Abstract
Although the impact of anxiety on patients with some types of cancer is well recognized, to the authors knowledge its impact on patients with prostate carcinoma has not been studied as thoroughly. The authors conducted a systematic review of the medical literature for high-quality articles that quantified anxiety levels in men with prostate carcinoma and identified 29 articles. Using the clinical timeline of prostate carcinoma to organize the articles, cross-sectional studies that reflected anxiety prevalence in populations and longitudinal studies that reflected changes in anxiety over time were identified. Anxiety appeared to fluctuate over the clinical timeline in response to stressors and uncertainty (such as at the time of screening and/or biopsy), rising before these times and falling afterward. Although anxiety levels in men age > 55 years who were at risk for prostate carcinoma were modest (10–15%), multiple studies found that these levels were substantially higher in men who presented for screening (> 50%), and “seeking peace of mind” was the motivation cited most frequently for pursuing screening. Most studies demonstrated a significant decrease in anxiety levels after a normal screening or biopsy result, although the proportion of men who remained anxious afterward did not fall to baseline levels (20–36%). Men who presented for prostate-specific antigen monitoring after treatment had elevated anxiety levels at the time of testing (23–33%). Many years after therapy for localized disease, anxiety levels were lower after prostatectomy (23%) compared with the levels after watchful waiting (31%).
Keywords: prostate carcinoma, anxiety, uncertainty, prostate-specific antigen monitoring, screening, stressors, treatment, watchful waiting, medical decision-making
Prostate carcinoma is the most commonly diagnosed, noncutaneous malignancy among men in the U.S. In 2004, there were > 230,000 new men diagnosed with prostate cancer and nearly 30,000 deaths.1 Nevertheless, routine screening for prostate carcinoma remains controversial. On the one hand, there is histologic evidence of prostate carcinoma in the vast majority of elderly men who die of other, noncancer diagnoses, which raises the concern for over-diagnosis of clinically insignificant disease with aggressive screening approaches. Conversely, the large number of deaths, the relatively slow growth rate of prostate carcinoma, and the improved availability and toxicity profile of definitive local therapy suggests that identification and treatment of early disease can have important benefits. In addition to numerous retrospective studies, early diagnosis and treatment is supported by a randomized trial that showed decreases in disease-specific and overall mortality after radical prostatectomy compared with watchful waiting.2
The importance of uncertainty about the mortality gains from earlier diagnosis and treatment is increased by the impact of prostate carcinoma screening, diagnosis, and treatment on health-related quality of life (HRQOL), including psychologic well being.3 One inadequately understood aspect of the HRQOL impact of prostate carcinoma and its treatments is psychological distress from anxiety. Among men who have a history of prostate carcinoma, clinicians are sufficiently familiar with men who are worried about their prostate-specific antigen (PSA) levels, that this psychologic state has been described as PSAdynia, which is described as a state of physical or emotional distress due to an elevated PSA level.4 Whereas the impact of anxiety on patients with a variety of cancer types is recognized and reviews exist,5 the role of anxiety in patients who specifically have or are at risk for prostate carcinoma is limited.
The primary objective of this report was to review systematically the existing data on anxiety levels in patients with prostate carcinoma and its variation over the clinical timeline of the disease. We did not include studies that focused only on the related topic of depression, because depression is distinct conceptually and clinically from anxiety. In the psychometrics literature, valid measures of anxiety are designed to distinguish it from depression.6 We chose to review the recent literature that focuses on quantifying anxiety levels in men with, or at risk for having, prostate carcinoma, using the initiation of PSA testing in the late 1980s as our starting point.7
First, we define anxiety, recognize the role of stress and uncertainty in triggering anxiety, and briefly describe validated scales that have been used to measure anxiety in patients with or at risk for prostate carcinoma. Based on this definition, we use the clinical timeline for prostate carcinoma to identify key times when stressors and uncertainty may affect anxiety levels. This timeline focuses on screening, early detection, and therapy for localized disease, largely because few studies assess anxiety in later stage disease. We use this timeline to organize and integrate the available data. The results are reported from a structured review of the literature on anxiety levels in patients with prostate carcinoma patients, or men at risk for prostate carcinoma, and we finish by summarizing these findings.
Defining and Measuring Anxiety
The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) defines anxiety as the apprehensive anticipation of future danger or misfortune accompanied by feelings of dysphoria or somatic symptoms of tension.8 This definition reflects two important aspects of anxiety: 1) the transient affective state of heightened emotions and 2) the importance of an anticipated situational stressor. A principal feature of anxiety-provoking events, or stressors, is the degree to which their outcome is perceived as threatening or uncertain. A third important possible aspect of anxiety is that it also may be a relatively stable background personality characteristic, or trait, of emotional-behavioral difficulties. This separation of anxiety into a background trait and a situational state matters in two ways: Men with high trait levels of anxiety will carry this predisposition throughout the clinical course of disease, distinct from the particular situation encountered (although potentially exacerbated by stressful situations), thereby making these individuals important to identify. In addition, over the clinical course, particular points in time will be more stressful than others, making them more likely to be anxiety provoking, thereby making these situations important to identify. For example, anxiety concerning screening is a commonly studied aspect of the anxiety related to prostate carcinoma. Despite the unconfirmed role of PSA screening in decreasing prostate carcinoma mortality/morbidity, the number of men screened with PSA has risen dramatically since its introduction.9
These features of anxiety helped structure our review. Having identified the circumstances that may affect anxiety levels, we systematically searched the literature to identify studies that 1) measured anxiety levels in specific populations in these circumstances (e.g., at the time of screening) and 2) measure changes in anxiety levels across such circumstances (e.g., before screening and after receiving screening results). We report results in two broad categories of studies: cross-sectional studies for identified populations and longitudinal studies for transitions between times.
Four validated scales for measuring anxiety have been used in studies of patients with prostate carcinoma (Table 1). Three instruments for generalized anxiety have been used to measure anxiety levels in prostate carcinoma patients: the State-Trait Anxiety Inventory (STAI),10,11 the Hospital Anxiety and Depression Scale (HADS),12,13 and the Impact of Event Scale (IES).14–16 Only one scale, The Memorial Anxiety Scale for Prostate Cancer (MAX-PC), has been developed and validated specifically to measure anxiety in patients with prostate carcinoma.17
TABLE 1.
