Abstract
OBJECTIVES
Published studies of gene transfer to mouse salivary glands have not employed the parotid glands. Parotid glands are the likely target tissue for most clinical applications of salivary gene transfer. The purpose of the present study was to develop a convenient and reproducible method of retroductal gene transfer to mouse parotid glands.
METHODS
The volume for vector delivery was assessed by infusion of Toluidine Blue into Stensen’s ducts of Balb/c mice after direct intraoral cannulation. Recombinant, serotype 5 adenoviral vectors, encoding either firefly luciferase or human erythropoietin (hEpo), were constructed and then administered to parotid glands (107 vector particles/gland). Transgene expression in vivo was measured by enzyme activity (luciferase) or an enzyme-linked immunosorbent assay (hEpo). Vector biodistribution was measured by real-time quantitative (Q) PCR.
RESULTS
The chosen volume for mouse parotid vector delivery was 20 µL. Little vector was detected outside of the targeted glands, with both QPCR and luciferase assays. Transgene expression was readily detected in glands (luciferase, hEpo), and serum and saliva (hEpo). Most secreted hEpo was detected in saliva.
CONCLUSION
These studies show that mouse parotid glands can be conveniently and reproducibly targeted for gene transfer, and should be useful for pre-clinical studies with many murine disease models.
Keywords: parotid, gene transfer, adenoviral vector, mouse
Introduction
There now are many studies showing the feasibility of therapeutic gene transfer targeting salivary glands in rodents and larger animals (for representative reviews see Baum et al, 2002, Kagami et al, 2008). In almost all published studies of gene transfer to rodent salivary glands, the submandibular glands were primarily targeted (e.g. Mastrangeli et al, 1994, Barka and van der Noen, 1996, Goldfine et al, 1997, Tucker et al, 2003, Shai et al, 2005, Vosters et al, 2009), while in all published large animal (miniature pig and rhesus macaque) studies, the salivary gland targeted for gene transfer was the parotid (e.g. Li et al, 2004, Voutetakis et al, 2008). Despite previous demonstration of successful gene transfer to rat parotid glands (Mastrangeli et al, 1994, Kagami et al, 1998), most investigators using rodent models have focused on the submandibular glands for two general reasons: (i) submandibular glands are a relatively large and well-encapsulated tissue in rats and mice, the latter feature theoretically providing a barrier helping to prevent the spread of vectors beyond the targeted gland, and (ii) parotid glands are diffuse, multi-segmental tissues in rodents, a characteristic that makes it relatively difficult to dissect out all gland tissue for quantification of gene transfer. The targeting of parotid glands for salivary gene transfer in miniature pig and rhesus macaque studies results from these glands being, like human parotid glands, very well-encapsulated, and having a main excretory duct that is fairly straight (e.g. O’Connell et al, 1999, Li et al, 2004). In humans, it appears that the parotid gland will be the main target for clinical applications of salivary gene transfer (e.g. Zheng et al, 2010).
In the current study, we have systematically examined the feasibility of direct, in vivo gene transfer to murine parotid glands. Mice, because of the existence of so many gene knockout models of human disease, provide important, initial pre-clinical models for feasibility in gene therapy (e.g. Muzzin et al, 1996, Oshima et al, 1997, Bohl et al, 2000, Ding et al, 2001, Lu et al, 2008, Trionfini et al, 2009) with potential applicability to salivary gene transfer. Using recombinant serotype 5, adenoviral (Ad5) vectors, we show herein that mouse parotid glands can be conveniently and reproducibly targeted for gene transfer.
Materials and Methods
Animal studies
The National Institute of Dental and Craniofacial Research Animal Care and Use Committee and the National Institutes of Health Biosafety Committee approved all animal experiments.
Fluid volume determination for parotid administrations
Prior to beginning the gene transfer studies, we evaluated a useful volume of fluid that could be administered to mouse parotid glands via retroductal delivery. To do this, Toluidine Blue (Fisher Scientific, Pittsburgh, PA, USA; 1%, w/v, solution in 0.1% acetic acid, v/v, 4.2% ethanol, v/v) was administered to right parotid glands in three volumes: 20, 35 and 50-µL, exactly as described below for the gene transfer procedure. After 1 h the Toluidine Blue distribution in the targeted glands was directly observed following a neck dissection.
