Summary
Purpose
High-frequency oscillations (HFOs) are an emerging biomarker for epileptic tissue. Yet the mechanism by which HFOs are produced is unknown, and their rarity makes them difficult to study. Our objective was to examine the occurrence of HFOs in relation to action potentials (APs) and the effect of microstimulation in the tetanus toxin (TT) model of epilepsy, a non-lesional model with a short latency to spontaneous seizures.
Methods
Rats were injected with TT into dorsal hippocampus and implanted with a 16 channel (8 × 2) multielectrode array, one row each in CA3 and CA1. After onset of spontaneous seizures (3-9 days), recordings were begun of APs and local field potentials, analyzed for the occurrence of interictal spikes and HFOs. Recordings were made during microstimulation of each electrode using customized, open-source software.
Results
Population bursts of APs during interictal spikes were phase-locked with HFOs, which were observable almost exclusively with high-amplitude interictal spikes. Further, HFOs could reliably be produced by microstimulation of the hippocampus, providing evidence that these oscillations can be controlled temporally by external means.
Discussion
We show for the first time the occurrence of HFOs in the TT epilepsy model, an attractive preparation for their experimental investigation and, importantly, one with a different etiology than status models, providing further evidence of the generality of HFOs. The ability to provoke HFOs with microstimulation may prove useful for better understanding HFOs by directly evoking them in the lab, and designing high-throughput techniques for pre-surgical localization of the epileptic focus.
Keywords: Oscillations, microelectrode, stimulation, interictal spike, electrocorticography, animal model
Introduction
Up to a third of patients with epilepsy continue to have seizures despite optimal medical therapy (Cockerell et al., 1995; Kwan and Brodie, 2000). Surgical resection is a potentially curative option for some of these patients but requires accurate localization of the ictal onset zone using electroencephalography (EEG) and/or intracranial electrocorticography (ECoG). A limitation of these methods is that they rely on recording paroxysmal seizures, sometimes requiring weeks of continuous in-patient monitoring associated with risks, substantial cost, and inconvenience. In comparison, the more frequently occurring interictal spikes (IISs) are useful in diagnosing seizure disorders, but have poor localizing capabilities (Hufnagel et al., 2000; Rosenow and Luders, 2001).
High-frequency oscillations (HFOs; 200-400 Hz) in the hippocampus are pathological field potentials detected in the local field potential (LFP), EEG, or ECoG, and are highly specific to epileptic regions in animal models and patients with epilepsy (Rampp and Stefan, 2006; Engel et al., 2009). Moreover, a recent study has shown that HFOs are a more accurate predictor of the seizure onset zone than IISs (Jacobs et al., 2008). As such, HFOs have potential as a more readily ascertainable biomarker for epileptic tissue than IISs or infrequent seizures.
Despite these implications, little is known about the mechanisms of HFOs. Thus, evoking the oscillations on demand in a high-throughput animal model will have clear experimental benefits. We address this in two ways. First, we describe the presence of HFOs in the tetanus toxin animal model of temporal lobe epilepsy, which has advantages over the status-epilepticus models currently used to study HFOs. Second, we show that HFOs are reliably triggered by electrical microstimulation, providing access to a more consistent evaluation of their presence and experimental perturbation. We use this model to address two questions concerning the nature of HFOs: 1) what is their relationship to IISs, and 2) what is their relationship to multiunit activity?
Materials and Methods
Surgery
Multielectrode arrays (MEAs) were chronically implanted in 8 male Sprague-Dawley rats (>350 g; Charles River Laboratories; Wilmington, MA), made epileptic with focal injections of tetanus toxin (TT), and 3 control rats. All work was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Emory University's Institutional Animal Care and Use Committee. Rats were anesthetized with 1.5-3.0% inhaled isoflurane and given buprenorphine (0.05 mg/kg) to minimize pain. A craniectomy was made over the right hippocampus, centered at 3.5 mm posterior and 2.8 mm lateral to bregma. The dura was incised with a sterile syringe needle and an injection of 25 ng of TT (Sigma Aldrich, St. Louis, MO) in 0.5 μl phosphate buffered saline with 0.2% bovine serum albumin was delivered (Jefferys and Walker, 2005). The injection, using a pulled glass pipette and a stereotactically mounted injector (Nanoject; Drummond Scientific Co., Broomall, PA), was located in the dorsal hippocampus (3.3 mm posterior and 3.2 mm lateral to bregma, and 3.1 mm ventral to pia). The needle was allowed to equilibrate for one minute prior to injection, the injection was delivered over 3 minutes, and the needle remained in place for 5 minutes following the injection to prevent reflux.