Characteristics of Anxiety Measurement Scales Used in Studies of Patients with Prostate Carcinoma
| Instrument/subscale | Original purpose | No. of items |
Possible score |
Thresholds for case identification |
|---|---|---|---|---|
| Hospital Anxiety and Depression Scale (HADS) | 14 | 0–42 | 7–10 (mild), 11–14 (moderate), 15–21 (severe) | |
| Anxiety (HADS-A) | 7 | 0–21 | ||
| Depression (HADS-D) | 7 | 0–21 | ||
| Impact of Event Scale (IES) | Intrusion and avoidance after traumatic event | 15 | 0–75 | 8.5–19.0 (medium), ≥ 20.0 (high) |
| Intrusiveness | ||||
| Avoidance | ||||
| State-Trait Anxiety Inventory (STAI) | Symptoms of anxiety as a clinical disorder | 40 | ||
| State (STAI-S) | 20 | 20–80 | ≥ 45 | |
| Trait (STAI-T) | 20 | 20–80 | ≥ 42 | |
| Memorial Anxiety Scale for Prostate Cancer (MAX-PC) | Anxiety in patients with PCa | 18 | 0–54 | None established |
| Prostate Cancer Anxiety | 11 | 0–33 | ||
| Fear of Recurrence | 4 | 0–12 | ||
| PSA Anxiety | 3 | 0–9 |
PSA: prostate-specific antigen.
Before describing the results on anxiety levels in men who are screened for or who have prostate carcinoma, it is important to know the baseline anxiety levels in the population at risk for prostate carcinoma as a point of comparison. Following current screening guidelines, the at-risk population includes men age > 50 years and men age > 45 years with the known risk factors of a first-degree relative with prostate carcinoma or African-American race. Unfortunately, to our knowledge, exact prevalence rates of baseline anxiety symptoms in this at-risk population do not exist, although there are estimates of the overall prevalence of anxiety diagnoses. The 1-year prevalence estimate of any anxiety disorder in the U.S. population age > 55 years is 11.4%.18 A recent estimate of anxiety symptoms in healthy, nondepressed men ages 70–79 years was 12%, with no difference between African Americans and whites.19 A nationally representative sample recently estimated the 12-month U.S. prevalence of any DSM-IV anxiety disorder at 11.08% (95% confidence interval 10.43–11.73%).20 These likely are overestimates because they include diagnoses, some of which are rare in this population (e.g., obsessive-compulsive disorder).
MATERIALS AND METHODS
Search Strategy
For identifying articles that focused on men who were at risk for or who had prostate carcinoma, the terms “prostate cancer,” “prostatic carcinoma,” “prostatic neoplasms,” “prostate specific antigen,” or “PSA” were used. For identifying articles that focused on anxiety, the terms “anxiety,” “anxiety disorder,” or “worry” were used. These two search strategy results were combined with an “AND” Boolean statement in the following data bases from January 1987 through April 2004: Medline, PsychINFO, the Cochrane Database on Systematic Reviews, American College of Physicians Journal Club, Database of Abstracts of Reviews of Effects, and the Cochrane Central Register of Controlled Trials. In total, 68 articles were identified that included assessments of anxiety in populations with or at risk for prostate carcinoma as one of their primary objectives.
Inclusion Criteria
We included only quantitative studies with sample sizes > 20 participants and that reported either mean scores or percentages of men who were anxious according to a clear standard on a given measure. Two authors (W. D. and P. B.) independently reviewed all of the articles to determine whether they met these inclusion criteria, and there was agreement on 62 of 68 articles (91%). Adjudication by discussion was necessary for the remaining six articles, which then were categorized. Twenty-nine articles met the criteria (Table 2).
TABLE 2.
Anxiety in Patients with Prostate Carcinoma, Published Studies from 1985 to 2004
| Study | No. of Patients | Population | Anxiety measures | Main anxiety finding(s) |
|---|---|---|---|---|
| Bisson et al., 200231 | 88 | Mean age 64.5 yrs; newly diagnosed, localized PCa | HADS | Anxiety on HADS-A, 8%; anxiety on IES, 14% |
| Bratt et al., 200028 | 110 | Age range 40–72 yrs; men with at least 3 relatives with a PCa history | HADS, IES | Thirty-one percent say worry about PCa affects daily life; HADS scores were below the population mean |
| Caffo et al., 199632 | 90 | Ages 49–83 yrs; nonmetastatic PCa patients treated with radiation | Original questionnaire | After treatment, 40% felt anxious, and 36% felt tense |
| Cantor et al., 200229 | 168 | Ages 45–70 yrs, no history of PCa, primary care patients | Original questionnaire | At least some reassurance from screening found by 99%; 87% thought not being screened was worst option |
| Clark et al., 199752 | 201 | Convenience sample, posttreatment for nonlocalized PCa | Original questionnaire | Three domains of life quality identified: self-perceptions, anxiety about the effects of treatment, concern with decision making |
| Clark et al., 200334 | 349 | Ages 42–87 yrs, posttreatment (various) PCa patients | Original questionnaire | On a scale from 0 to 100, mean “health worry” scores by treatment were: surgery, 24.3; hormones, 27.6; radiation, 29.6; watchful waiting, 33.1 |
| Cohen et al., 200325 | 1635 | Ages 34–79 yrs, participants in an annual free PCa screening program | Original questionnaire | Having higher scores on PCa-specific worry is associated significantly with abnormal PSA results (P = 0.004) |
| Cormier et al., 200243 | 220 | Ages 40–70 yrs, first-degree relatives of PCa patients | STAI | “Moderate deterioration” in anxiety for 20% of respondents; high scores on STAI-T predicted deterioration |
| Cormier et al., 200227 | 277 | Ages 40–70 yrs, first-degree relatives of PCa patients | Original questionnaire | Moderate or extreme worry about their own genetic susceptibility to PCa in 36% |
| Davison et al., 199930 | 100 | Ages 50–79 yrs, primary care patients; screening education | STAI | Receiving screening information before primary care visit did not affect anxiety, but did result in “active” screening decisions |
| Davison et al., 200346 | 74 | Mean age 62.2 yrs; early stage PCa patients and partners | STAI | Patients experienced significantly lower levels of anxiety by 4 mos after diagnosis |