Adenoviral vector preparation
Ad5 vectors encoding either firefly luciferase (AdLuc) or human erythropoietin (hEpo; AdhEpo) were prepared and amplified as previously reported (Zheng et al, 2000, Voutetakis et al, 2005, respectively). Vectors were tested in vitro by transduction of 293 cells. Transduction was assessed on the following day either by a luciferase enzymatic assay or a human hEpo-specific enzyme-linked immunosorbent assay (ELISA; see below).
Ad5 vector administration to male Balb/c mice
Balb/c mice (8 weeks old) were anesthetized with a mixture of 100-mg ml−1 ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA) and 20-mg ml−1 xylazine (Phoenix Scientific, St. Joseph, MO, USA) given intramuscularly (1 µl g−1 of body weight). For each animal, one or occasionally both Stensen’s duct of the parotid glands was cannulated with modified polyethylene tubing (Intramedic PE-10, BD Diagnostic Systems, Sparks, MD, USA), and atropine (intramuscular injection, 0.5 mg kg−1 body weight; Sigma, St. Louis, MO, USA) was administered to decrease salivary flow. After an additional 10 min, either AdLuc or AdhEpo (107 vector particles, vp/gland) was administered by retrograde ductal delivery to the cannulated glands in a 20-µL volume. The dose of vector chosen was based on previous results with murine submandibular glands (e.g. see Wang et al, 2000, Yamano et al, 2002, Voutetakis et al, 2004), and our hypothesis was that 107 vp/gland would lead to significant transgene expression with little to no inflammation in the targeted parotid glands. For the present study at least 15 mice received the AdLuc vector, and at least 20 mice received the AdhEpo vector, to their parotid glands. The data presented herein are experiments performed with five mice/vector and are representative of all experiments performed. Additionally, in some experiments, 20-µL of saline was administered to the contralateral parotid gland. After 2 days, for experiments with AdLuc, mice were again anesthetized and first imaged to visualize in situ the tissue distribution of the luciferase expression using a Xenogen IVIS system (see below) and thereafter sacrificed, and the parotid glands and liver removed. Following administration of the AdhEpo vector, mice were anesthetized and then given a subcutaneous injection of pilocarpine (0.5 mg ml−1, 1 µl g−1 body weight; Sigma) to stimulate salivary flow. Whole saliva was collected from the oral cavity with a microhematocrit capillary tube (Fisher Scientific, Hampton, NH, USA; Baum et al, 2002). Blood samples were next obtained from mice by retro-orbital plexus bleeding. Thereafter, saliva and serum were stored at −80 °C until assayed for hEpo by the ELISA described below.
Assessment of transgene expression
For in vitro assessment of vector transduction, 293 cells were transduced with Ad5 vectors, and both AdLuc and AdhEpo mediated transgene expression well above background (data not shown). With in vivo studies, parotid and liver tissue extracts were obtained after parotid gland transduction, as described above. For luciferase assays, cells or tissues were lysed in cell lysis buffer (Promega, Madison, WI, USA) for 15 min. Fifty µL of the cell or tissue lysates were added to 100 µL of luciferase substrate, and light output was measured with a luminometer. Results were expressed as relative light units (RLU) per cell number (in vitro) or per mg protein (in vivo). A commercially available ELISA kit was used to determine the expression levels of hEpo in cell culture media and in mouse parotid glands (also homogenized in the above cell lysis buffer), serum and saliva (Stem Cell Technologies, Vancouver, BC, Canada). The lower limit of hEpo detection was 0.6 mU ml−1. Assays were performed in duplicate according to the manufacturer’s instructions.
Imaging of Luciferase expression in vivo
The tissue distribution of luciferase transgene expression was visualized using the Xenogen IVIS Imaging System (Caliper Life Sciences, Hopkington, MA, USA). Mice were examined 2 days after administration of AdLuc to a single parotid gland. After being anesthetized, as described above, each mouse received an intraperitoneal injection of luciferin (4 mg/100 µl in PBS) substrate prior to imaging.
Determination of AdLuc and AdhEpo copy numbers in parotid gland and liver
Genomic DNA from parotid glands and livers was extracted with the Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA). One hundred ng DNA was used per quantitative (Q) PCR reaction. The primers E3Taq1 (5′-GAGTTGGCACCCCTATTCGA-3′) and E3Taq2 (5′-ATGCCACATCCGTTGACTTG-3′), and probe E3Taqprobe (5′-/56-FAM/CCACCCGTGTGTACCTGGTGGACA/36-TSMTSp/-3′), for the adenoviral E3 region were used to measure vector copy number. These sequences were selected using Primer Express™ Primer Design software (PE Applied Biosystems, Foster City, CA, USA), and the assay done in an ABI Prism 7700 Sequence Detector (PE Applied Biosystems). The conditions used were as follows: 95 °C for 2 min, 95 °C for 8 min, 95 °C for 15 s and 60 °C for 1 min for 40 cycles.
