Abstract
Here we report a cavitand with a photochemical switch as one of the container walls. The azo-arene switch undergoes photoisomerization when subjected to UV light producing a self-fulfilled cavitand. This process is thermally and photochemically reversible. The reported cavitand binds small molecules and these guests can be ejected from the cavitand through this photochemical process.
Molecular devices on the nanoscale continue to attract attention,1 and a variety of stimuli to drive the machinery are available. Changes in redox,2 pH,3 metal ion presence,4 and other chemical inputs5 have been used to cycle between well-defined molecular states, but perhaps the oldest—and one of the most frequently used stimuli—is light. In supramolecular devices the trans/cis photoisomerism of the azo benzene module provided the first switching mechanism applied to the binding behavior of cyclodextrins6 and crown ethers.7 Its ease of introduction, reliable shape and distance changes and broad applicability, even to foldamers8 and biological molecules such as proteins comprising ion channels,9 have insured the popularity of the photoisomerization process. Surprisingly, the azobenzene module has not appeared in deep cavitands and we correct that omission here.10
In contrast to most photo-switchable devices—where the photo-responsive unit is appended on the structure’s periphery to impart function—we integrated it into the cavitand’s structure. We devised two light-responsive cavitands that exhibit very different guest binding behavior when exposed to light. Control of guest uptake and release is achieved through an unusual conformational preference, but only when a tert-butyl group is present on the azo-arene substituent. The switching is dictated by weak attractive forces and not through typical covalent bonds or steric constraints.
We prepared azo cavitands 1 and 2 in one step from the known mono-amine cavitand 311 (Scheme 1). Mixing nitrosoarenes12 at room temperature in glacial acetic acid with cavitand 3 resulted in the precipitation of pure azo-arene cavitands as orange solids (see ESI† for 1H, 13C NMR and mass spectrometry characterization data).
Scheme 1.
Synthesis of azo cavitands 1 and 2.
In the resting configuration azo-arene cavitands trans-1 and trans-2 present deep cavities for guest binding. Upon irradiation with UV light, the azo substituent undergoes photoisomerization to produce cis-1 and cis-2. The tert-butyl substituent of 1 was expected to fold into and occupy the cavitand void where it can enjoy stabilizing CH–π interactions. In contrast, the unsubstituted azo cavitand cis-2 can neither reach the same CH–π distances nor adequately fill the space.
