity and partial characterization of rat liver UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 1992 May 5;267(13):9236–9240. [PubMed] [Google Scholar]
- Tufaro F., Snider M. D., McKnight S. L. Identification and characterization of a mouse cell mutant defective in the intracellular transport of glycoproteins. J Cell Biol. 1987 Aug;105(2):647–657. doi: 10.1083/jcb.105.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyms A. S., Berrie E. M., Ryder T. A., Nash R. J., Hegarty M. P., Taylor D. L., Mobberley M. A., Davis J. M., Bell E. A., Jeffries D. J. Castanospermine and other plant alkaloid inhibitors of glucosidase activity block the growth of HIV. Lancet. 1987 Oct 31;2(8566):1025–1026. doi: 10.1016/s0140-6736(87)92588-8. [DOI] [PubMed] [Google Scholar]
- Unnasch T. R., Gallin M. Y., Soboslay P. T., Erttmann K. D., Greene B. M. Isolation and characterization of expression cDNA clones encoding antigens of Onchocerca volvulus infective larvae. J Clin Invest. 1988 Jul;82(1):262–269. doi: 10.1172/JCI113581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C., Grossi C. E., Milstein C., Sitia R. Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J Cell Biol. 1991 Nov;115(4):983–994. doi: 10.1083/jcb.115.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I., Rindress D., Cameron P. H., Ou W. J., Doherty J. J., 2nd, Louvard D., Bell A. W., Dignard D., Thomas D. Y., Bergeron J. J. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991 Oct 15;266(29):19599–19610. [PubMed] [Google Scholar]
- West C. M. Current ideas on the significance of protein glycosylation. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):3–20. doi: 10.1007/BF00230632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. M., Enns C. A. A region of the C-terminal portion of the human transferrin receptor contains an asparagine-linked glycosylation site critical for receptor structure and function. J Biol Chem. 1993 Jun 15;268(17):12780–12786. [PubMed] [Google Scholar]
- Withka J. M., Wyss D. F., Wagner G., Arulanandam A. R., Reinherz E. L., Recny M. A. Structure of the glycosylated adhesion domain of human T lymphocyte glycoprotein CD2. Structure. 1993 Sep 15;1(1):69–81. doi: 10.1016/0969-2126(93)90009-6. [DOI] [PubMed] [Google Scholar]
- de Virgilio C., Bürckert N., Neuhaus J. M., Boller T., Wiemken A. CNE1, a Saccharomyces cerevisiae homologue of the genes encoding mammalian calnexin and calreticulin. Yeast. 1993 Feb;9(2):185–188. doi: 10.1002/yea.320090209. [DOI] [PubMed] [Google Scholar]