Morphological evaluation of parotid glands following Ad5 vector administration
Parotid glands from control and treated mice were fixed in 10% formalin and then paraffin-embedded. Ten µm sections were stained with hematoxylin and eosin (H&E). For immunohistochemistry of hEpo, gland sections were dewaxed, rehydrated in a gradient of ethanol, washed in PBS and the immunostaining performed using an anti-hEpo antibody (H-162; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) as primary antibody following the procedures described in Histostain®-SP kits (Invitrogen, Carlsbad, CA, USA).
Results and discussion
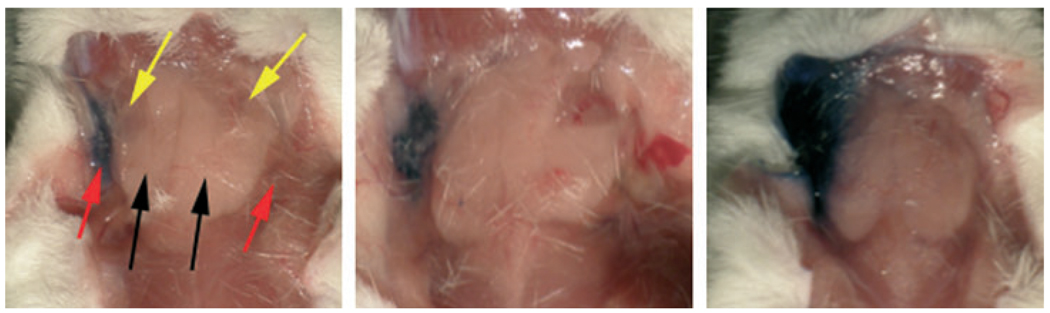
Initially, we evaluated the volume of fluid that could be infused into a mouse parotid gland, without causing gross damage, by using a solution of Toluidine Blue. Three different volumes were infused into single parotid glands of mice. As shown in Fig. 1, when 20-µl was infused, all of the visible fluid remained in the targeted right parotid gland (left panel), while when either 35-µl (middle panel) or 50-µl (right panel) fluid was infused visible extravasations were observed, slight and considerable, respectively. Based on these results, we decided to deliver all Ad5 vectors suspended in a fixed volume of 20-µL.
Figure 1.
Determination of fluid volume for use in murine parotid gland vector infusions. The right parotid glands of mice were cannulated and infused with a solution of Toluidine Blue as described in Materials and Methods. The figure shows a mouse infused either with 20- (left), 35- (center) or 50- (right) µl of Toluidine Blue. Red arrows point to parotid glands. Black arrows point to submandibular glands. Yellow arrows point to sublingual glands. No fluid extravasation was seen with the 20-µl volume
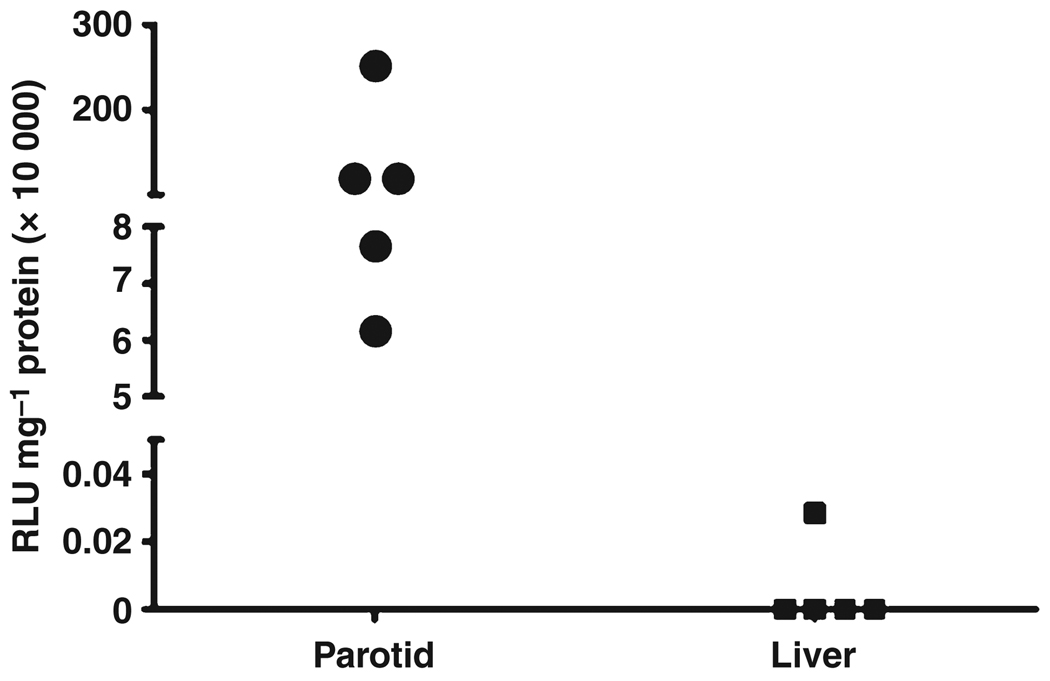
We next administered the AdLuc vector to mouse parotid glands. Since transgenic luciferase protein will be localized intracellularly, after 48 h mice were sacrificed and the targeted parotid glands, and a portion of liver, were obtained. Luciferase activity essentially was seen only in extracts from the parotid glands (Fig. 2). No luciferase activity was found