| Demark-Wahnefried et al., 199522 | 268 (black), 1218 (white) | Median age 64 yrs; men attending free PCa screening | Original questionnaire | Sixty-three percent of white men and 50% of black men seek peace of mind from screening |
| Essink-Bot et al., 199821 | 625 | Ages 55–74 yrs, nonparticipants in PCa screening | STAI | Only men with a high predisposition to anxiety showed high levels of anxiety at later time points. |
| Ficarra et al., 200036 | 30 | Mean age 64 yrs; PCa patients postsurgery | HADS | Pathological levels of anxiety were present in 16.5% of patients |
| Fowler et al., 199635 | 621 | Localized PCa patients from SEER registry | Original questionnaire | Anxiety in 17% of patients receiving radiation therapy, 10% of patients undergoing prostatectomy were anxious |
| Gustafsson et al., 199544 | 307 (biopsy), 100 (initial screening) | 55–70 years, randomly selected subsets of 2400 who have PCa diagnostic tests | Original questionnaire; serum cortisol levels | Serum cortisol levels highest postbiopsy; return to baseline with biopsy results |
| Heim and Oei, 199342 | 47 | Mean age 72 yrs, PCa patients, 80% non-metastatic, 43% have some pain | STAI | Patients in pain had higher State anxiety levels (P < 0.05) |
| Helgesen et al., 199947 | 400 | Mean age 75; patients with newly diagnosed or previously known PCa | HADS | Anxiety ranged from 3.2% to 8.3% over 3 yrs with no differences over time |
| Lintz et al., 200340 | 210 | Ages 43–92 yrs, PCa posttreatment (82% radiation therapy) | HADS | Anxious at a cut-off of 11, 1.9%; anxious at a cut-off of 8, 14.3%; no anxiety at all, 85.7% |
| Lofters et al., 200237 | 52 | Ages 55–86 yrs, patients with metastatic PCa, 81% with hormone-refractory disease | HADS | Fifteen percent report “extreme anxiety” at time of PSA test; 83% “agree/strongly agree” they would feel anxious if PSA tests stopped |
| Nordin et al., 200145 | 118 | Patients with newly diagnosed, mixed-stage PCa | HADS | Twelve percent anxious at diagnosis vs. 14% anxious 6 mos later; 18% had advanced disease at diagnosis vs. 12% after 6 mos; 9% had localized disease at diagnosis vs. 15% after 6 mos |
| Pedersen et al., 199348 | 131 | Ages 49–73 yrs, localized PCa patients undergoing prostatectomy | Original questionnaire using a visual analogue scale from 0 mm to 100 mm | Median value on the scale was slightly greater before surgery than after for the population (median, 10 mm [range, 0–73 mm] vs. 0 mm [range 0–35 mm]) |
| Potosky et al., 200141 | 431 | Ages 40–89 yrs; localized disease, 6 mos postandrogen ablation | Original questionnaire | Overall, 62% of patients worry; orchiectomy, 51%; hormone ablation, 67% |
| Roth et al., 199839 | 93 | Ages 52–88 yrs, 81% Stage D, 4 yrs postdiagnosis | HADS: Distress Thermometer | Thirty-three percent of men judged “anxious” on HADS-A; 29% of men above Distress Thermometer cut-off score for distress |
| Roumier et al., 200426 | 420 | Ages 40–70 yrs; asymptomatic relatives in a 3-yr screening program | STAI | Less anxious men more likely to come for all screenings (P = 0.01); mean scores for STAI-S: 33 for those who had all PSA tests, 37 for those who missed a PSA test |
| Steineck et al., 20023 | 326 | Ages 48–74 yrs, localized PCa, 4 yrs after randomized to surgery vs. watchful waiting | STAI | STAI-S scores 10% above the 90th percentile; no differences between groups |
| Taylor et al., 200223 | 136 | Median age 59 yrs; free screening | IES | “Seeking peace of mind” most important reason for pursuing screening |
| Ward et al., 199724 | 340 | Ages 40–80 yrs, Sydney community members chosen randomly | Original questionnaire | Twenty-nine percent “ever worry” about having PCa; anxiety a predictor for getting a PSA test |
| Zabora et al., 200138 | 4496 | Ages 20–80 yrs, general cancer patients | Beck’s Depression Inventory | In all, 30.5% positive cases of depression/anxiety |
PCa: prostate carcinoma; HADS: Hospital Anxiety and Depression Scale; IES: Impact of Event Scale; HADS-A: HADS Anxiety subscale; PSA: prostate-specific antigen; STAI: State-Trait Anxiety Inventory; STAI-S: STAI State subscale; STAI-T: STAI Trait subscale; SEER: Surveillance, Epidemiology, and End Results Program.
Data Reporting
First, we describe cross-sectional studies of anxiety levels in defined populations. Then, we describe longitudinal studies of changes in anxiety levels across time. The cross-sectional studies present point-in-time estimates of anxiety levels for defined populations, and the longitudinal studies present changes-over-time estimates.
RESULTS
Cross-Sectional Estimates of Anxiety Levels
Prescreening
In a population that was screened for prostate carcinoma, high anxiety levels on the STAI were found among those with a high predisposition to anxiety, but relatively low, transient elevations were found among others (Table 3).21 By far the most important screening motivation for men who presented to a PSA screening event was “peace of mind,” which was mentioned by 50% of African-American men and by > 60% of white men.22 In a prospective study of men who were registered for PSA screening, “seeking peace of mind” was rated higher than a large list of other potential cognitive and emotional motivations for attending screenings. 23 Nearly all of these men anticipated that they would have a negative test result. Anxiety, as measured by a single question, was a predictor for PSA screening in a randomly chosen community sample of men, with 29% reporting that they “ever worried about having prostate cancer.”24 Forty-two percent of the men who were worried had been screened in the past year, whereas only 13% of the men who did not worry sought screening. Another study found that men who had abnormal PSA test results had higher prescreening anxiety (on a validated, three-item instrument) compared with men who had normal PSA results.25 Although anxiety may be relieved by a negative test, one study found that higher levels of anxiety may be associated with avoiding screening.26 In that study, asymptomatic relatives of patients with prostate cancer were evaluated for anxiety prior to entering a 3-year screening program, and mean scores on the STAI-State subscale (STAI-S) were higher in men who later missed one or more of their screening tests.
TABLE 3.