The MEA (Tucker Davis Technologies; Alachua, FL) was implanted after the toxin injection. The array had sixteen 33 μm diameter tungsten electrodes with polyimide insulation arranged in two rows of 8 electrodes, with 175 μm between electrodes within a row and 1 mm between rows. The two rows had different lengths, 4.0 mm and 3.0 mm, with the former directed at the CA3 region of the hippocampus, and the latter at the more dorsal CA1 region. The array had stainless steel wires for ground and reference electrodes, which were wound around stainless steel skull screws (Plastics One; Roanoke, VA, USA) at the time of surgery. The reference screw was located slightly posterior to lambda, over the cerebellum.
The array was positioned at a 50° angle to midline (counter-clockwise rotation, with the posterior end swung laterally) to match the contours of the hippocampus (Deadwyler et al., 1996). Electrodes were lowered while recording activity to attain correct positioning (Deadwyler et al., 1996; Gross et al., 2006; Rolston et al., 2009a), usually ending when the longer electrodes were ∼3 mm ventral to pia.
When the recordings stabilized, the craniectomy was sealed with cyanoacrylate glue (Loctite; Rocky Hill, CT) and dental acrylic (OrthoJet; Lang Dental; Wheeling, IL) was applied to secure the array's connector. The rats returned to their normal housing, and rested and recovered post-operatively for 5-8 days before recordings began. All animals exhibited spontaneous seizures within 3-9 days. No mortality or morbidity from the injections was observed, consistent with previous reports (Jefferys and Walker, 2005).
Electrophysiology
Electrophysiological recordings were made using the open-source NeuroRighter system (Rolston et al., 2009a). Data were sampled at 25 kHz with a 1-9000 Hz bandwidth. Raw data was digitally processed to separate LFPs from action potentials (APs). To record the LFP, the raw recording was band-pass filtered from 1-500 Hz (3-pole Butterworth) and downsampled to 2000 Hz. To detect APs, the raw recording was filtered from 500-5000 Hz (3-pole Butterworth), the common median reference was subtracted across channels (Rolston et al., 2009b), and APs were detected by finding deviations greater than 5× the signal's RMS (root-mean-square) noise or less than −5×RMS noise. Power spectra, spectrograms, and coherence measurements were computed with the Chronux analysis software (http://chronux.org/).
Data for analysis was obtained from recordings conducted on post-operative days 5-36. The dates for each animal varied, depending on the date of the first detected seizure and the stability of recorded single unit activity (which changes over the span of days). Data was recorded in 30 minute sessions with 4-10 sessions per animal. The maximum number of sessions depended on the animal's ability to tolerate further recording and the stability of recorded single unit activity. No data sets with stable single unit activity that were recorded after seizure onset were discarded for any reason. Detailed information is available in Table 1.
Table 1. Recording information for analyzed rodents.
| Animal | Day(s) post-injection analyzed | Number of sessions analyzed* |
|---|---|---|
| 1 | 8 | 4 |
| 2 | 9 | 4 |
| 3 | 6 | 5 |
| 4 | 6-36 | 6 |
| 5 | 5-9 | 10 |
| 6 | 5-9 | 5 |
| 7 | 10-11 | 5 |
| 8 | 15-28 | 7 |
30 minute recording sessions
Interictal spike and HFO detection
IISs were detected by filtering the LFP between 10-100 Hz (4th order zero-phase Butterworth), producing a signal V(t). IISs were identified as excursions of this signal ≥ 7.5 × median(|V(t)|) / 0.6745 (Donoho and Johnstone, 1994). The constant of 0.6745 is used to remove bias from the noise estimate (Donoho and Johnstone, 1994; Huber and Ronchetti, 2009). Using the median, rather than the RMS, prevents spurious elevation of the threshold when the IIS rate increases, since the median is less sensitive to outliers than the mean (Quiroga et al., 2004).