in liver extracts of four transduced mice, and very low (0.47% of the lowest parotid value) activity seen in the liver extract from one of the transduced mice. The median luciferase activity seen in parotid glands was 1.1 × 106 RLU mg−1 protein, while that for the liver was zero (background). These results strongly suggest that vector delivered to a targeted parotid gland overwhelmingly remained in the gland and did not escape significantly into the circulation, as following intravascular delivery, Ad5 vectors predominantly transduce liver in rodents (e.g. Kay et al, 1995, Amalfitano et al, 1999, Johnson et al, 2006). This result was also consistent with that obtained following the infusion of 20-µL Toluidine Blue (above).
Figure 2.
Luciferase activity levels achieved following AdLuc delivery to murine parotid glands. Mice were administered AdLuc (107 vector particles) in a single parotid gland. After 48 h mice were sacrificed and parotid and liver tissue was removed. Extracts of tissue were prepared and luciferase activity assayed as described in Materials and Methods. Data shown are individual results from five mice (same animals as shown in Fig. 3)
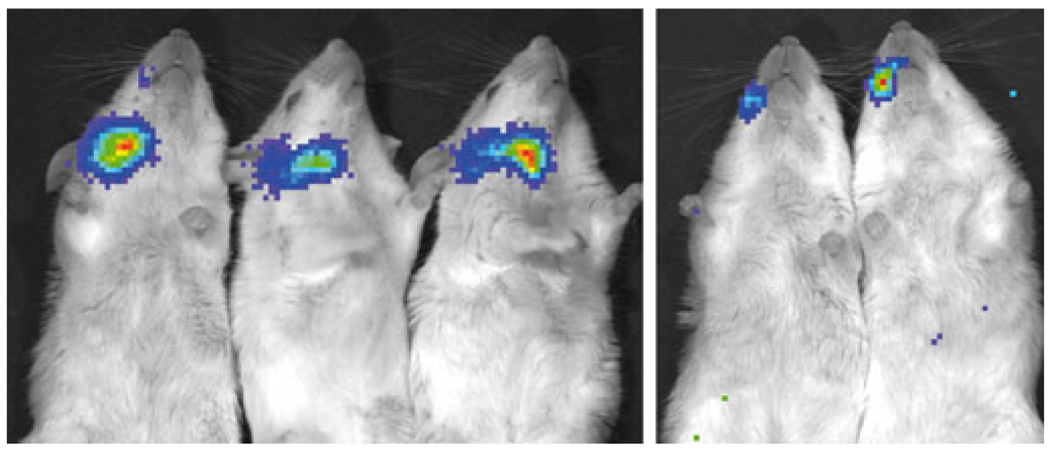
Since luciferase activity (i.e. the oxidation of luciferin) results in the generation of a photon, it is also possible to inject animals with a bioluminescent substrate and, in a living animal, directly visualize the tissue sites where functional luciferase is expressed (e.g. Dunn et al, 2005, Johnson et al, 2006). The five mice, whose gland extracts were obtained and assayed for the data shown in Fig. 2, were imaged in this highly sensitive manner prior to sacrifice to visualize any luciferase expressing tissues. All mice exhibited considerable luciferase activity in their targeted right parotid glands (Fig. 3; three very high, two moderate, just as seen with the gland extract assay, Fig. 2). Most importantly, at the sensitivity level of this image, little to no luciferase activity could be seen in any other tissues, including the liver, again a result consistent with the AdLuc vector being localized to and retained in the targeted glands after delivery.