The switching capabilities of 1 and 2 were investigated in d12-mesitylene. Both cavitands present only trans configuration by 1H NMR after heating to reflux and cooling to room temperature in the dark. Under ambient conditions a small percentage of cis cavitand is observed, 8% for 1 and 5% for 2.13
Computational and crystallographic studies reveal that cis-azobenzene derivatives adopt a non-planar conformation with a CCNN dihedral angle of ~53°.14 Photoisomerization converts both cavitands to their respective cis configurations but with different consequences. Irradiating cavitand 2 with 365 nm light for 15 minutes converts it to cis-2 where it can adopt numerous cis conformations. In contrast, the photoisomerization of cavitand 1 places the tert-butyl substituent inside the cavitand in a contorted conformational preference.
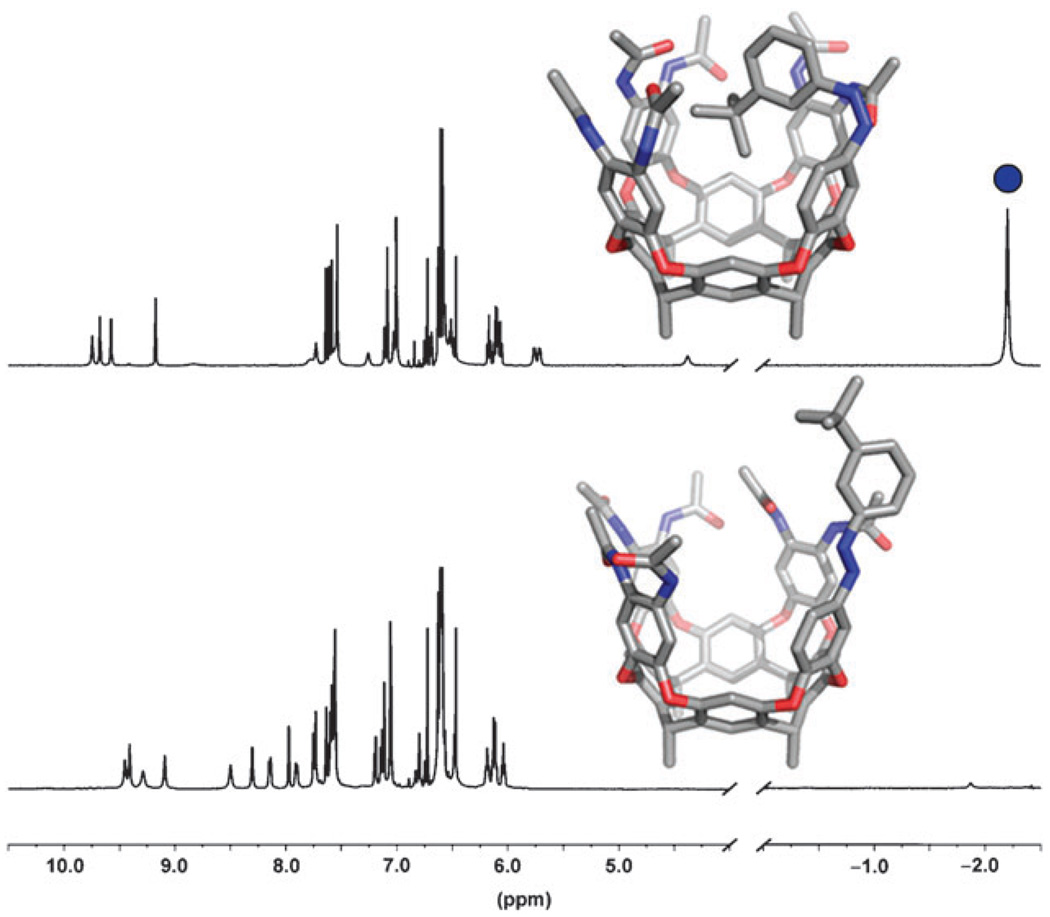
This isomerization can be monitored by 1H NMR. Exposing trans-1 (Fig. 1, bottom) to 365 nm light for 15 minutes converts it to the self-contained cis-1 (Fig. 1, top); the shielded magnetic environment of the cavitand’s interior causes the characteristic upfield shift of the tert-butyl group to −2.2 ppm (see ESI† for NOE analysis). Cis-1 and cis-2 revert to their trans configurations by heating to 164 °C for 5 minutes or irradiating with 450+ nm light for 20 minutes. This switching cycle can be repeated 5 times without detectable degradation of the system.
Fig. 1.
1H NMR spectra in d12-mesitylene showing trans-1 (bottom) and self-contained cis-1 (top). The inverted tert-butyl signal is marked by a blue circle.
Cavitands 1 and 2 feature a cavity defined by 8 aromatic panels. This concave surface is suited to complement neutral or cationic small molecules of appropriate shape and size. Adamantane derivatives, for example, are taken up by trans-1 in d12-mesitylene with binding constants ranging from 5 to >600 M−1 (Table 1). The exchange rates of these guests in and out of the cavity are slow on the NMR time scale and separate (upfield) signals are seen for the bound adamantane guests. The largest association constants are observed for adamantanes 7 and 8 which are stabilized by hydrogen bonding and polar interactions with the upper rim of the cavitand. Cavitand 2 binds the same adamantane guests with similar trends in affinity and magnitude as those observed for 1 with association constants ranging from 6 to >700 M−1.
Table 1.