HFOs were detected by filtering the LFP between 200-400 Hz (4th order zero-phase Butterworth), squaring each sample, low-pass filtering the result below 200 Hz (2-pole zero-phase), and taking the square root of each sample (Stark and Abeles, 2007). The median of the signal was then subtracted, producing a time-varying signal H(t) which provided an estimate of the energy in the 200-400 Hz band at any given time. HFOs were detected by finding excursions ≥ 5 × median(|H(t)|) / 0.6745 (as was done with IIS detection). Additionally, events were rejected if they persisted less than 15 ms. Having a minimum duration of elevated energy (as we do) helps guard against spurious event detections.
The sensitivity and specificity for each method was determined by showing 1500 separate 1 s windows (randomly drawn from all data) to an evaluator, blinded to the automated markings. The evaluator marked the data as containing HFOs and IISs, and these markings were compared to the automated markings of the same data. For IIS detection, this analysis yielded an estimated sensitivity of 92.9 % and specificity of 99.0 % (275 events autodetected, 283 detected manually). For HFO detection, the estimated sensitivity was 78.7 % and specificity was 100 % (37 events autodetected, 47 detected manually), consistent with previous automated methods (Staba et al., 2002).
Microstimulation
To test the effects of microstimulation on HFOs, we stimulated each animal (after all spontaneous recordings) with randomly distributed current-controlled pulses, using a custom-built stimulator (Rolston et al., 2009c). Importantly, the device stimulated through the same electrodes used for recording, with stimulation artifacts ∼1-2 ms in duration (Rolston et al., 2009a). Pulses had amplitudes of ±2, 4, 6, 8, 10, 15, 20, or 50 μA (cathodic phase first) and pulse widths of 800 μs. Each pulse amplitude was delivered to each electrode a total of 10 times (i.e., 10 trials for each amplitude and electrode combination). To prevent neural adaptation to repeated pulses, the trial order (trial × electrode × pulse amplitude) was randomized (e.g., electrode 5 at 10 μA, then electrode 1 at 2 μA, etc.). The pulses were delivered at 1 second intervals (1 Hz).
The resulting data was then searched for HFOs, using the automated method described above, for the 100 ms before and 100 ms after each stimulus pulse. Conservatively, the 5 ms immediately after each stimulus was blanked (samples set to zero) to avoid any possible contamination by artifact (artifacts usually lasted 1-2 ms (Rolston et al., 2009a)). Additionally, the 5 ms immediately before each stimulus was blanked to prevent possible confounding effects of asymmetric blanking when comparing pre- and post-stimulus data.
Histology
Electrode locations first identified through microelectrode recording during surgery were verified via postmortem histology. After experimentation, rats were deeply anesthetized with an overdose of Euthasol (5ml/kg) injected intraperitoneally and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1M phosphate buffer at pH 7.2. The heads were removed and post-fixed at 4°C. The following day, the brains were removed and cryoprotected with 30% sucrose at 4°C. Frozen transverse sections 50μm thick were cut on a sliding microtome and collected in 0.1M phosphate buffered saline. Alternating sections were mounted onto gelatin-coated glass slides, allowed to dry overnight, stained with Cresyl Violet, dehydrated and coverslipped. Sections were visualized using a Nikon Eclipse E400 research microscope equipped with a Nikon DS-Fi1 camera and NIS-Elements: BR 3.00 software.
Results
A temporal lobe epileptic focus was induced by injecting a small quantity of TT (25 ng) into the dorsal hippocampus of 8 male Sprague-Dawley rats. Epilepsy was characterized by chronic spontaneous seizures and frequent interictal discharges. The rats were also implanted with 16-electrode microwire arrays targeted by microelectrode mapping (Gross et al., 2006) to the dorsal hippocampus (Fig. 1). These electrodes were used to record both the hippocampal local field potential (LFP) and multiunit activity (i.e., APs recorded across all hippocampal electrodes; Fig. 2).