Percentage of Prostate Carcinoma Patients with Anxiety
| Study | Measure | Case definition | Anxiety (%) |
|---|---|---|---|
| Prescreening/screening | |||
| Bratt et al., 200028 | One question (yes or no) | PCa worry affects daily life? | 31 |
| Cormier et al., 200227 | STAI | Scale deterioration > 2 SE measurement | 21 |
| Demark-Wahnefried et al., 199522 | One question (yes or no) | “Provides peace of mind” | 50, 63 |
| Taylor et al., 200223 | IES | 34/35+ | 50 |
| Ward et al., 199724 | One question (yes or no) | “Have you ever worried that you might have PCa?” | 29 |
| Postscreening | |||
| Cormier et al., 200227 | STAI | Scale deterioration > 2 SE measurement | 13 |
| Cormier et al., 200243 | One question, 5-item response | Top 3 of 5 items | 36 |
| Taylor et al., 200223 | IES | Any intrusive thoughts? | 35 |
| Treatment, localized | |||
| Bisson et al., 200231 | HADS | 11+ | 10 |
| IES | 35+ | 14 | |
| Caffo et al., 199632 | One question (yes or no) | “Yes” to “felt worried” | 40 |
| Ficarra et al., 200036 | HADS | 11+ | 17 |
| Nordin et al., 200145 | HADS | 8+ | 9 (DX), 15 (6 mos) |
| Treatment, nonlocalized | |||
| Lintz et al., 200340 | HADS | 8+ | 14 |
| 11+ | 2 | ||
| Nordin et al., 200145 | HADS | 8+ | 18 (DX), 12 (6 mos) |
| Potosky et al., 200141 | One question | “Experience any worry about PCa” | 62 |
| PSA monitoring | |||
| Steineck et al., 20023 | STAI-T | >90th percentile | 9 (surgery), 10 (WW) |
| One question, 7-item response | Top 5 of 7 items | ||
| Roth et al., 199839 | HADS-A | 7+ | 33 |
| Distress Thermometer | 5+ | 29 | |
| Lofters et al., 200237 | One question, 5-item response | Top 2 of 5 items, 24 hrs before PSA check | 31 |
| “I would feel anxious” discontinuing PSA tests, 5-item response | Top 2 of 5 items, 24 hrs before PSA check | 83 |
PCa: prostate carcinoma; STAI: State-Trait Anxiety Inventory; SE: standard error; IES: Impact of Event Scale; DX: diagnosis; HADS: Hospital Anxiety and Depression Scale; HADS-A: HADS Anxiety subscale; WW: watchful waiting; PSA: prostate-specific antigen.
Having a family member with prostate carcinoma may raise anxiety about one’s own risk for cancer. Among men who had first-degree family members diagnosed with prostate carcinoma, an additional 20% experienced “significant anxiety” about having prostate carcinoma on the STAI-S compared with men who had no family history of prostate carcinoma.27 Another study of men in families with three or more relatives who had prostate carcinoma found that 82% of participants worried about an inherited predisposition for prostate carcinoma. 28 In that study, 31% of the cohort found that worry affected their daily life.
Postscreening
Two cross-sectional studies addressed postscreening populations. After screening, it would be expected that anxiety levels would be lower for men who had normal PSA values and, possibly, would be higher for men who had elevated PSA values. A study that compared the degree of reassurance from a normal result on a screening test with “no testing” and “reassurance from a negative biopsy result” revealed these expected patterns. Reassurance was strongest from a negative biopsy, next strongest for normal screening result, and weakest for no testing.29 In contrast, men who presented to a family practice clinic for general preventive care had low anxiety levels on the STAI-S both before and after a visit in which prostate cancer screening was completed.30
Treatment and monitoring: localized disease
Four articles evaluated anxiety levels in men who were diagnosed with clinically localized prostate carcinoma. Among patients who had been diagnosed recently with prostate carcinoma and were referred to a specialty clinic for treatment decisions, 18% of patients had significant anxiety at presentation on the HADS Anxiety subscale (HADS-A).31 According to a questionnaire that measured worry and tension, 40% of men who had been treated with radiation reported feeling “anxious,” and 36% “felt tense” after treatment.32 After treatment for prostate carcinoma, it has been hypothesized that testing with PSA to monitor for asymptomatic disease recurrence (i.e., rising PSA) causes higher anxiety levels.33 It is debatable whether the treatment modality affects anxiety levels during posttreatment PSA monitoring. One study found no significant differences between treatment groups, including surgery, radiation therapy, watchful waiting, and hormone therapy.34 A different study found that anxiety was a “medium to big problem” for 17% of patients after radiation therapy, whereas the same was true for only 10% of patients after undergoing prostatectomy.35
Anxiety with monitoring may be reduced many years after therapy due to adaptation. One study compared anxiety levels on the HADS-A an average of 22 months after prostatectomy for patients with prostate carcinoma or with benign prostatic hyperplasia (control group).36 Among the men with prostate carcinoma, 16.5% exceeded the HADS threshold of 8, whereas only 2% of men in the control group exceeded that threshold. Among men who were surveyed 4 years after enrollment in a randomized, controlled trial of prostatectomy versus watchful waiting, no statistically significant difference was found between the two groups on the STAI-S.3
Treatment and monitoring: nonlocalized disease
Eight studies of anxiety levels in populations of men with nonlocalized prostate carcinoma were identified. In a sample of men with metastatic prostate carcinoma who were being monitored with PSA for disease progression, 16% were “extremely” anxious, and 31% were “very” anxious or “extremely” anxious on a non-standardized, 4-point scale.37 In another survey that included mixed-stage cancer patients (including patients with prostate carcinoma) who presented for comprehensive cancer care, it was found that 24% had clinically significant anxiety levels on a subscale of the Brief Symptom Inventory, although the patients with prostate carcinoma had low overall psychologic distress compared with other patients.38 In a clinic for patients with prostate carcinoma in which > 80% had metastatic disease, 33% of patients exceeded the HADS-A screening threshold for anxiety.39 In 2 studies, it was found that age was associated inversely with anxiety regardless of disease stage, with men age < 65 years more worried about their disease.40,41 However, the overall presence of anxiety in those studies varied greatly. In a study of anxiety at 6 months after initiation of hormone therapy to prevent disease progression, it was found that > 50% of men were “worried.”41 In another study, it was found that only 14% of patients with mixed-stage cancer experienced at least some anxiety on the HADS-A, and 2% scored above the “anxiety case” threshold.40 It is interesting to note that, in the latter study, it also was found that there was no significant difference in anxiety between patients with localized or advanced cancer, although men with advanced disease were depressed more frequently. Another study in a mixed-stage patient population showed that, although diagnosis or staging of the disease itself was not correlated with anxiety on the STAI, patients with pain had higher levels of anxiety.42
Longitudinal Changes in Anxiety
Although it is important to determine anxiety levels in a specific population at a particular point in time, such comparisons may be misleading if individuals with different levels of baseline anxiety make different screening or treatment decisions (Table 4). Consequently, it is equally important to know how anxiety changes over time, especially in response psychologic stressors. Knowing about these changes requires longitudinal assessments with the same measures in the same population before and after an event, such as PSA screening, biopsy, deciding between treatment options, or PSA monitoring.