| Guests | 1 | 2Ka/M−1 |
|---|---|---|
| 1-Adamantanemethanol (4) | 5 | 6 |
| 1-Chloroadamantane (5) | 37 | 45 |
| 1-Adamantaneamine (6) | 166 | 145 |
| 1-Adamantanecarbonitrile (7) | 311 | 226 |
| 2-Adamantanone (8) | 653 | 703 |
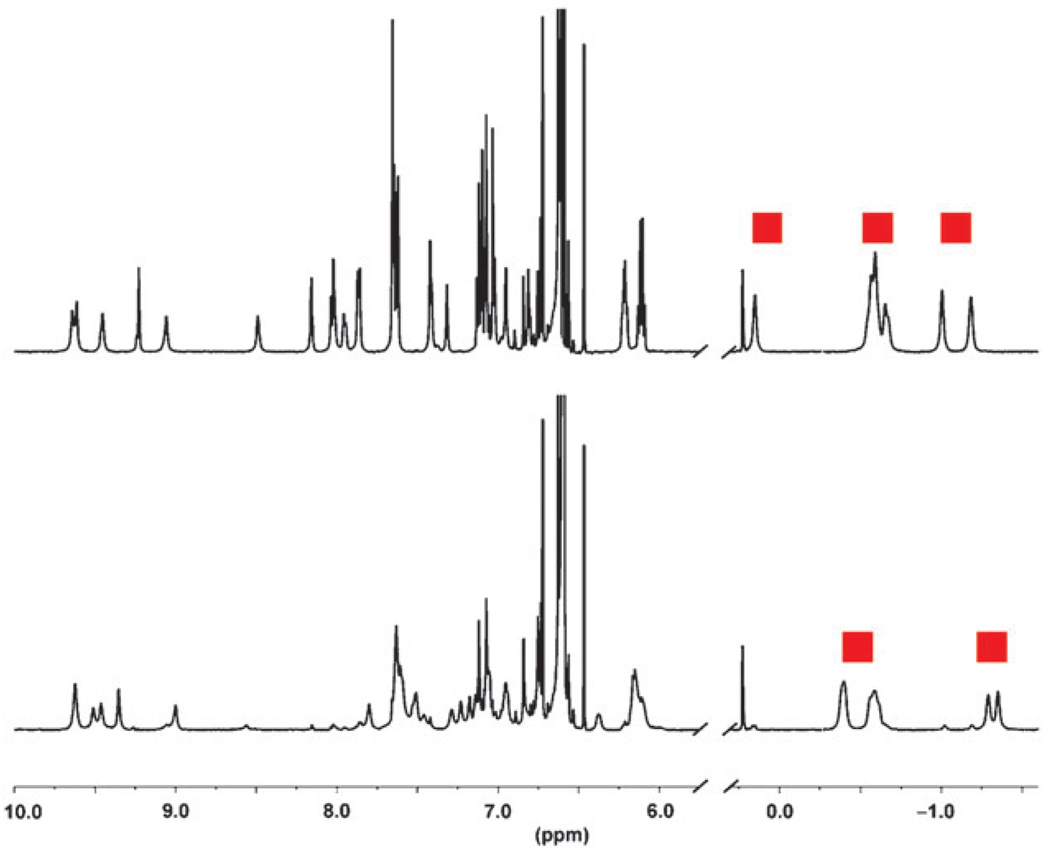
The effect of UV radiation on guest binding was different for the two types of cavitands. When cavitand 2 adamantane complexes were subjected to UV light, there was no change in the concentrations of bound guest.15 Only the magnetic environment of the guest was altered. Fig. 2 shows the relevant spectra of cavitand 2 containing 2-adamantanone (8) in both the trans (top) and cis (bottom) configuration. The broadened 1H NMR spectrum observed for cis-2 is due to the multiple cis conformations that cavitand 2 adopts when a guest molecule is present. The rate of the switching process in the presence of guest molecules is slightly retarded and can be achieved by heating or application of light (ESI†). However, bound adamantane signals are present in either cavitand configuration confirming that light cannot be used to control guest uptake and release in 2.
Fig. 2.
1H NMR spectra in d12-mesitylene showing cavitand 2 binding 2-adamantanone (8, 9.3 eq.) both in the trans configuration (top) and cis configuration (bottom). The guest remains bound when cavitand 2 is in either configuration and the bound guest signals are marked with red squares.
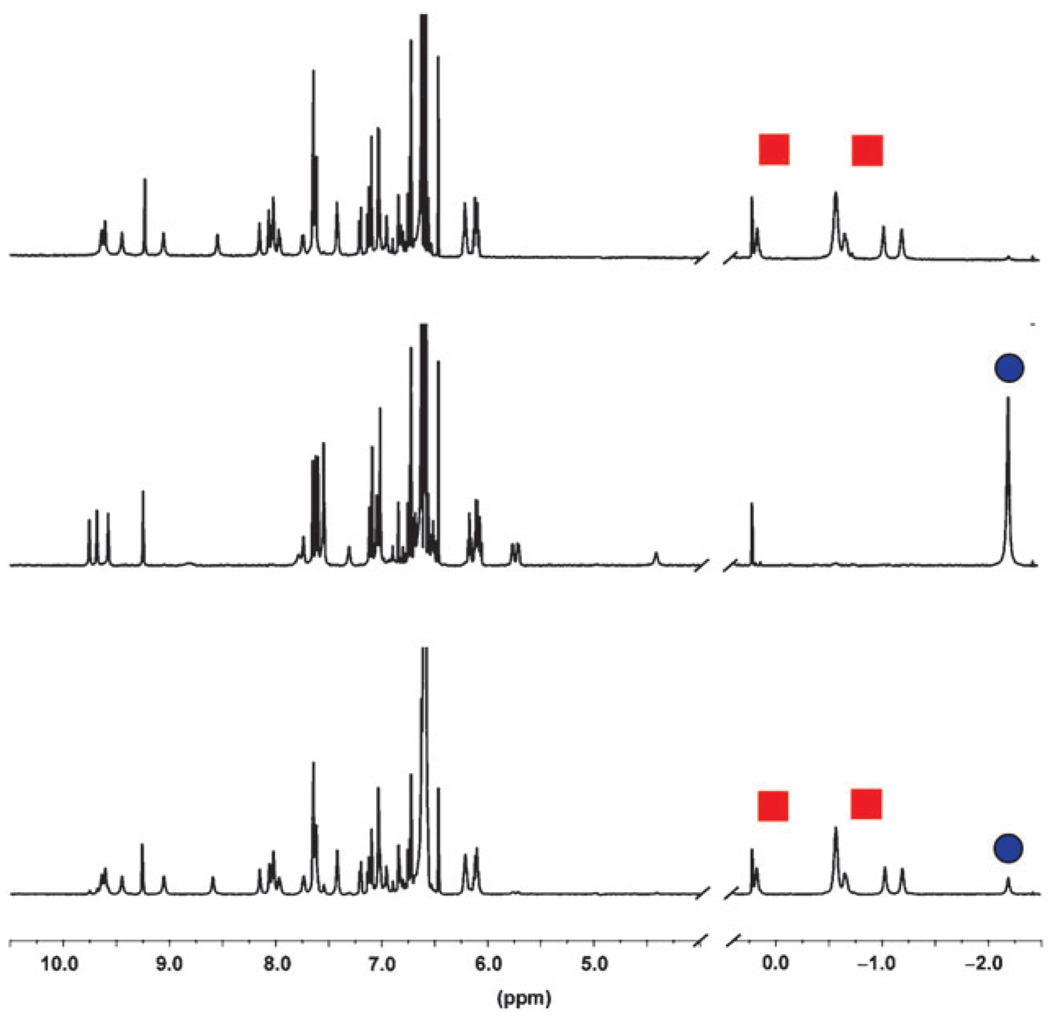
In contrast, light does control the uptake and release of adamantane guests in cavitand 1. Exposing its host/guest complexes to UV light for 15 minutes forces out the resident guests and replaces them with the inwardly-directed tert-butyl group. For instance, the bound guest 2-adamantanone (8) exhibits 5 upfield signals (Fig. 3, red squares, bottom); after UV irradiation, these signals disappear as 2-adamantanone is