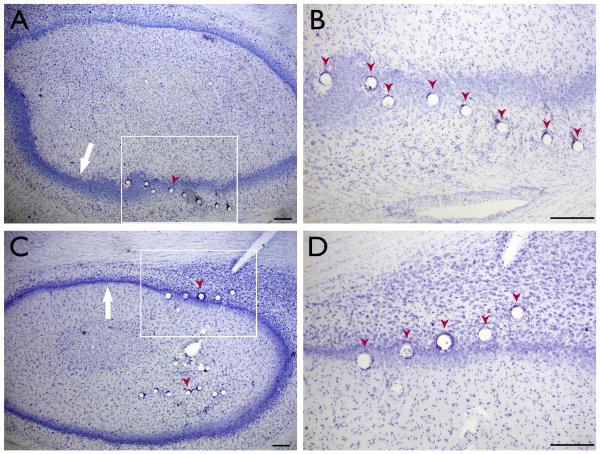
Figure 1. Histological confirmation of electrode location.
(A, C) Nissl-stained transverse sections through the hippocampus in two animals. The tissue damage due to electrode placement (red arrowheads) can be seen along the pyramidal cell layer (white arrow). (B, D) Higher magnification of the boxed portions of (A) and (C), respectively. All scale bars 100μm.
Figure 2. Interictal spikes (IISs) coincide with high-frequency oscillations (HFOs) and population bursts.
(A) The LFP shows a sample IIS recorded in the frequency band of 1-500 Hz. (B) Filtering the LFP between 200-400 Hz shows a large increase in fast-ripple power during the negative peak of the IIS. (C) A raster of multiunit activity, aligned to the peak of all detected IISs (in this recording), shows a high degree of bursting during the IIS.
In all epileptic animals studied, LFP power spectra revealed large increases in power in the “fast ripple” range defined as 200-400 Hz, representing the presence of high-frequency oscillations (HFOs; Figs. 2, 3). Spectrograms of the LFP further revealed that these HFOs temporally coincided with interictal spikes (IISs; Fig. 3A). Moreover, multiunit activity showed the presence of large-scale population bursting during interictal spikes, coinciding with—but not limited to—HFOs (Fig. 2C).
Figure 3. High-frequency oscillations are present and coincide with interictal spikes.
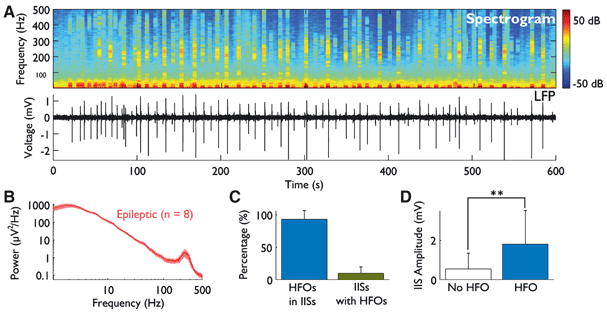
(A) A spectrogram shows that HFO power is limited to defined bursts (top). By comparing the spectrogram to the time-domain LFP trace (bottom), a relationship between HFOs and IISs is suggested. (B) The average power spectrum is shown for all 8 epileptic animals. Spectra include data from all interictal activity (i.e., with and without IISs and HFOs). A large peak in the fast ripple frequency band of 200-400 Hz is present. Standard error is indicated by shading. (C) Most HFOs occur within IISs, but most IISs do not contain HFOs. (D) The amplitude of IISs containing HFOs is significantly greater than that of IISs without HFOs (P < 0.0001).
To quantify these observations, we used automated methods to detect both IISs and HFOs, and then determined the number of HFOs occurring within IISs. Across the 8 animals, 1999 HFOs were identified and 97.6% occurred during IISs. However, of the 9000 IISs detected, only 10.2% coincided with one or more HFOs (Fig. 3C).
To see if there were differences in the IISs that contained HFOs and those that did not, we examined the peak amplitude of each IIS (absolute value) and compared those containing and those not containing HFOs. The average amplitude of IISs with HFOs was 1.8 mV, 3.3-fold greater than the mean amplitude of 544 μV for IISs without HFOs (P < 0.0001, Wilcoxon rank-sum test; Fig. 3D).
