Abstract
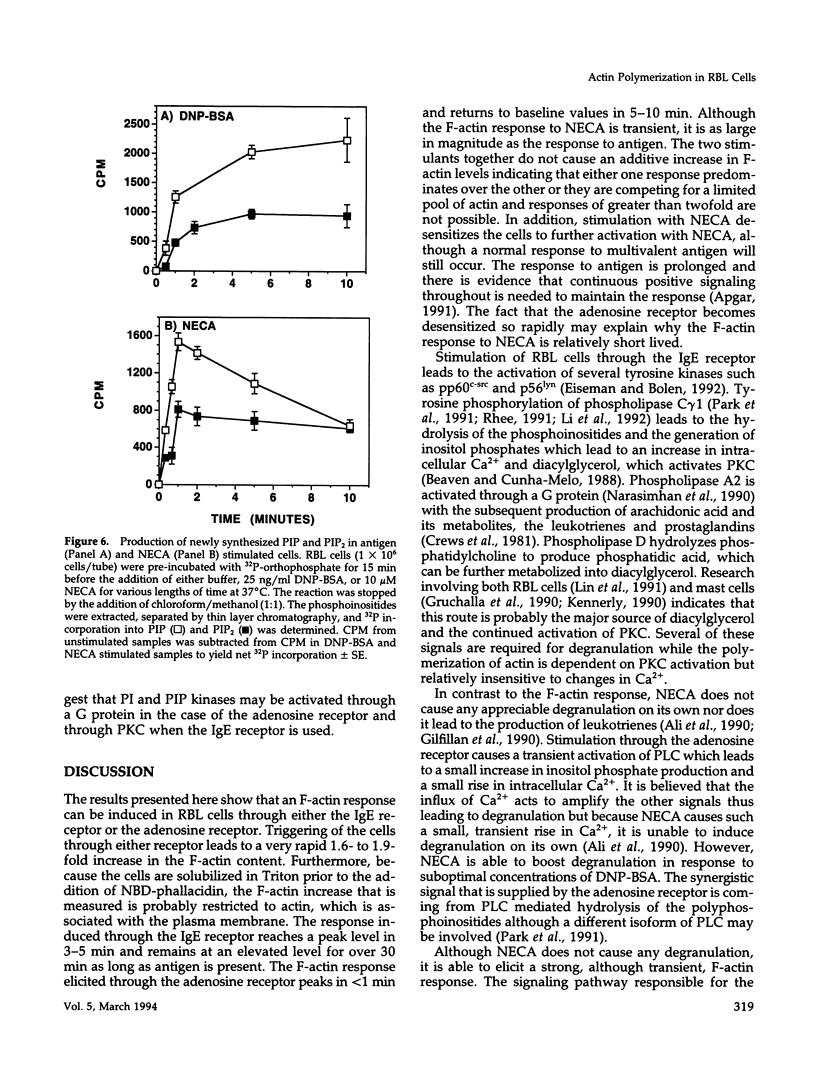
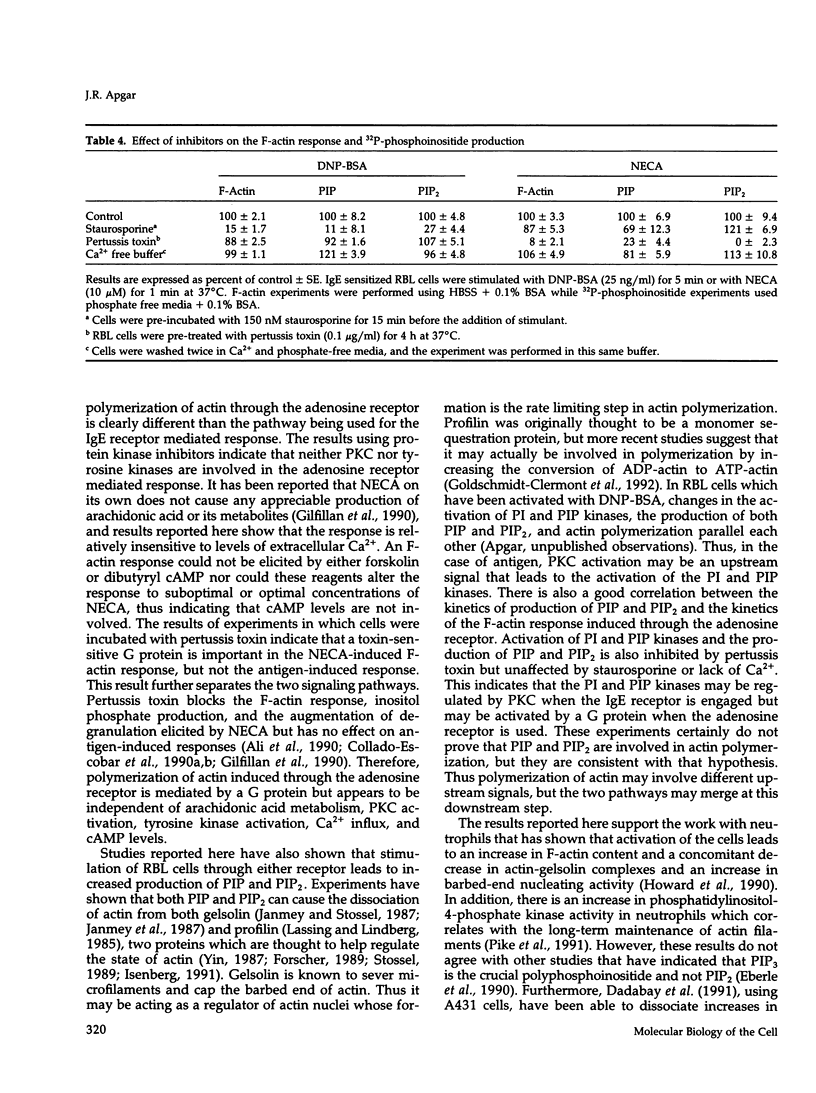
Crosslinking of the IgE receptor on rat basophilic leukemia (RBL) cells using the multivalent antigen DNP-BSA leads to a rapid and sustained increase in the filamentous actin content of the cells. Stimulation of RBL cells through the adenosine receptor also induces a very rapid polymerization of actin, which peaks in 45-60 s and is equivalent in magnitude to the F-actin response elicited through stimulation of the IgE receptor. However, in contrast to the IgE mediated response, which remains elevated for over 30 min, the F-actin increase induced by the adenosine analogue 5'-(N-ethylcarboxamido)-adenosine (NECA) is relatively transient and returns to baseline values within 5-10 min. While previous work has shown that the polymerization of actin in RBL cells stimulated through the IgE receptor is mediated by protein kinase C (PKC), protein kinase inhibitors have no effect on the F-actin response activated through the adenosine receptor. In contrast, pretreatment of the cells with pertussis toxin completely inhibits the F-actin response to NECA but has relatively little effect on the response induced through the IgE receptor. Stimulation of RBL cells through either receptor causes increased production of phosphatidylinositol mono-phosphate (PIP) and phosphatidylinositol bis-phosphate (PIP2), which correlates with the F-actin response. Production of PIP and PIP2 may be important downstream signals since these polyphosphoinositides are able to regulate the interaction of gelsolin and profilin with actin. Thus the polymerization of actin can be triggered through either the adenosine receptor or the IgE receptor, but different upstream signaling pathways are being used. The IgE mediated response requires the activation of PKC while stimulation through the adenosine receptor is PKC independent but involves a G protein.