released into solution (Fig. 3 middle). The process is reversed by heating the system to 164 °C for 5 minutes (Fig. 3, top) or by application of visible light for 20 minutes where it reaches a photostationary state of 71% trans-1. The process was cycled 5 times without noticeable degradation. Light can be used to completely control the uptake and release of all adamantane guests (4–8) in cavitand 1.
Fig. 3.
Select regions of the 1H NMR spectra in d12-mesitylene illustrate how UV light affects guest binding in 1. Complex with 8 (7.8 eq.) before UV irradiation (bottom), after UV irradiation (middle) and after heating to 164 °C for 5 minutes (top). Signals for bound 2-adamantanone (8) are marked by red squares; the introverted tert-butyl is marked by a circle.
The well-defined molecular environments of cavitands have afforded numerous applications.16 Although the conformations of these molecules can be manipulated by temperature,17 pH18 or metal ions,19 there are few methods that reversibly control guest binding. Diederich and co-workers used acid/base chemistry to control the uptake and release of cycloalkanes,20 and we have used metal ions to manipulate a self-included “ouroborand” cavitand.21 These systems function well, but less invasive switching processes are also desirable.22 The two new cavitands presented here respond to light and heat by changing their conformations, but only the tert-butyl substituted 1 forces guests out upon irradiation. The process is reversible and can be cycled numerous times. The control of guest binding underscores the subtle influence of appropriate filling of space in recognition processes.23
Supplementary Material
Acknowledgments
We are grateful to the Skaggs Institute and the NIGMS (GM 27953) for financial support. A postdoctoral fellowship for O.B.B. was provided by NIH (F32GM087068). A.C.S. is a Skaggs Pre-doctoral Fellow.