TABLE 4.
Longitudinal Studies of Anxiety in Patients with Prostate Carcinoma
| Study | Measure | Time 1 | Time 2 | Anxiety levels |
|---|---|---|---|---|
| Screening | ||||
| Davison et al., 199930 | STAI | Prescreen | Postscreen, unknown result | No change |
| Taylor et al., 200223 | IES | Prescreen | Postscreen, normal result | Decrease |
| Essink-Bot et al., 199821 | STAI | Prescreen | Postscreen, normal result | Decrease |
| Cormier et al., 200227 | STAI | Prescreen | Postscreen, normal result | Decrease |
| Gustafsson et al., 199544 | Serum cortisol | Prescreen | Postscreen, normal result | Decrease |
| Diagnosis | ||||
| Davison et al., 200346 | STAI | At positive biopsy | 4 mos later | Decrease |
| Essink-Bot et al., 199821 | STAI | Postscreen, abnormal result | Postbiospy, unknown result | No change |
| Essink-Bot et al., 199821 | STAI | Postbiopsy, unknown result | Postbiospy, normal result | Decrease |
| Gustafsson et al., 199544 | Serum cortisol | Postscreen, abnormal result | 16 weeks later | No change |
| Nordin et al., 200145 | HADS | At positive biopsy | 6 mos later | Decrease |
| Treatment | ||||
| Bisson et al., 200231 | HADS | Localized disease, first visit | Two weeks after first visit | Decrease |
| Bisson et al., 200231 | IES | Localized disease, first visit | Two weeks after first visit | No change |
| Helgesen et al., 200047 | HADS | Postdiagnosis or during treatment | Annually for 3 yrs | No change |
STAI: State-Trait Anxiety Inventory; IES: Impact of Event Scale; HADS: Hospital Anxiety and Depression Scale.
Prescreen to postscreen
There are significant decreases in the level of distress after a normal result on a PSA screening test, a decrease that is largest for younger men and for African-American men.23 In one study, patients were assessed with the STAI-S at three points over the course of prostate carcinoma screening with PSA: at the time of screening, while waiting for screening results, and after receiving normal results.43 Anxiety levels at the time of screening were lower than in population-matched norms. In addition, there was a significant overall decrease in anxiety on the STAI-S between the pre-PSA result and post-PSA result time points. However, between these 2 times, 20% of the participants experienced a significant increase in anxiety levels. In a multivariate model, predictors of worsening anxiety included younger men, men with more than two relatives who had a history of prostate carcinoma, and men with high anxiety levels according to the STAI-Trait subscale. Another study documented changes in cortisol levels, a common biologic marker of a stress response, in two different time sequences: 1) before and after undergoing PSA screening and 2) prior to undergoing a biopsy, after biopsy but before results were known, and after biopsy when pathology results were known.44 In the screening group, cortisol levels before screening were higher than in an age-matched control group and then dropped to cohort-equivalent levels at 2 weeks if the test was normal.
Postscreen to postbiopsy
In a population of men who were being screened for prostate carcinoma, anxiety levels were highest among those who had undergone a biopsy but still were awaiting the results.21 Although anxiety levels were unchanged by the abnormal PSA screening procedure alone, they dropped significantly between a prebiopsy state of uncertainty about disease status and a post-biopsy normal pathology result. In the study of cortisol levels discussed above, the highest levels were found immediately after biopsy but before patients were informed of the biopsy result.44 After the men were informed of pathology findings, anxiety levels dropped to near baseline, regardless of the biopsy results.
Diagnosis through treatment
The changes in anxiety levels among populations postdiagnosis and during or after treatment were less consistent. Three studies assessed anxiety both at the time of diagnosis and 4–6 months later. In 118 patients with mixed-stage prostate carcinoma, a marginally significant increase was found in the proportion of patients who were anxious according to the HADS-A (12% at diagnosis vs. 14% after 6 months; P = 0.05).45 When the populations were separated into patients with advanced cancer versus localized cancer, there was a trend toward patients with localized cancer becoming more anxious (9% at diagnosis vs. 15% after 6 months; P value not significant), whereas patients with advanced cancer became less anxious (18% at diagnosis vs. 12% after 6 months; P value not significant) after treatment.45 In another study that used the STAI-S to assess 74 patients with early-stage prostate carcinoma, mean scores were