Full text
PDF

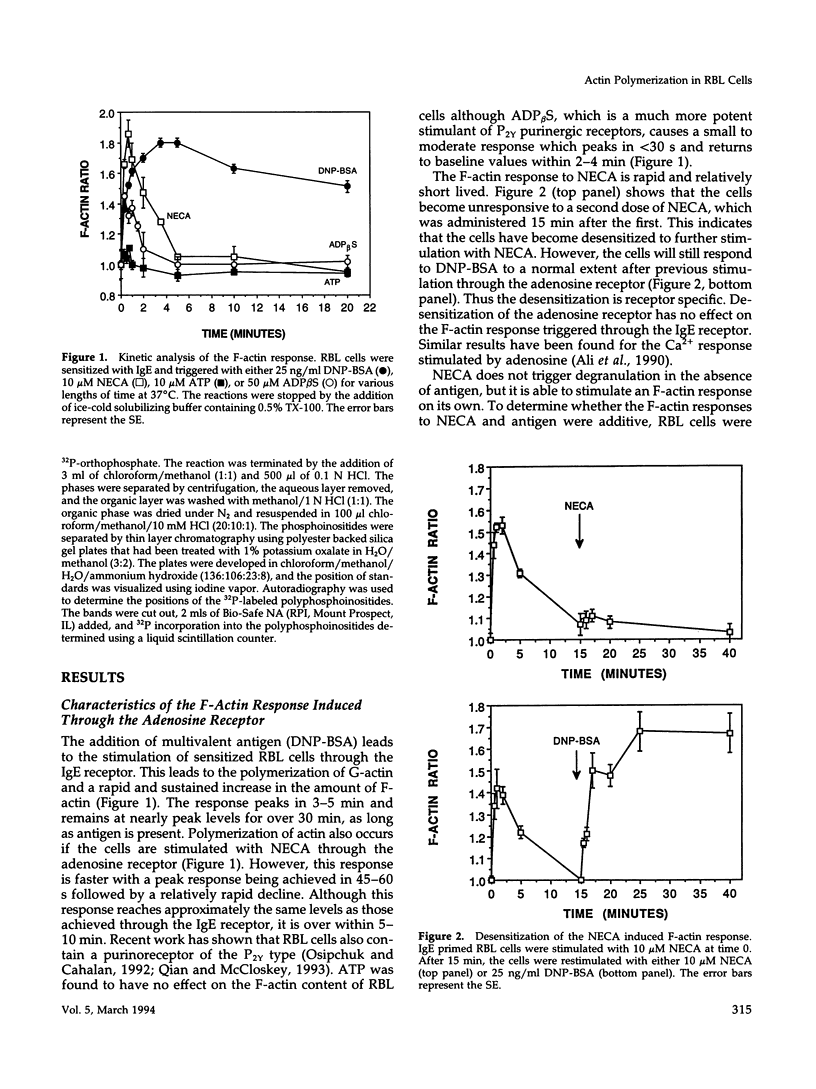


Selected References
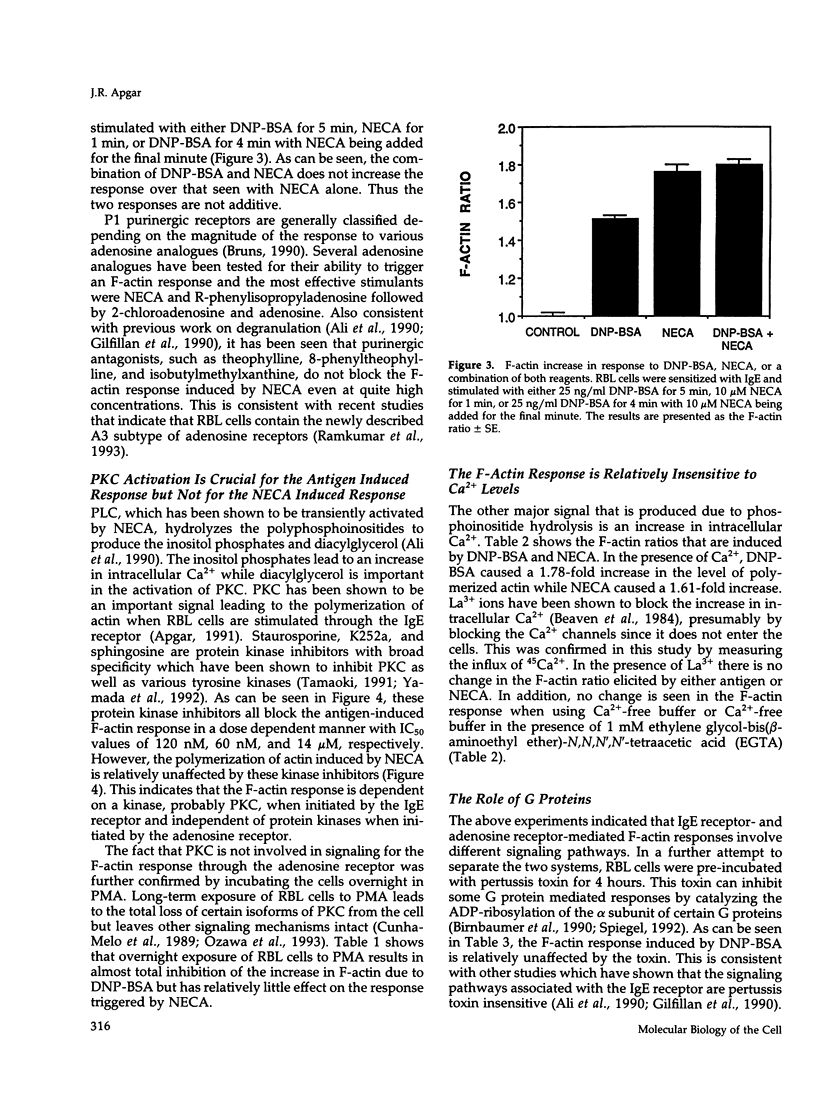
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Apgar J. R. Regulation of the antigen-induced F-actin response in rat basophilic leukemia cells by protein kinase C. J Cell Biol. 1991 Mar;112(6):1157–1163. doi: 10.1083/jcb.112.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
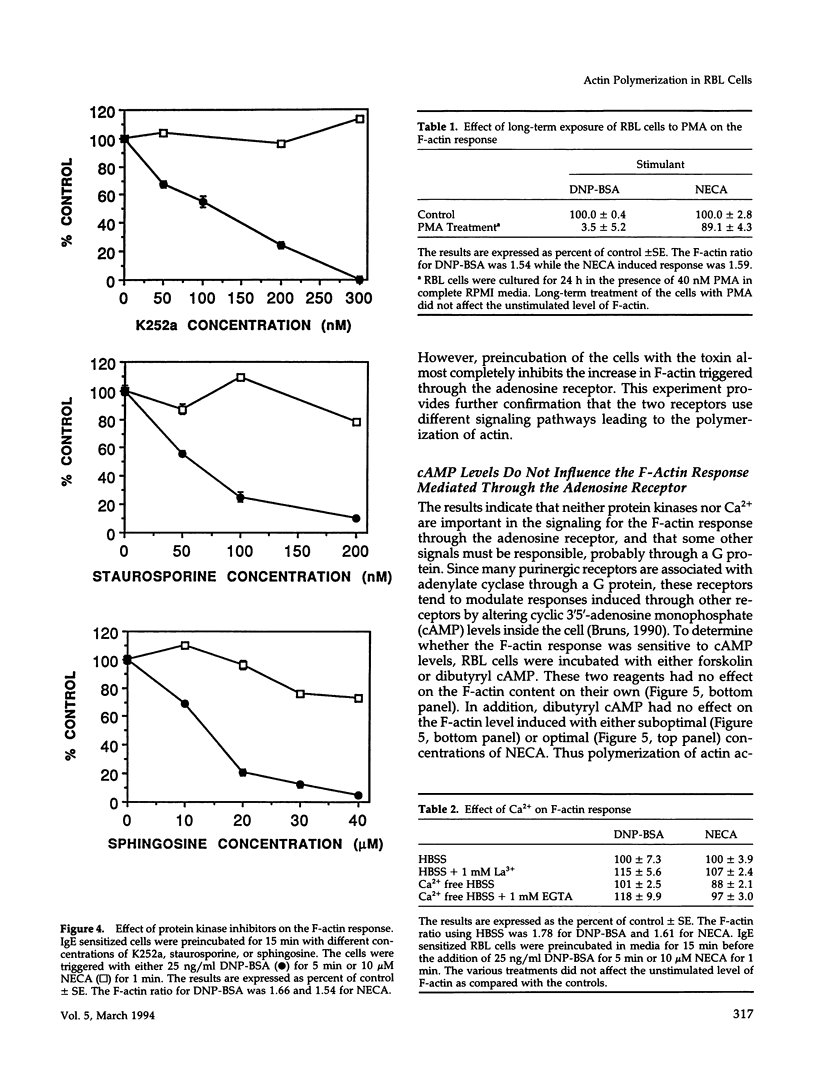
- Beaven M. A., Cunha-Melo J. R. Membrane phosphoinositide-activated signals in mast cells and basophils. Prog Allergy. 1988;42:123–184. [PubMed] [Google Scholar]
- Beaven M. A., Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993 May;14(5):222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7137–7142. [PubMed] [Google Scholar]
- Benhamou M., Siraganian R. P. Protein-tyrosine phosphorylation: an essential component of Fc epsilon RI signaling. Immunol Today. 1992 Jun;13(6):195–197. doi: 10.1016/0167-5699(92)90152-w. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Zigmond S. H., Schreiber A. D., Smith E. R., Southwick F. S. Binding of IgG containing immune complexes to human neutrophil Fc gamma RII and Fc gamma RIII induces actin polymerization by a pertussis toxin-insensitive transduction pathway. J Immunol. 1991 Jun 15;146(12):4282–4288. [PubMed] [Google Scholar]
- Bruns R. F. Adenosine receptors. Roles and pharmacology. Ann N Y Acad Sci. 1990;603:211–226. doi: 10.1111/j.1749-6632.1990.tb37674.x. [DOI] [PubMed] [Google Scholar]
- Collado-Escobar D., Ali H., Beaven M. A. On the mechanism of action of dexamethasone in a rat mast cell line (RBL-2H3 cells). Evidence for altered coupling of receptors and G-proteins. J Immunol. 1990 May 1;144(9):3449–3457. [PubMed] [Google Scholar]
- Collado-Escobar D., Cunha-Melo J. R., Beaven M. A. Treatment with dexamethasone down-regulates IgE-receptor-mediated signals and up-regulates adenosine-receptor-mediated signals in a rat mast cell (RBL-2H3) line. J Immunol. 1990 Jan 1;144(1):244–250. [PubMed] [Google Scholar]