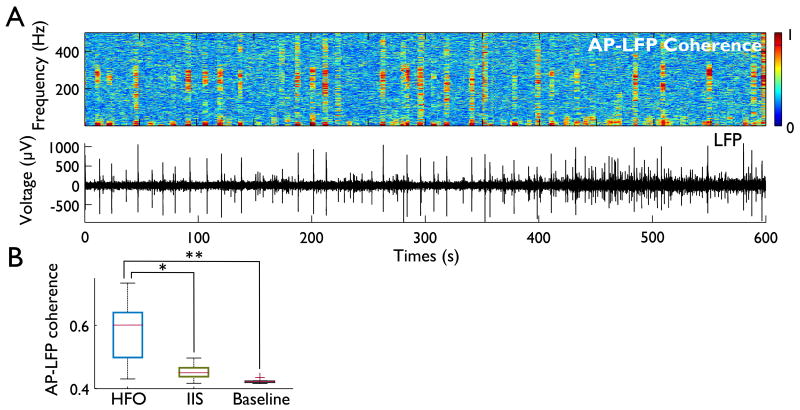
Since we observed that IISs coincide with population multiunit bursts (Fig. 2C), we next asked whether these bursts were correlated to HFOs. To find bursting at particular frequencies, we calculated the AP-LFP coherence (also known as “spike-field” coherence) between the population multiunit activity (unsorted APs recorded from all hippocampal electrodes) and the LFP in 500 ms sliding windows with 400 ms overlap (Mitra and Bokil, 2008) (Fig. 4A, B; results were unchanged when using 100 ms windows with 50 ms overlap). We then averaged the coherence in the HFO band (200-400 Hz) and compared the spike-field coherence during HFOs to that during IISs without HFOs, and both to the baseline (non-HFO, non-IIS). The mean coherence during HFOs (across all 8 animals) was 0.58 ± 0.10 (values range from 0, i.e. no coherence, to 1, i.e. complete phase-locking), which was significantly greater than the coherence during non-HFO-containing IISs (0.45 ± 0.02; Wilcoxon sign-rank P = 0.016) or baseline (0.42 ± 0.01, P = 0.008; Fig. 4B).
Figure 4. AP-LFP coherence is increased during HFOs.
(A) A cohereogram between the LFP and multiunit activity (top) shows increases in coherence limited to the HFO frequency range (larger 5 s sliding window with 4 s overlap, for visualization purposes). These increases in coherence appear associated with a subset of IISs, as shown in the time-locked LFP trace (bottom). (B) Coherence in the HFO bandwidth (200-400 Hz) is increased during HFOs as compared to IISs (P < 0.05) or baseline recordings (P < 0.01), as is indicated in this box plot of mean coherence for each animal.
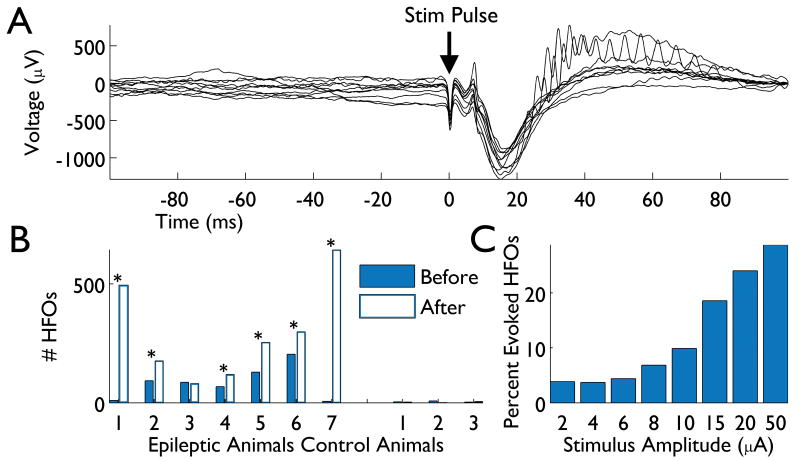
The sporadic nature of HFO occurrence makes them difficult to study. We therefore sought a method by which HFOs could be more reliably generated. Using microstimulation of the implanted array, we screened 7 epileptic animals (single session per animal; each exhibiting spontaneous HFOs) with a variety of pulse amplitudes (2-50 μA; see Methods). We then searched the 100 ms after each stimulus pulse for HFOs, along with the 100 ms preceding each pulse. In 6 of the 7 animals tested, there were significantly more HFOs present after stimulus pulses than before (P < 0.001, χ2 test), showing that microstimulation evokes HFOs with short latency (Fig. 5). To test the specificity of these results, we also stimulated 3 control animals in an identical fashion. While some spurious detections of HFOs occurred in these non-epileptic animals, there was no significant difference between pre- and post-stimulus counts (Fig. 5B). Because we stimulated using a range of current amplitudes, we were also able to analyze the number of HFOs evoked as a function of intensity. This analysis revealed a clear dependence on stimulus amplitude (higher amplitudes evoked more HFOs), with >25% of the evoked HFOs due to 50 μA stimuli (Fig. 5C).