significantly lower by 4 months after diagnosis.46 In another study, in which clinically stable patients with prostate carcinoma were evaluated either at or near the time of diagnosis and then 5 more times over 3 years, it was found that the percentage of patients with considerable anxiety on the HADS-A was low (3.2– 8.3%), and there was no clear increase or decrease in anxiety over the study period.47 The same was found in a study that assessed anxiety using a 0–100-mm visual analogue scale before prostatectomy and again 3 months, 6 months, 12 months, and 18 months later; anxiety was low overall, and patients were somewhat more anxious before surgery (median score, 10 mm) than after surgery (median score, 0 mm).48
DISCUSSION
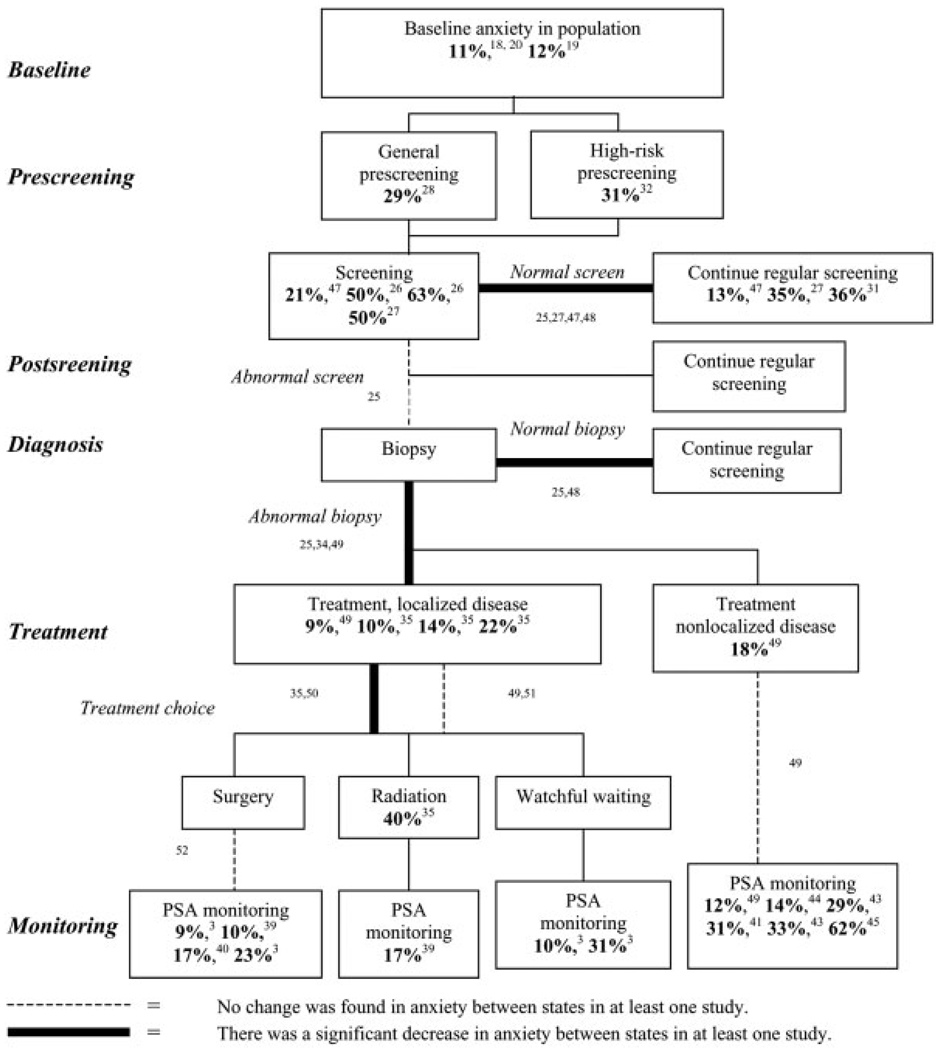
In this report, we have summarized and integrated the known data from both cross-sectional and longitudinal studies on anxiety levels concerning prostate carcinoma screening, detection, and management (Fig. 1). From studies that included case identification of men with anxiety, the percentages are listed in bold in the appropriate box representing a population. From studies that assessed longitudinal changes in anxiety levels, bold lines indicate a proven decrease in anxiety levels between the states, a dashed line indicates studies where no change in anxiety was found between the states, and standard thin lines indicate unstudied (and therefore unknown) relations between these states. There were no studies that documented rises in anxiety over time. As with any attempt to summarize limited, nonuniform data, we admit several caveats about this way of reporting the information. Anxiety levels were assessed using different scales across studies (see Table 3), making comparisons across populations problematic. In some cases, no standardized scale was used at all, and only an original questionnaire with unknown psychometric properties was employed. In Figure 1, the relations that identify significant changes in anxiety across situations do not necessarily correspond to the listed percentages in the boxes; that is, the relations found were or were not significant, but this finding was proven in different studies. With these limitations in mind, we draw the following conclusions.
FIGURE 1.
This chart illustrates the timeline of anxiety in prostate carcinoma and summarizes findings from the literature using standardized anxiety measures. Text boxes represent relevant patient populations at selected times in the potential course of screening, diagnosis, treatment, and follow-up. Cross-sectional studies of population anxiety levels are reported in these boxes. Lines connecting boxes represent transition points between populations when anxiety levels may change, and longitudinal studies that assessed changes in a population over time provided evidence for these relations. Finally, italicized text represents important decision-making points, such as screening tests, biopsy results, and treatment choices. Except for “Baseline,” details for the reported percentages are included in Table 3, including the anxiety measurement scales used and the definitions of cut-off values for determining a “case” of anxiety. For details on longitudinal changes in anxiety levels across populations, see Table 4 for studies and measurement instruments. Superscript numerals indicate references. PSA: prostate-specific antigen.
Anxiety levels appear to vary plausibly over the clinical timeline in response to stress and uncertainty. For example, in screening for prostate carcinoma, whereas baseline levels of anxiety in older men are relatively low (10–15%),18,19 these levels are significantly higher in men who present for screening (> 50%).22 Reducing anxiety is one potential motivation for screening, because individuals may hope for reassurance from a normal test result. Furthermore, “seeking peace of mind” appears to be a significant motivation for pursuing screening; and, when they present for screening, most men anticipate a normal test result. Consistent with this anticipation, studies have shown a significant decrease in anxiety levels after a negative screening test, although the proportion of men who remain anxious afterward does not appear to return to cohort baseline levels.23,27 All of these findings are consistent with the hypothesis that anxiety relief plays a significant role in the motivation to pursue screening for prostate carcinoma, and it possibly helps explain the high rates of PSA screening since its introduction, despite concerns about the efficacy of early intervention. Whether such anxiety reduction justifies the rise in screening practices remains an open question.