- Crews F. T., Morita Y., McGivney A., Hirata F., Siraganian R. P., Axelrod J. IgE-mediated histamine release in rat basophilic leukemia cells: receptor activation, phospholipid methylation, Ca2+ flux, and release of arachidonic acid. Arch Biochem Biophys. 1981 Dec;212(2):561–571. doi: 10.1016/0003-9861(81)90399-4. [DOI] [PubMed] [Google Scholar]
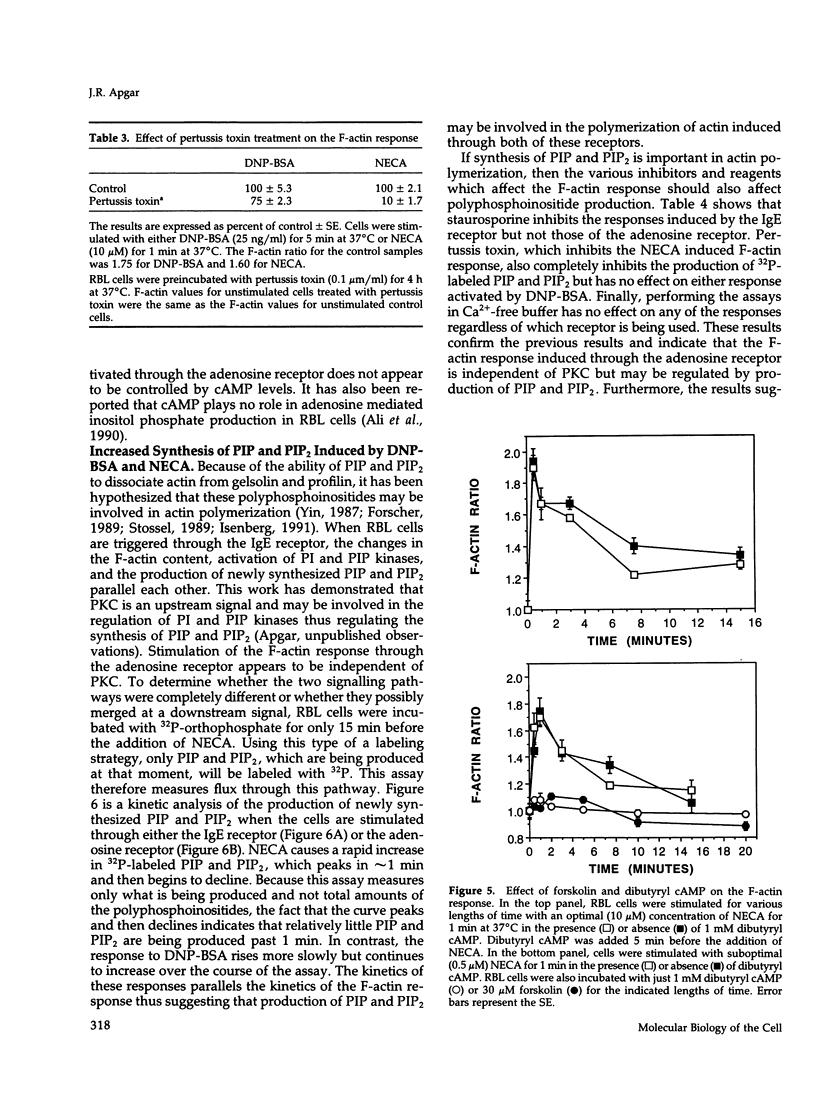
- Cunha-Melo J. R., Gonzaga H. M., Ali H., Huang F. L., Huang K. P., Beaven M. A. Studies of protein kinase C in the rat basophilic leukemia (RBL-2H3) cell reveal that antigen-induced signals are not mimicked by the actions of phorbol myristate acetate and Ca2+ ionophore. J Immunol. 1989 Oct 15;143(8):2617–2625. [PubMed] [Google Scholar]
- Dadabay C. Y., Patton E., Cooper J. A., Pike L. J. Lack of correlation between changes in polyphosphoinositide levels and actin/gelsolin complexes in A431 cells treated with epidermal growth factor. J Cell Biol. 1991 Mar;112(6):1151–1156. doi: 10.1083/jcb.112.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G. P., Chan C. K., Lea P., Takai A., Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J Cell Biol. 1992 Feb;116(3):695–706. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle M., Traynor-Kaplan A. E., Sklar L. A., Norgauer J. Is there a relationship between phosphatidylinositol trisphosphate and F-actin polymerization in human neutrophils? J Biol Chem. 1990 Oct 5;265(28):16725–16728. [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Forscher P. Calcium and polyphosphoinositide control of cytoskeletal dynamics. Trends Neurosci. 1989 Nov;12(11):468–474. doi: 10.1016/0166-2236(89)90098-2. [DOI] [PubMed] [Google Scholar]