Figure 5. Microstimulation evokes HFOs.
(A) The LFP in response to a microstimulation pulse of 20 μA is shown (10 trials are overlaid). HFOs can be seen in a subset of responses. (B) Across animals, more HFOs are observed in the 100 ms immediately after each stimulus pulse (open blue bars) than in the 100 ms preceding the pulse (solid blue bars). Significance (P < 0.001, χ2 test) is indicated with asterisks. While spurious HFOs were detected in non-epileptic control animals, these animals never exhibited a significant increase in HFOs from stimulation. (C) The proportion of HFOs generated by each pulse amplitude is indicated, showing that increasing amplitudes evoke progressively more HFOs.
Discussion
To date, only a few animal models have been used to study HFOs, notably kainic acid (Bragin et al., 2004) and pilocarpine injection (Foffani et al., 2007). Both are status epilepticus models that induce hippocampal sclerosis, and both exhibit latent periods of weeks to months before seizure onset (Nadler, 1981; Tremblay and Ben-Ari, 1984; Cavalheiro, 1995; Pitkanen et al., 2006)(Nadler, 1981; Tremblay and Ben-Ari, 1984; Cavalheiro, 1995; Pitkanen et al., 2006)(Nadler, 1981; Tremblay and Ben-Ari, 1984; Cavalheiro, 1995; Pitkanen et al., 2006)(Nadler, 1981; Tremblay and Ben-Ari, 1984; Cavalheiro, 1995; Pitkanen et al., 2006). The tetanus toxin (TT) model of temporal lobe epilepsy (Jefferys et al., 1995), in contrast, produces no neuropathologically-apparent lesions (Mellanby et al., 1977; Jefferys et al., 1992; Benke and Swann, 2004). We therefore asked whether a non-status model, without neuropathological lesions like hippocampal sclerosis, might also produce HFOs. Indeed, as shown above, TT-treated animals showed frequent HFOs, making it apparent that overt neuropathology is not a prerequisite for HFOs. Their origins likely lie, instead, in the shared pathophysiological alterations of function in the TT, kainate, and pilocarpine models. Further study should be directed at uncovering this shared substrate.
More practically, the TT model produces frequent seizures (∼2 per hour) within 3-9 days of toxin injection (Jefferys et al., 1995). Status models are usually reported as producing seizures within weeks to months of the experimental intervention (Pitkanen et al., 2006), although recent continuous monitoring showed that infrequent seizures can occur earlier (Goffin et al., 2007). While similar continuous monitoring studies have not been carried out with TT, the higher frequency of seizures early on provides a greater number of observable seizures in a briefer time, shortening the waiting period between surgery and data acquisition. This shortened lead time is crucial when using fixed microwire electrodes, which tend to degrade in performance over several weeks. As another practical benefit, the tetanus toxin model has a near complete success rate in inducing seizures, compared to status models, reducing the number of animals required for study. Finding HFOs in this model, as we did, provides an attractive preparation for their further investigation and, importantly, one with a different etiology than previously used models.