Footnotes
Electronic supplementary information (ESI) available: Characterization details, equilibrium studies, computations and kinetics of photoisomerization. See DOI: 10.1039/c0cc03865b
Notes and references
- 1.(a) Kay ER, Leigh DA, Zerbetto F. Angew. Chem., Int. Ed. 2007;46:72–191. doi: 10.1002/anie.200504313. [DOI] [PubMed] [Google Scholar]; (b) Balzani V, Credi A, Venturi M, editors. Molecular Devices and Machines—A Journey into the Nanoworld. Wiley-VCH; 2003. pp. 288–328. [Google Scholar]; (c) Molecular Machines Special Issue. Acc. Chem. Res. 2001;34:409–522. [Google Scholar]
- 2.Asakawa M, Ashton PR, Balzani V, Credi A, Hamers C, Mattersteig G, Montalti M, Shipway AN, Spencer N, Stoddart JF, Tolley MS, Venturi M, White AJP, Williams DJ. Angew. Chem., Int. Ed. 1998;37:333–337. doi: 10.1002/(SICI)1521-3773(19980216)37:3<333::AID-ANIE333>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Bissell RA, Cordova E, Kaifer AE, Stoddart JF. Nature. 1994;369:133–137. [Google Scholar]
- 4.(a) Leigh DA, Lusby PJ, Slawin AMZ, Walker DB. Angew. Chem., Int. Ed. 2005;44:4557–4564. doi: 10.1002/anie.200500004. [DOI] [PubMed] [Google Scholar]; (b) Armaroli N, Balzani V, Collin J-P, Gaviza P, Sauvage J-P, Ventura B. J. Am. Chem. Soc. 1999;121:4397–4408. [Google Scholar]; (c) Vignon SA, Jarrosson T, Iijima T, Tseng H-R, Sanders JKM, Stoddart JF. J. Am. Chem. Soc. 2004;126:9884–9885. doi: 10.1021/ja048080k. [DOI] [PubMed] [Google Scholar]
- 5.(a) Fletcher SP, Dumur F, Pollard MM, Feringa BL. Science. 2005;310:80–82. doi: 10.1126/science.1117090. [DOI] [PubMed] [Google Scholar]; (b) Hernandez JV, Kay ER, Leigh DA. Science. 2004;306:1532–1537. doi: 10.1126/science.1103949. [DOI] [PubMed] [Google Scholar]
- 6. Ueno A, Yoshimura H, Saka R, Osa T. J. Am. Chem. Soc. 1979;101:2779–2780. Fukushima M, Osa T, Ueno A. J. Chem. Soc., Chem. Commun. 1991:15–17. Wang Y, Ma N, Wang Z, Zhang X. Angew. Chem., Int. Ed. 2007;46:2823–2826. doi: 10.1002/anie.200604982. (d) For a review see: Luboch E, Poleska-Muchlado Z, Jamrogiewicz M, Biernat J. In: Macrocyclic Chemistry Current Trends and Future Perspectives. Gloe K, editor. Dordrecht: Springer; 2005. pp. 203–218.
- 7.(a) Shinkai S, Nakaji T, Nishida Y, Ogawa T, Manabe O. J. Am. Chem. Soc. 1980;102:5860–5865. [Google Scholar]; (b) Shinkai S, Ogawa T, Nakaji T, Kusano Y, Manabe O. Tetrahedron Lett. 1979;20:4569–4572. [Google Scholar]; (c) Shinkai S, Nakaji T, Ogawa T, Shigematsu K, Manabe O. J. Am. Chem. Soc. 1981;103:111–115. [Google Scholar]
- 8.Hua Y, Flood AH. J. Am. Chem. Soc. 2010;132:12838–12840. doi: 10.1021/ja105793c. [DOI] [PubMed] [Google Scholar]
- 9.(a) Banghart MR, Mourot A, Fortin DL, Yao JZ, Kramer RH, Trauner D. Angew. Chem., Int. Ed. 2009;48:9097–9101. doi: 10.1002/anie.200904504. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Nat. Chem. Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Two examples of azo-arenes attached to resorcin[4]arenes have been reported. However, these molecules are not deep cavitands and their guest binding function was not investigated, see: Husaru L, Gruner M, Wolff T, Habicher WD, Salzer R. Tetrahedron Lett. 2005;46:3377–3379. Jain VK, Kanaiya PH. J. Inclusion Phenom. Macrocyclic Chem. 2008;62:111–115.
- 11. Lledo A, Rebek J., Jr Chem. Commun. 2010;46:1637–1639. doi: 10.1039/b927031k. For other uniquely functionalized cavitands see: Brody MS, Schalley CA, Rudkevich DM, Rebek J., Jr Angew. Chem., Int. Ed. 1999;38:1640–1644. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1640::AID-ANIE1640>3.0.CO;2-Y.