- Geiger B. Membrane-cytoskeleton interaction. Biochim Biophys Acta. 1983 Aug 11;737(3-4):305–341. doi: 10.1016/0304-4157(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Gilfillan A. M., Wiggan G. A., Welton A. F. Pertussis toxin pretreatment reveals differential effects of adenosine analogs on IgE-dependent histamine and peptidoleukotriene release from RBL-2H3 cells. Biochim Biophys Acta. 1990 May 22;1052(3):467–474. doi: 10.1016/0167-4889(90)90157-9. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Furman M. I., Wachsstock D., Safer D., Nachmias V. T., Pollard T. D. The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 1992 Sep;3(9):1015–1024. doi: 10.1091/mbc.3.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruchalla R. S., Dinh T. T., Kennerly D. A. An indirect pathway of receptor-mediated 1,2-diacylglycerol formation in mast cells. I. IgE receptor-mediated activation of phospholipase D. J Immunol. 1990 Mar 15;144(6):2334–2342. [PubMed] [Google Scholar]
- Hartwig J. H., Kwiatkowski D. J. Actin-binding proteins. Curr Opin Cell Biol. 1991 Feb;3(1):87–97. doi: 10.1016/0955-0674(91)90170-4. [DOI] [PubMed] [Google Scholar]
- Howard T. H., Oresajo C. O. A method for quantifying F-actin in chemotactic peptide activated neutrophils: study of the effect of tBOC peptide. Cell Motil. 1985;5(6):545–557. doi: 10.1002/cm.970050609. [DOI] [PubMed] [Google Scholar]
- Howard T. H., Wang D. Calcium ionophore, phorbol ester, and chemotactic peptide-induced cytoskeleton reorganization in human neutrophils. J Clin Invest. 1987 May;79(5):1359–1364. doi: 10.1172/JCI112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T., Chaponnier C., Yin H., Stossel T. Gelsolin-actin interaction and actin polymerization in human neutrophils. J Cell Biol. 1990 Jun;110(6):1983–1991. doi: 10.1083/jcb.110.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Actin binding proteins--lipid interactions. J Muscle Res Cell Motil. 1991 Apr;12(2):136–144. doi: 10.1007/BF01774032. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Plasmalemmal undercoat: the cytoskeleton supporting the plasmalemma. Arch Histol Cytol. 1988 May;51(2):127–145. doi: 10.1679/aohc.51.127. [DOI] [PubMed] [Google Scholar]