The relationship between interictal spikes (IISs) and HFOs has not been defined. In the TT model, 98% of HFOs occur within IISs (Fig. 3C). This is dissimilar to the rate (48%) reported by Jacobs et al. (2008) in humans, but more in line with that of Worrell et al. (2008), who reported 84%. Numerous differences exist between these studies—etiology, species, HFO and IIS detection methods, electrode size (micro- vs. macroelectrodes), and electrode location, to name a few—so comparisons are not direct. Regardless, the association between IISs and HFOs is theoretically appealing. If HFOs indicate the synchronized bursting of pyramidal cells (Bragin et al., 2000; Rampp and Stefan, 2006; Foffani et al., 2007; Engel et al., 2009), these discharges would be more likely to occur when groups of pyramidal cells are depolarized, as in IISs, which represent large-scale depolarizations of neural tissue (de Curtis and Avanzini, 2001). Indeed, we see evidence of this: 1) HFOs are more likely to occur during IISs, 2) larger IISs are more likely to coincide with, and possibly evoke, HFOs (Fig. 3D), and 3) HFOs are more likely to occur when cells are depolarized exogenously with microstimulation (Fig. 5). Regarding the second point above, it is important to entertain the possibility that the dearth of HFOs in low amplitude IISs reflects low amplitude HFOs below our detection threshold, rather than HFO absence. In this case, the point might be rephrased, such that HFO amplitude is related to IIS amplitude, rather than HFO presence being related to IIS amplitude. Future experiments might analyze the amplitude relationships between IISs and HFOs in greater detail.
Does the bursting of pyramidal cells underlie HFOs? Using AP-LFP coherence, we measured the phase-locked firing of hippocampal neurons in relation to the HFO frequency band. Coherence was significantly greater than baseline during HFOs. Thus, neural populations are bursting phase-locked to HFOs when they occur, consistent with their involvement in HFO genesis.
Aside from the spontaneous recording of HFOs in the TT model, we were able to reliably evoke HFOs using microstimulation of CA3 (Fig. 5). Evoking HFOs via microstimulation of the recording electrodes has not yet been reported, perhaps due to the difficulties arising from stimulation artifact (Rolston et al., 2009a). However, anecdotal evidence of HFOs triggered by perforant path stimulation was presented by Bragin et al. (2002) in the kainate model, using larger stimulus currents (0.1–0.5 mA). Our finding extends that of Bragin et al. by statistically quantifying the ability to elicit HFOs, showing a dependence on stimulus amplitude, defining a new circuit element whose stimulation elicits HFOs (CA vs. perforant path), and providing a simplified system wherein the stimulation and recording electrodes are the same. It should also be noted that triggering HFOs with microstimulation was not necessarily an expected finding, prior to the current work or the work of Bragin et al. While microstimulation has been known to produce single population spikes, created by the superimposition of multiple APs (Andersen et al., 1971), that these population spikes would spontaneously recur at HFO frequencies in models of epilepsy, well after the initial stimulus lapsed, speaks to the internal dynamics of the epileptic versus intact hippocampal network.
This ability to electrically evoke HFOs has potential uses. For example, automated methods could be developed to microstimulate tissue while monitoring HFOs. If successful, such a technique would help determine epileptic onset zones when preparing patients for resective surgery. While still invasive, this procedure could be done in a single session, rather than awaiting spontaneous seizures during weeks of expensive, time-consuming in-patient monitoring. Though a similar approach could be developed with spontaneous HFOs, artificially increasing their rate would allow for more rapid and reliable tests. Moreover, the fact that we observed spontaneous and microstimulation-evoked HFOs in a non-lesional animal model augurs well for the usefulness of this technique in the many non-lesional patients undergoing intracranial monitoring. Other applications might include high-throughput drug screening (to evaluate effects on HFOs) and increased experimental control for basic science experiments. All such applications will require further investigation of evoked HFOs, both to characterize them more fully and validate their relevance to human epilepsy. But despite the difficulty of the experiments, by providing a novel form of control over HFOs, these experiments are now possible.