Figure 3.
Visualization of transgene expression in vivo after AdLuc administration to murine parotid glands. AdLuc (107 particles/gland) was delivered to the right parotid glands of five mice and, after 48 h, the tissue distribution of luciferase transgene expression was visualized using the Xenogen IVIS Imaging System. In three mice (left) there were high levels of luciferase activity detected, while in two mice (right) only moderate luciferase activity was detected. No significant luciferase activity can be visualized in any other tissue. See Materials and Methods for additional details
Although a highly sensitive reporter gene and quite useful experimentally, luciferase is without any therapeutic application or clinical value. To assess the ability of mouse parotid glands to produce and secrete a therapeutically important protein, we next administered the AdhEpo vector. After AdhEpo delivery, the transduced mouse parotid glands make significant levels of hEpo, secreting it into both serum, where it could be biologically useful (Voutetakis et al, 2005) and saliva, where it would not. On average, in extracts of the targeted parotid gland, we could detect considerable hEpo expression, nearly 382 ± 115 mU hEpo mg−1 protein. The concentration of hEpo found in saliva was nearly 50-fold that found in serum (1940 ± 460 mU ml−1 versus 32 ± 4 mU ml−1). Interestingly, these latter results are quite different from those seen following transduction of mouse submandibular glands, where after AdhEpo administration almost all of the secreted transgenic hEpo is found in the bloodstream (Voutetakis et al, 2004, 2005), and suggests that differences in the sorting of transgenic secretory proteins may exist between these two closely related exocrine glands.
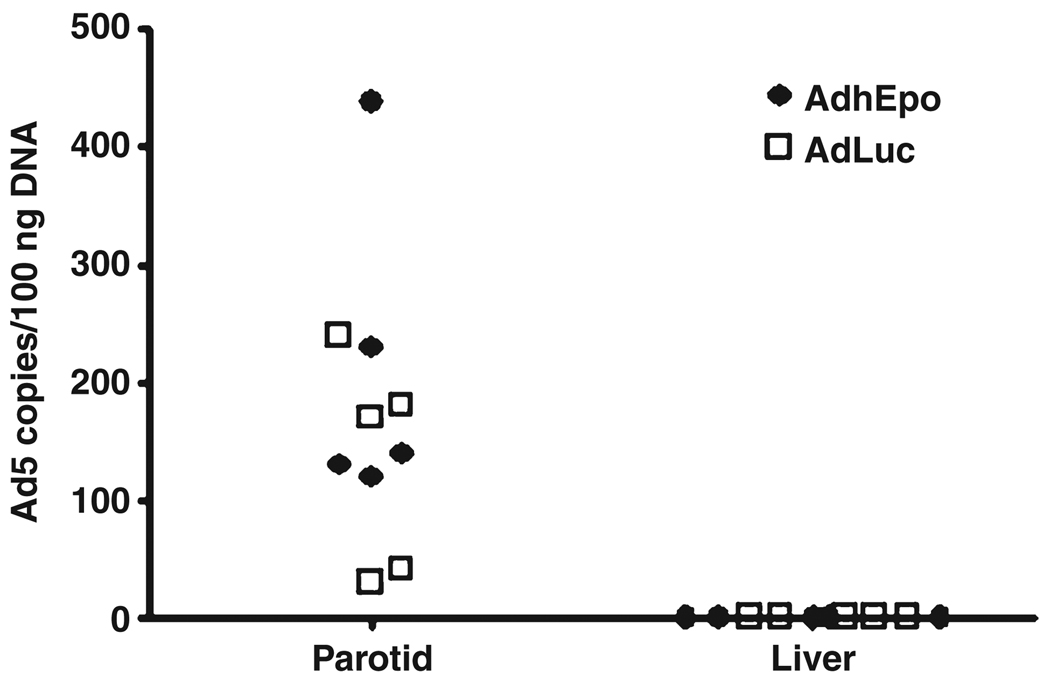
To directly assess Ad5 vector distribution following parotid gland administration of 107 vp/gland, we obtained portions of transduced parotid glands, as well as the livers, from all animals 48-h after vector delivery and extracted genomic DNA. Using QPCR, we then determined the number of vector copies present in each tissue. As shown in Fig. 4, similar levels of vector could readily be detected in all parotid gland samples after AdLuc and AdhEpo administration (median value ~150 copies/100 ng DNA), but no vector copies could be detected in any DNA extracted from liver samples. As in the above experiments, this result strongly suggests that gene transfer vectors can be localized to parotid glands following intraoral administration via Stensen’s duct.