- 12.The synthesis of 3-tert-butyl nitrosobenzene is adapted from the literature procedure: Defoin A. Synthesis. 2004:706–710. Experimental details can be found in the ESI†.
- 13.The photostationary state is dependent on the conditions employed. For instance, after heating in the dark, nearly all of 1 persists in the trans conformation for extended periods of time (Fig. 1, bottom). Under ambient conditions a small amount of cis-1 is present. The quartz metal halide light employed in later experiments produces even more cis-1 at that photostationary state.
- 14.For computational analysis see: Crecca CR, Roitberg AE. J. Phys. Chem. A. 2000;110:8188–8203. doi: 10.1021/jp057413c. Chen PC, Chieh YC. J. Mol. Struct. (Theochem) 2003;624:191–200. For a structural study see: Mostad A, Romming C. Acta Crystallogr., Sect. C. 1983;39:1121.
- 15.Only in experiments where a minimal amount of the weakest guests are present did we observe a slight decrease in the amount of bound guest.
- 16.(a) Ichimura K, Oh SK, Nakagawa M. Science. 2000;288:1624–1626. doi: 10.1126/science.288.5471.1624. [DOI] [PubMed] [Google Scholar]; (b) Hooley RJ, Biros SM, Rebek J., Jr Angew. Chem., Int. Ed. 2006;45:3517–3519. doi: 10.1002/anie.200600405. [DOI] [PubMed] [Google Scholar]; (c) Oh SK, Nakagawa M, Ichimura K. J. Mater. Chem. 2002;12:2262–2269. [Google Scholar]; (d) Schafer C, Eckel R, Ros R, Mattay J, Anselmetti D. J. Am. Chem. Soc. 2007;129:1488–1489. doi: 10.1021/ja067734h. [DOI] [PubMed] [Google Scholar]; (e) Azov VA, Schlegel A, Diederich F. Angew. Chem., Int. Ed. 2005;44:4635–4638. doi: 10.1002/anie.200500970. [DOI] [PubMed] [Google Scholar]; (f) Butterfield SM, Rebek J., Jr J. Am. Chem. Soc. 2006;128:15366–15367. doi: 10.1021/ja0663374. [DOI] [PubMed] [Google Scholar]; (g) Iwasawa T, Rebek J., Jr Science. 2007;317:493–496. doi: 10.1126/science.1143272. [DOI] [PubMed] [Google Scholar]
- 17.(a) Moran JR, Karbach S, Cram DJ. J. Am. Chem. Soc. 1982;104:5826–5828. [Google Scholar]; (b) Cram DJ, Cram JM. Container Molecules and Their Guests. Cambridge: Royal Society of Chemistry; 1994. [Google Scholar]
- 18.Skinner PJ, Cheetham AG, Beeby A, Gramlich V, Diederich F. Helv. Chim. Acta. 2001;84:2146–2153. [Google Scholar]
- 19.Azov VA, Beeby A, Cacciarini M, Cheetham AG, Diederich F, Frei M, Gimzewski JK, Gramlich V, Hecht B, Jaun B, Latychevskaia T, Lieb A, Lill Y, Marotti F, Schlegel A, Schlittler RR, Skinner PJ, Seiler P, Yamakoshi Y. Adv. Funct. Mater. 2006;16:147–156. [Google Scholar]
- 20.Gottschalk T, Jaun B, Diederich F. Angew. Chem., Int. Ed. 2007;46:260–264. doi: 10.1002/anie.200603366. [DOI] [PubMed] [Google Scholar]
- 21.Durola F, Rebek J., Jr Angew. Chem., Int. Ed. 2010;49:3189–3191. doi: 10.1002/anie.200906753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letzel MC, Schäfer C, Novara FR, Speranza M, Rozhenko AB, Schoeller WW, Mattay J. J. Mass Spectrom. 2008;43:1553–1564. doi: 10.1002/jms.1464. [DOI] [PubMed] [Google Scholar]
- 23.(a) Mecozzi S, Rebek J., Jr Chem.–Eur. J. 1998;4:1016–1022. [Google Scholar]; (b) Shivanyuk A, Rebek J., Jr J. Am. Chem. Soc. 2003;125:3432–3433. doi: 10.1021/ja027982n. [DOI] [PubMed] [Google Scholar]; (c) Hof F, Nuckolls C, Rebek J., Jr J. Am. Chem. Soc. 2000;122:4251–4252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.