- Jacobson B. S. Interaction of the plasma membrane with the cytoskeleton: an overview. Tissue Cell. 1983;15(6):829–852. doi: 10.1016/0040-8166(83)90053-8. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Iida K., Yin H. L., Stossel T. P. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem. 1987 Sep 5;262(25):12228–12236. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Kennerly D. A. Phosphatidylcholine is a quantitatively more important source of increased 1,2-diacylglycerol than is phosphatidylinositol in mast cells. J Immunol. 1990 May 15;144(10):3912–3919. [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Li W., Deanin G. G., Margolis B., Schlessinger J., Oliver J. M. Fc epsilon R1-mediated tyrosine phosphorylation of multiple proteins, including phospholipase C gamma 1 and the receptor beta gamma 2 complex, in RBL-2H3 rat basophilic leukemia cells. Mol Cell Biol. 1992 Jul;12(7):3176–3182. doi: 10.1128/mcb.12.7.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. Y., Wiggan G. A., Gilfillan A. M. Activation of phospholipase D in a rat mast (RBL 2H3) cell line. A possible unifying mechanism for IgE-dependent degranulation and arachidonic acid metabolite release. J Immunol. 1991 Mar 1;146(5):1609–1616. [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992 Feb;125:37–48. doi: 10.1111/j.1600-065x.1992.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Narasimhan V., Holowka D., Baird B. A guanine nucleotide-binding protein participates in IgE receptor-mediated activation of endogenous and reconstituted phospholipase A2 in a permeabilized cell system. J Biol Chem. 1990 Jan 25;265(3):1459–1464. [PubMed] [Google Scholar]