Figure 4.
Detection of adenoviral vector in parotid gland and liver tissue of mice following AdLuc or AdhEpo administration. AdLuc or AdhEpo (107 particles/gland) was delivered to single glands (n = 5 mice/vector group), parotid and liver tissue was removed, genomic DNA extracted and QPCR assays performed as described in Materials and Methods. Data from individual mice are shown in a scatter plot (open squares, AdLuc; closed diamonds, AdhEpo)
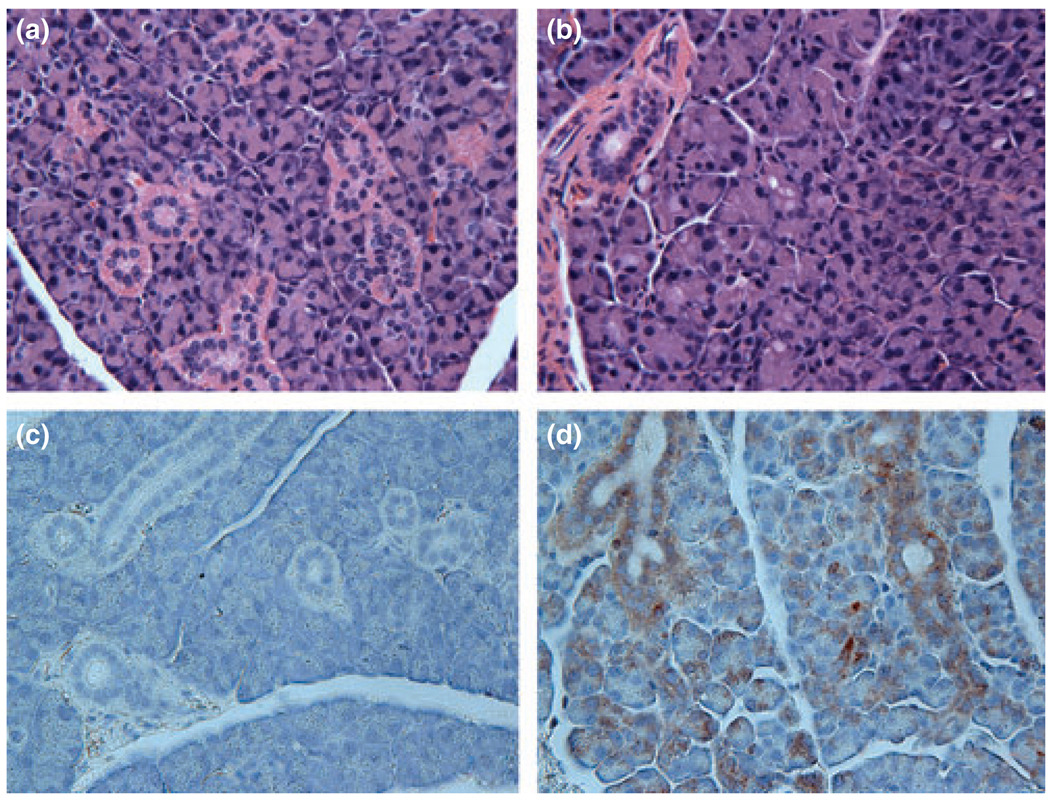
Finally, we evaluated paraffin-embedded sections of transduced parotid glands, stained with H&E, for possible pathological changes occurring with vector administration. As shown in Fig. 5, there was no difference in the histological appearance of sections obtained from untreated mouse parotid glands and those administered 107 vp of AdhEpo, i.e. there was no evidence of a significant inflammatory response Fig. 5a,b). This is not surprising, given previous reports of studies on the administration of different doses of Ad5 vectors to rat submandibular glands (Adesanya et al, 1996). In addition, gland sections were immunostained for the presence of hEpo. As shown in Fig. 5c,d, hEpo was detected in both acinar and duct cells in the transduced parotid glands.
Figure 5.