Acknowledgments
This work was funded by the Wallace H. Coulter Foundation (http://www.whcf.org/), the Epilepsy Research Foundation (http://www.epilepsy.com/etp/scientific_research), a Neurology/Biomedical Engineering seed grant from Emory University and Georgia Tech (http://www.bme.gatech.edu/), a University Research Council grant from Emory University (http://www.emory.edu), the National Institute of General Medical Sciences (NIGMS; http://www.nigms.nih.gov/) to JDR and NGL (GM08169), and, from the National Institute of Neurological Disorders and Stroke (NINDS; http://www.ninds.nih.gov/), a Ruth L. Kirschstein National Research Service Award to JDR (NS060392), a translational research fellowship to JDR (NS007480), a career development award to REG (NS046322), and a research grant to SMP and REG (NS054809). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: None of the authors have conflicts of interest to declare. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Andersen P, Bliss TV, Skrede KK. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13:208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Benke TA, Swann J. The tetanus toxin model of chronic epilepsy. Adv Exp Med Biol. 2004;548:226–238. doi: 10.1007/978-1-4757-6376-8_16. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41 6:S144–152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci. 1995;16:33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346:140–144. doi: 10.1016/s0140-6736(95)91208-8. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Progress in Neurobiology. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J Neurosci. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho DL, Johnstone IM. Ideal Spatial Adaptation by Wavelet Shrinkage. Biometrika. 1994;81:425–455. [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Goffin K, Nissinen J, Van Laere K, Pitkanen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol. 2007;205:501–505. doi: 10.1016/j.expneurol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson's disease and tremor. Mov Disord. 2006;21 14:S259–283. doi: 10.1002/mds.20960. [DOI] [PubMed] [Google Scholar]
- Huber PJ, Ronchetti E. Robust statistics. 2nd. Hoboken, N.J.: Wiley; 2009. [Google Scholar]
- Hufnagel A, Dumpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–478. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80-500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG, Walker MC. Tetanus Toxin Model of Focal Epilepsy. In: Pitkanen A, Schwartzkroin PA, Moshé SL, editors. Models of Seizures and Epilepsy. Burlington, MA: Elsevier; 2005. [Google Scholar]
- Jefferys JG, Borck C, Mellanby J. Chronic focal epilepsy induced by intracerebral tetanus toxin. Ital J Neurol Sci. 1995;16:27–32. doi: 10.1007/BF02229071. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Evans BJ, Hughes SA, Williams SF. Neuropathology of the chronic epileptic syndrome induced by intrahippocampal tetanus toxin in rat: preservation of pyramidal cells and incidence of dark cells. Neuropathol Appl Neurobiol. 1992;18:53–70. doi: 10.1111/j.1365-2990.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. The New England journal of medicine. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Mellanby J, George G, Robinson A, Thompson P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J Neurol Neurosurg Psychiatry. 1977;40:404–414. doi: 10.1136/jnnp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed brain dynamics. New York: Oxford University Press; 2008. [Google Scholar]
- Nadler JV. Minireview. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Schwartzkroin PA, Moshé SL. Models of Seizures and Epilepsy. Burlington, MA: Elsevier Academic Press; 2006. [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- Rampp S, Stefan H. Fast activity as a surrogate marker of epileptic network function? Clin Neurophysiol. 2006:2111–2117. doi: 10.1016/j.clinph.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Rolston JD, Gross RE, Potter SM. A low-cost multielectrode system for data acquisition enabling real-time closed-loop processing with rapid recovery from stimulation artifacts. Frontiers in Neuroengineering. 2009a;2:12. doi: 10.3389/neuro.16.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston JD, Gross RE, Potter SM. Common Median Referencing for Improved Action Potential Detection with Multielectrode Arrays. 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Minneapolis, MN. 2009b. [DOI] [PubMed] [Google Scholar]
- Rolston JD, Gross RE, Potter SM. NeuroRighter: Closed-loop Multielectrode Stimulation and Recording for Freely Moving Animals and Cell Cultures. 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Minneapolis, MN. 2009c. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80-500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Stark E, Abeles M. Predicting movement from multiunit activity. J Neurosci. 2007;27:8387–8394. doi: 10.1523/JNEUROSCI.1321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay E, Ben-Ari Y. Usefulness of parenteral kainic acid as a model of temporal lobe epilepsy. Rev Electroencephalogr Neurophysiol Clin. 1984;14:241–246. doi: 10.1016/s0370-4475(84)80011-8. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]