- Oliver J. M., Berlin R. D. Mechanisms that regulate the structural and functional architecture of cell surfaces. Int Rev Cytol. 1982;74:55–94. doi: 10.1016/s0074-7696(08)61169-9. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Seagrave J., Stump R. F., Pfeiffer J. R., Deanin G. G. Signal transduction and cellular response in RBL-2H3 mast cells. Prog Allergy. 1988;42:185–245. [PubMed] [Google Scholar]
- Osipchuk Y., Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992 Sep 17;359(6392):241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Szallasi Z., Kazanietz M. G., Blumberg P. M., Mischak H., Mushinski J. F., Beaven M. A. Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993 Jan 25;268(3):1749–1756. [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. IgE-induced tyrosine phosphorylation of phospholipase C-gamma 1 in rat basophilic leukemia cells. J Biol Chem. 1991 Dec 25;266(36):24237–24240. [PubMed] [Google Scholar]
- Pfeiffer J. R., Seagrave J. C., Davis B. H., Deanin G. G., Oliver J. M. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985 Dec;101(6):2145–2155. doi: 10.1083/jcb.101.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Costello K., Southwick F. S. Stimulation of human polymorphonuclear leukocyte phosphatidylinositol-4-phosphate kinase by concanavalin A and formyl-methionyl-leucyl-phenylalanine is calcium-independent. Correlation with maintenance of actin assembly. J Immunol. 1991 Oct 1;147(7):2270–2275. [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Qian Y. X., McCloskey M. A. Activation of mast cell K+ channels through multiple G protein-linked receptors. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7844–7848. doi: 10.1073/pnas.90.16.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L., Beaven M. A., Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993 Aug 15;268(23):16887–16890. [PubMed] [Google Scholar]