Morphological evaluation of murine parotid glands following Ad5 vector administration. Parotid glands from control and treated mice were fixed in 10% formalin and then paraffin-embedded. Ten µm sections were stained with either H&E (a, b) or immunostained with a primary antibody directed to hEpo (c, d), see Materials and Methods for additional details. (a, c) Sections from a control parotid gland. (b, d) sections from a parotid gland transduced with AdhEpo
The experiments described herein show that it is readily possible to administer gene transfer vectors directly into the parotid glands of 20–25 g mice. Importantly, essentially all vector administered to these glands remains localized in the tissue under the conditions utilized, i.e. little to no vector was detected beyond the targeted tissue using two very sensitive tools, bioluminescent imaging and QPCR. In particular, the absence of any substantive vector copies or transgene expression in the liver is notable. If parotid-administered vector were to leak into the circulation due, for example, to cannula perforation of the gland lumen, the most likely place to detect it would be in the liver, as intravascular administration of Ad5 vectors primarily targets the liver (e.g. Kay et al, 1995, Amalfitano et al, 1999, Johnson et al, 2006). Of note, we did use a very low vector dose in these experiments, 107 vp/gland, which, as hypothesized, led to essentially no detectable inflammatory response in the transduced glands. Nonetheless, all targeted gland tissue expressed significant levels of transgenic protein. Furthermore, when AdhEpo was used, the transgenic hEpo protein was secreted into both serum and saliva. Extrapolating the vector dose and infusion volume used herein to a human parotid gland, for an ‘average’ infusion volume used in an ongoing clinical trial (approximately 750 µl; Baum et al, 2010, Zheng et al, 2010), the present dose would correspond to an Ad5 vector dose in humans of 3.75 × 108 vp/gland. This is slightly higher than the second dosage group in the ongoing clinical trial, i.e. suggesting reasonable scaling between two species whose average body weight differs by a factor of >3000-fold.
In summary, these studies show that mouse parotid glands can be conveniently and reproducibly targeted for pre-clinical gene transfer experiments. Since it is likely that most clinical applications of salivary gland gene transfer will focus on parotid glands, particularly for gene therapeutics of single protein deficiency disorders (see e.g. Voutetakis et al, 2008, 2010), this approach to gene transfer should allow testing relevant therapeutic paradigms in pre-clinical studies with many murine gene knockout disease models (e.g. Muzzin et al, 1996, Oshima et al, 1997, Bohl et al, 2000, Ding et al, 2001, Lu et al, 2008, Trionfini et al, 2009).
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research.
References
- Adesanya MR, Redman RS, Baum BJ, O’Connell BC. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–1093. doi: 10.1089/hum.1996.7.9-1085. [DOI] [PubMed] [Google Scholar]
- Amalfitano A, McVie-Wylie AJ, Hu H, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-α-glucosidase. Proc Natl Acad Sci USA. 1999;96:8861–8866. doi: 10.1073/pnas.96.16.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka T, van der Noen H. Retrovirus-mediated gene transfer into salivary glands in vivo. Hum Gene Ther. 1996;7:613–618. doi: 10.1089/hum.1996.7.5-613. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Zheng C, Alevizos I, et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncol. 2010;46:4–8. doi: 10.1016/j.oraloncology.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl D, Bosch A, Cardona A, Salvetti A, Heard JM. Improvement of erythropoiesis in beta-thalassemic mice by continuous erythropoietin delivery from muscle. Blood. 2000;95:2793–2798. [PubMed] [Google Scholar]
- Ding EY, Hodges BL, Hu H, et al. Long-term efficacy after [E−, polymerase −] adenovirus-mediated transfer of human acid-α-glucosidase gene into glycogen storage disease type II knockout mice. Hum Gene Ther. 2001;12:955–965. doi: 10.1089/104303401750195917. [DOI] [PubMed] [Google Scholar]
- Dunn CA, Jin Q, Taba M, Jr, Francheschi RT, Rutherford RB, Giannobile WV. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther. 2005;11:294–299. doi: 10.1016/j.ymthe.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine ID, German MS, Tseng HC, et al. The endocrine secretion of human insulin and growth hormone by exocrine glands of the gastrointestinal tract. Nat Biotechnol. 1997;15:1378–1382. doi: 10.1038/nbt1297-1378. [DOI] [PubMed] [Google Scholar]