Moving along the clinical timeline, a normal biopsy result provides reassurance and decreases anxiety.21 The highest anxiety levels are reported among men who are awaiting biopsy results, and the levels decrease only after normal results are received, suggesting that it is the uncertainty about the results, and not the procedure itself, that induces anxiety. In fact, anxiety levels also decrease after a cancer diagnosis, although not as much. This demonstrates a general anxiety-relief value to biopsy results for men who have elevated PSA values, perhaps suggesting an important anxiolytic role of information receipt to eliminate uncertainty, even when the information received is not the preferred outcome.49,50
Nevertheless, living with the knowledge of an elevated PSA level may impact HRQOL by persistently increasing anxiety. This is true despite the fact that, after a diagnosis of prostate carcinoma, anxiety levels return toward baseline levels given sufficient time, suggesting that patients adapt to the situation.3 However, regardless of treatment choices, men who present for PSA testing to monitor for disease spread have much higher reported anxiety levels, so-called PSAitis.37 On-going anxiety from fear of recurrence or spread is an important part of the HRQOL impact expected from any of the treatments, and it should be accounted for in choosing among these outcomes. For instance, it appears that, after surgery, men have somewhat lower levels of anxiety compared with men who choose watchful waiting.3 Hope for minimizing PSA monitoring and the associated anxiety about cancer spread by undergoing surgery (to “get rid of the cancer”) may influence the choice of surgery over watchful waiting and radiation therapy.51
In this report, we have characterized the impact of anxiety over the clinical course of prostate carcinoma based on a systematic review of the medical literature. We have identified important times of stress and uncertainty over the clinical disease course when anxiety may change. The data on anxiety levels in patients with prostate carcinoma has been collected piecemeal, using different scales or even unvalidated instruments, making comparisons across populations difficult, if not impossible. Most studies are small in size, and few are prospective in design. In areas where it has been studied most carefully, particularly in the realm of pursuing screening, anxiety changes are consistent with the hypothesis that more anxious men are more likely to pursue screening, especially younger men and those who have relatives with a history of prostate carcinoma. It also may influence the choice of treatments for localized disease, with men who are more anxious more likely to undergo surgery to avoid worry about cancer spread. One neglected area of our current understanding for HRQOL and decision making in prostate carcinoma is the role of patient anxiety, and our current knowledge remains fragmentary. Future work should further assess the role anxiety may play in men’s decisions about prostate carcinoma detection and treatment.
Acknowledgments
The authors thank the following individuals who reviewed the article and offered helpful comments and suggestions: Greg Sachs, M.D.; Carol Stocking, Ph.D.; Joseph Shega, M.D.; George Loewen-stein, Ph.D.; and Walter Stadler, M.D. They also thank three anonymous reviewers for improving the organization and focus of the article.
REFERENCES
- 1.American Cancer Society. Cancer facts and figures. Atlanta: American Cancer Society; 2004. [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 3.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 4.Klotz LH. PSAdynia and other PSA-related syndromes: a new epidemic: a case history and toxonomy. Urology. 1997;50:831–832. doi: 10.1016/S0090-4295(97)00490-1. [DOI] [PubMed] [Google Scholar]
- 5.Noyes R, Hold CS, Massie MJ. Anxiety disorders. In: Holland JC, editor. Psycho-oncology. Oxford: Oxford University Press; 1998. pp. 548–563. [Google Scholar]
- 6.Antony MM, Orsillo SM, Roemer L. Practitioner’s guide to empirically based measures of anxiety. New York: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- 7.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM IV. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 9.Hankey BF, Jeuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–1024. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 10.Speilberger CD, Gorschuch RL, Lushene RE. STAI manual for the state-trait anxiety inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 11.Okun A, Stein RD, Gauman LJ, Silver EJ. Content validity of the psychiatric symptom index, CES-depression scale, and the State-Trait Anxiety Inventory from the perspective of the DSM-IV. Psychological Rep. 1996;79(3 Pt 1):1059–1069. doi: 10.2466/pr0.1996.79.3.1059. [DOI] [PubMed] [Google Scholar]
- 12.Smith AB, Selby PJ, Velikova G, et al. Factor analysis of the Hospital and Depression Scale form a large cancer population. Psychol Psychother Theory Res Prac. 2002;75:165–176. doi: 10.1348/147608302169625. [DOI] [PubMed] [Google Scholar]
- 13.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale, an update literature review. J Psychosomatic Res. 2001;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Weiss ES, Marmar CR. The Impact of Event Scale—revised. In: Wilson JP, Keane TM, editors. Assessing psychological trauma in PTSD. New York: Guilford; 1997. pp. 399–411. [Google Scholar]
- 16.Orsillo SM, Batten SV, Hammond C. Acute stress disorder and post-traumatic stress disorder: a brief overview and guide to assessment. In: Antony MM, Orsillo SM, Roemer L, editors. Practitioner’s guide to empirically based measures of anxiety. New York: Kluwer Adademic/Plenum Publishers; 2001. pp. 245–254. [Google Scholar]
- 17.Roth AJ, Rosenfeld B, Kornblith AB, et al. The Memorial Anxiety Scale for Prostate Cancer: validation of a new scale to measure anxiety in men with prostate cancer. Cancer. 2003;97:2910–2918. doi: 10.1002/cncr.11386. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. Mental health: a report of the Surgeon General. Rockville: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health; 1999. [Google Scholar]
- 19.Mehta KM, Simonsick EM, Penninx B, et al. Prevalence and correlates of anxiety symptoms in well-functioning older adults: findings from the health aging and body composition study. J Am Geriatr Soc.y. 2003;51:499–504. doi: 10.1046/j.1532-5415.2003.51158.x. [DOI] [PubMed] [Google Scholar]
- 20.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatr. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 21.Essink-Bot M-L, Koning HJ, Hijs HG, Kirkels WJ, Van der Maas PJ, Schroder FH. Short-term effects of population-based screening for prostate cancer on health-related quality of life. J Natl Cancer Inst. 1998;90:925–931. doi: 10.1093/jnci/90.12.925. [DOI] [PubMed] [Google Scholar]