- Rhee S. G. Inositol phospholipids-specific phospholipase C: interaction of the gamma 1 isoform with tyrosine kinase. Trends Biochem Sci. 1991 Aug;16(8):297–301. doi: 10.1016/0968-0004(91)90122-c. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., Dabiri G. A., Paschetto M., Zigmond S. H. Polymorphonuclear leukocyte adherence induces actin polymerization by a transduction pathway which differs from that used by chemoattractants. J Cell Biol. 1989 Oct;109(4 Pt 1):1561–1569. doi: 10.1083/jcb.109.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A. M. G proteins in cellular control. Curr Opin Cell Biol. 1992 Apr;4(2):203–211. doi: 10.1016/0955-0674(92)90034-a. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Tamaoki T. Use and specificity of staurosporine, UCN-01, and calphostin C as protein kinase inhibitors. Methods Enzymol. 1991;201:340–347. doi: 10.1016/0076-6879(91)01030-6. [DOI] [PubMed] [Google Scholar]
- Weatherbee J. A. Membranes and cell movement: interactions of membranes with the proteins of the cytoskeleton. Int Rev Cytol Suppl. 1981;12:113–176. doi: 10.1016/b978-0-12-364373-5.50014-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Neufeld E. J., Majerus P. W. Phosphoinositide interconversion in thrombin-stimulated human platelets. J Biol Chem. 1985 Jan 25;260(2):1046–1051. [PubMed] [Google Scholar]
- Yamada K., Jelsema C. L., Beaven M. A. Certain inhibitors of protein serine/threonine kinases also inhibit tyrosine phosphorylation of phospholipase C gamma 1 and other proteins and reveal distinct roles for tyrosine kinase(s) and protein kinase C in stimulated, rat basophilic RBL-2H3 cells. J Immunol. 1992 Aug 1;149(3):1031–1037. [PubMed] [Google Scholar]
- Yin H. L. Gelsolin: calcium- and polyphosphoinositide-regulated actin-modulating protein. Bioessays. 1987 Oct;7(4):176–179. doi: 10.1002/bies.950070409. [DOI] [PubMed] [Google Scholar]