- Johnson M, Huyn S, Burton J, Sato M, Wu L. Differential biodistribution of adenoviral vector in vivo as measured by bioluminescence imaging and quantitative polymerase chain reaction. Hum Gene Ther. 2006;17:1262–1269. doi: 10.1089/hum.2006.17.1262. [DOI] [PubMed] [Google Scholar]
- Kagami H, Atkinson JC, Michalek SM, et al. Repetitive administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther. 1998;10:305–313. doi: 10.1089/hum.1998.9.3-305. [DOI] [PubMed] [Google Scholar]
- Kagami H, Wang S, Hai B. Restoring the function of salivary glands. Oral Dis. 2008;14:15–24. doi: 10.1111/j.1601-0825.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- Kay MA, Graham F, Leland F, Woo SLC. Therapeutic concentrations of human alpha-1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology. 1995;21:815–819. [PubMed] [Google Scholar]
- Li J, Zheng C, Zhang X, et al. Developing a convenient large animal model for gene transfer to salivary glands in vivo (2004) J Gene Med. 2004;6:55–63. doi: 10.1002/jgm.476. [DOI] [PubMed] [Google Scholar]
- Lu H, Chen L, Wang J, et al. Complete correction of hemophilia A with adeno-associated viral vectors containing a full size expression cassette. Hum Gene Ther. 2008;19:648–654. doi: 10.1089/hum.2007.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangeli A, O’Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol. 1994;266:G1146–G1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- Muzzin P, Eisensmith RC, Copeland KC, Woo SL. Correction of obesity and diabetes in genetically obese mice by leptin gene therapy. Proc Natl Acad Sci USA. 1996;93:11804–11808. doi: 10.1073/pnas.93.25.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell AC, Baccaglini L, Fox PC, et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999;6:506–513. doi: 10.1038/sj.cgt.7700078. [DOI] [PubMed] [Google Scholar]
- Oshima T, Murray GJ, Swaim WD, et al. Alpha-galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai E, Palmon A, Panet A, et al. Prolonged transgene expression in murine salivary glands following non-primate lentiviral vector transduction. Mol Ther. 2005;12:137–143. doi: 10.1016/j.ymthe.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Trionfini P, Tomasoni S, Galbusera M, et al. Adenoviral-mediated gene transfer restores plasma ADAMTS13 antigen and activity in ADAMTS13 knockout mice. Gene Ther. 2009;16:1373–1379. doi: 10.1038/gt.2009.98. [DOI] [PubMed] [Google Scholar]
- Tucker SN, Lin K, Stevens S, Scollay R, Bennett MJ, Olson DC. Systemic and mucosal antibody responses following retroductal gene transfer to the salivary gland. Mol Ther. 2003;8:392–399. doi: 10.1016/s1525-0016(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Vosters JL, Yin H, Roescher N, Kok M, Tak PP, Chiorini JA. Local expression of tumor necrosis factor-receptor 1: immunoglobulin G can induce salivary gland dysfunction in a murine model of Sjögren’s syndrome. Arthritis Res Ther. 2009;11:R189. doi: 10.1186/ar2888. Epub Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutetakis A, Bossis I, Kok MR, et al. Salivary gland as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol. 2005;185:363–372. doi: 10.1677/joe.1.06171. [DOI] [PubMed] [Google Scholar]
- Voutetakis A, Kok MR, Zheng C, et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci U S A. 2004;101:3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutetakis A, Zheng C, Cotrim AP, et al. AAV5-mediated gene transfer to the parotid glands of non-human primates. Gene Ther. 2010;17:50–60. doi: 10.1038/gt.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutetakis A, Zheng C, Metzger M, et al. Sorting of transgenic secretory proteins in rhesus macaque parotid glands following adenoviral mediated gene transfer. Hum Gene Ther. 2008;19:1401–1405. doi: 10.1089/hum.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Baum BJ, Yamano S, et al. Adenoviral-mediated gene transfer to mouse salivary glands. J Dent Res. 2000;79:701–708. doi: 10.1177/00220345000790020201. [DOI] [PubMed] [Google Scholar]
- Yamano S, Huang LY, Ding C, et al. Recombinant adeno-associated virus serotype 2 vectors mediate stable interleukin 10 secretion from salivary glands into the bloodstream. Hum Gene Ther. 2002;13:287–298. doi: 10.1089/10430340252769806. [DOI] [PubMed] [Google Scholar]
- Zheng C, Baum BJ, Iadarola MJ, O’Connell BC. Genomic integration and gene expression by a modified adenoviral vector. Nat Biotechnol. 2000;18:176–180. doi: 10.1038/72628. [DOI] [PubMed] [Google Scholar]
- Zheng C, Nikolov NP, Alevizos I, et al. Transient detection of E1-containing adenovirus in saliva after delivery of a first-generation adenoviral vector to human parotid gland. J Gene Med. 2010;12:3–10. doi: 10.1002/jgm.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]