- 22.Demark-Wahnefried W, Strigo T, Catoe K, et al. Knowledge, beliefs, and prior screening behavior among blacks and whites reporting for prostate cancer screening. Urology. 1995;46:346–351. doi: 10.1016/S0090-4295(99)80218-0. [DOI] [PubMed] [Google Scholar]
- 23.Taylor KL, Shelby R, Kerner J, Redd W, Lynch J. Impact of undergoing prostate carcinoma screening on prostate carcinoma-related knowledge and distress. Cancer. 2002;95:1037–1044. doi: 10.1002/cncr.10781. [DOI] [PubMed] [Google Scholar]
- 24.Ward JE, Hughes A-M, Hirst GHL, Winchester L. Men’s estimates of prostate cancer risk and self-reported rates of screening. Med J Aust. 1997;167:250–253. doi: 10.5694/j.1326-5377.1997.tb125048.x. [DOI] [PubMed] [Google Scholar]
- 25.Cohen L, Fouladi RT, Babaiam RJ, et al. Cancer worry is associated with abnormal prostate-specific antigen levels in men participating in a community screening program. Cancer Epidemiol Biomarkers Prev. 2003;12:610–617. [PubMed] [Google Scholar]
- 26.Roumier X, Azzouzi R, Valeri A, et al. Adherence to an annual PSA screening program over 3 years for brothers and sons of men with prostate cancer. Eur Urol. 2004;45:280–286. doi: 10.1016/j.eururo.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Cormier L, Valeri A, Azzouzi R, et al. Worry and attitude of men in at-risk families for prostate cancer about genetic susceptibility and genetic testing. Prostate. 2002;51:276–285. doi: 10.1002/pros.10092. [DOI] [PubMed] [Google Scholar]
- 28.Bratt O, Damber J-E, Emanuelsson M, et al. Risk perception, screening practice and interest in genetic testing among unaffected men in families with hereditary prostate cancer. Eur J Cancer. 2000;36:235–241. doi: 10.1016/s0959-8049(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 29.Cantor SB, Volk RJ, Cass AR, Gilani J, Spann SJ. Psychological benefits of prostate cancer screening: the role of reassurance. Health Expect. 2002;5:104–113. doi: 10.1046/j.1369-6513.2002.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davison JB, Kirk P, Degner LF, Hassard TH. Information and patient participation in screening for prostate cancer. Patient Educ Counsel. 1999;37:255–263. doi: 10.1016/s0738-3991(98)00123-2. [DOI] [PubMed] [Google Scholar]
- 31.Bisson JI, Chubb HL, Bennett S, Mason M, Jones D, Kynaston H. The prevalence and predictors of psychological distress in patients with early localized prostate cancer. BJU Int. 2002;90:56–61. doi: 10.1046/j.1464-410x.2002.02806.x. [DOI] [PubMed] [Google Scholar]
- 32.Caffo O, Fellin G, Graffer U, Luciani L. Assessment of quality of life after radical radiotherapy for prostate cancer. Br J Urol. 1996;78:557–563. doi: 10.1046/j.1464-410x.1996.14812.x. [DOI] [PubMed] [Google Scholar]
- 33.Slovin S. Biochemical relapse in prostate cancer: is PSA promoting stress and anxiety? Medscape Gen Med. 2002;4:11. [PubMed] [Google Scholar]
- 34.Clark JA, Inui TS, Siliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol. 2003;21:3777–3784. doi: 10.1200/JCO.2003.02.115. [DOI] [PubMed] [Google Scholar]
- 35.Fowler FJ, Barry MJ, Lu-Yao G, Wasson JH, Bin L. Outcomes of external-beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three Surveillance, Epidemiology and End Results areas. J Clin Oncol. 1996;14:2258–2265. doi: 10.1200/JCO.1996.14.8.2258. [DOI] [PubMed] [Google Scholar]
- 36.Ficarra V, Righetti R, D’Amico A, et al. General state of health and psychological well-being in patients after surgery for urological malignant neoplasms. Urol Int. 2000;65:130–134. doi: 10.1159/000064857. [DOI] [PubMed] [Google Scholar]
- 37.Lofters A, Juffs HG, Pond GR, Tannock IF. “PSA-itis”: knowledge of serum prostate specific antigen and other causes of anxiety in men with metastatic prostate cancer. J Urol. 2002;168:2516–2520. doi: 10.1016/S0022-5347(05)64180-8. [DOI] [PubMed] [Google Scholar]
- 38.Zabora J, Brintzenhofeszoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82:1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Lintz K, Moynihan C, Steginga S, et al. Prostate cancer patients' support and psychological care needs: survey from a non-surgical oncology clinic. Psychooncology. 2003;12:769–783. doi: 10.1002/pon.702. [DOI] [PubMed] [Google Scholar]
- 41.Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19:3750–3757. doi: 10.1200/JCO.2001.19.17.3750. [DOI] [PubMed] [Google Scholar]
- 42.Heim HM, Oei TP. Comparison of prostate cancer patients with and without pain. Pain. 1993;53:159–162. doi: 10.1016/0304-3959(93)90075-Z. [DOI] [PubMed] [Google Scholar]
- 43.Cormier L, Guillenmin F, Valeri A, et al. Impact of prostate cancer screening on health-related quality of life in at-risk families. Urology. 2002;59 doi: 10.1016/s0090-4295(02)01552-2. 901–806. [DOI] [PubMed] [Google Scholar]
- 44.Gustafsson O, Theorell T, Norming A, Persk A, Ohstrom M, Nyman CR. Psychological reactions in men screened for prostate cancer. Br J Urol. 1995;75:631–636. doi: 10.1111/j.1464-410x.1995.tb07422.x. [DOI] [PubMed] [Google Scholar]
- 45.Nordin K, Berglund G, Glimelius B, Sjoden PO. Predicting anxiety and depression among cancer patients: a clinical model. Eur J Cancer. 2001;37:376–384. doi: 10.1016/s0959-8049(00)00398-1. [DOI] [PubMed] [Google Scholar]
- 46.Davison JB, Boldenberg SL, Gleave ME, Degner LF. Provision of individualized information to men and their partners to facilitate treatment decision making in prostate cancer. Oncol Nurs Forum. 2003;30:107–114. doi: 10.1188/03.ONF.107-114. [DOI] [PubMed] [Google Scholar]
- 47.Helgesen F, Andersson S-O, Varenhorst E, et al. Follow-up of prostate cancer patients by on-demand contacts with a specialist nurse. Scand J Urol Nephrol. 1999;34:55–61. doi: 10.1080/003655900750016904. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen KV, Carlsson P, Rahmquist M, Varenhorst E. Quality of life after radical retropubic prostatectomy for carcinoma of the prostate. Eur Urol. 1993;24:7–11. doi: 10.1159/000474254. [DOI] [PubMed] [Google Scholar]
- 49.Mishel MH. Uncertainty in illness. IMAGE: J Nurs Scholar. 1988;20:225–232. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 50.Rainey LC. Effects of preparatory patient education for radiation oncology patients. Cancer. 1985;56:1056–1061. doi: 10.1002/1097-0142(19850901)56:5<1056::aid-cncr2820560516>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 51.Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what's important. J Gen Intern Med. 2000;15:694–701. doi: 10.1046/j.1525-1497.2000.90842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark JA, Wray N, Brody B, Ashton C, Biesler B, Watkins H. Dimensions of quality of life expressed by men treated for metastatic prostate cancer. Soc Sci Med. 1997;45:1299–1309. doi: 10.1016/s0277-9536(97)00058-0. [DOI] [PubMed] [Google Scholar]