Abstract
Background
The use of docetaxel prolongs survival for patients with castrate resistant prostate cancer (CRPC). Inhibition of vascular endothelial growth factor (VEGF) with bevacizumab may further enhance the anti-tumor effect of docetaxel and estramustine in patients with CRPC.
Patients and Methods
This cooperative group trial enrolled men with CRPC. Patients received oral estramustine 280 mg three times per day on days 1-5 of every cycle, with 70 mg/m2 of docetaxel and bevacizumab at 15 mg/kg on day 2, every three weeks. PSA values were monitored every cycle and imaging was performed every 3 cycles. The primary endpoint was progression free survival (PFS) with safety, prostate specific antigen decline, measurable disease response, and overall survival secondary objectives.
Results
Seventy-nine patients were enrolled; 77 received a median of 8 cycles and were evaluable. A 50% PSA decline was observed in 58 patients (75%). Twenty-three of 39 patients with measurable disease had a partial response (59%) The median time of PFS was 8.0 months with an overall median survival of 24 months. Neutropenia without fever (69%), fatigue (25%), thrombosis\emboli (9%) were the most common severe toxicities. Twenty-four of 77 patients were removed from protocol treatment due to disease progression, 35/77 for physician or patient decision and 15 patients secondary to toxicity.
Conclusion
The combination of docetaxel, estramustine and bevacizumab was tolerable but complicated by toxicity. Although progression free survival did not meet the desired endpoint, encouraging anti-tumor activity and overall survival was observed. Further phase III evaluation of the role of bevacizumab in CRPC is ongoing.
Keywords: Docetaxel, Bevacizumab, castrate resistant prostate cancer
INTRODUCTION
Metastatic prostate cancer is often treated by castration which provides temporary palliation of symptoms and control of tumor growth.1 In the past, treatment for patients with castrate resistant prostate cancer (CRPC) was focused on palliative therapy, but the introduction of docetaxel based therapies has led to survival improvement. The use of docetaxel has shown a survival advantage over mitoxantrone and has become the standard for patients with CRPC.2,3 The overall survival advantage of 2.9 months is modest and further therapies that improve this survival of patients treated with docetaxel based therapies are needed.4
Prior to the completion of the phase III trials of docetaxel versus mitoxantrone, the Cancer and Leukemia Group B (CALGB) developed a series of sequential studies to evaluate the addition of novel agents to the docetaxel backbone. The addition of carboplatin was tolerated but did increase the myelosuppression of the regimen without a clear signal of increased activity.5 Exisulind which was thought to induce apoptosis in malignant cells was also combined with docetaxel and estramustine.6 Significant morbidity was associated with this combination without a substantial improvement in the efficacy.
Vascular endothelial growth factor (VEGF) is a glycoprotein important in promoting tumor angiogenesis 7,8 and plays a critical role in the progression of human prostate cancer. Flk-1/KDR receptors are expressed in human prostate cancer which correlates with higher grade lesions and outcome.9 Expression of vascular endothelial growth factor (VEGF) is observed in prostate tumors as well as in the plasma and urine of patients with metastatic disease with increasing expression correlating with disease progression.10,11 Studies have shown that plasma and urine VEGF levels in CRPC patients are independent predictors of survival.12,13 Bevacizumab, a humanized murine monoclonal antibody that neutralizes VEGF activity was therefore felt to be a reasonable therapeutic approach for advanced prostate cancer. A single-center study administering bevacizumab 10 mg/kg every 2 weeks in 15 patients with CRPC was well tolerated. However, as expected with this class of agents, minimal evidence of activity were observed with monotherapy.14 Evolving data in other cancers suggested that the overall benefit of VEGF blockade is more apparent when bevacizumab is combined with a cytotoxic agent. Based on the compelling in vivo data, the importance of circulating VEGF levels in CRPC and safety of bevacizumab, a multi-institutional trial of docetaxel, estramustine and bevacizumab was conducted.
PATIENTS AND METHODS
Patient Selection
This study was approved by the Executive Committee of the CALGB, and by the Institutional Review Board of each participating site. All patients provided written, informed consent. All patients were required to have evidence of metastatic prostate cancer despite castrate levels of testosterone (≤ 50 ng/ml). Patients were required to have evidence of progressive metastatic disease with documented measurable disease progression on cross-sectional imaging, new lesions on bone scan, or two sequential rises in PSA with baseline PSA being greater than 5 ng/ml. Anti-androgens and megestrol acetate were required to be discontinued for at least 4 weeks prior to registration with evidence of progression noted after their discontinuation. All patients were required to continue luteinizing hormone releasing hormone (LHRH) agonist if they have not had an orchiectomy. Patients were not allowed to have received prior cytotoxic therapy or other anti-angiogenesis agents including thalidomide. Patients were required to be at least 4 weeks from major surgery or radiation therapy, and at least 8 weeks since radionuclide therapy. All patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; no significant peripheral neuropathy; and no prior myocardial infarction, deep venous thrombosis, pulmonary embolus, or other major thromboembolic event within 1 year of entry. Patients requiring full dose anti-coagulation were also excluded. Patients were required to have a granulocytes >1500/μl, platelets > 100,000/μl, creatinine < 1.5 times the upper limit of normal (ULN), bilirubin < ULN, AST < 1.5 times the ULN, and urinalysis ≤ 1+ protein on dipstick. Patients receiving stable bisphosphonate therapy for at least four weeks prior to entry were allowed to continue, but initiation of bisphosphonate therapy was not allowed.
Treatment and Evaluation
Therapy was based on 21 day cycles. Patients received 280 mg of estramustine phosphate (Emcyt, Pharmacia Oncology, Peapack, NJ) TID on days 1-5, decadron 8 mg BID on days 1-3, docetaxel (Taxotere, Sanofi-Aventis, Bridgewater, NJ) 70 mg/m2 on day 2 intravenously over one hour, and bevacizumab (supplied by Genentech, distributed by NCI) 15 mg/kg intravenously administered after docetaxel on day 2. The first infusion of bevacizumab was administered over 90 minutes, the second over 60 minutes, and the third and subsequent doses were administered over 30 minutes if well tolerated. The dose was 5 mg/kg/week consistent with most other malignancies, and given every three weeks coordinated with chemotherapy administration. Warfarin, 2 mg daily was encouraged but not mandated as prophylaxis for thrombosis from estramustine, unless a contraindication existed. Hematological growth factors were allowed per ASCO guidelines, but prophylactic use was not allowed.
Patients were evaluated every cycle with a PSA, and every 3rd cycle with bone scans and CT scans of the abdomen and pelvis. Patients were encouraged to have blood pressure monitored weekly, and weekly blood counts were required. Liver function tests, including bilirubin, transaminase levels and alkaline phosphatase, along with urinalysis were analyzed prior to administration of the chemotherapy every cycle.
The therapy was held for ANC<1500/μl, platelets <100,000/μl with dose reduction to 75% of the starting docetaxel dose upon recovery, or for any episode of febrile neutropenia. For elevations of SGOT >1.5 ULN, the dose of docetaxel was reduced to 60 mg/m2, and for bilirubin levels >1.5 ULN or SGOT >5 ULN, the docetaxel dose was held. When the docetaxel dose was held, the estramustine and bevacizumab doses were also held. For grade 2 neurotoxicity, the estramustine was reduced by 50% and held with grade 3 toxicity. For grade 3 neurotoxicity, the docetaxel was reduced to 75%. For persistant grade 3 neurotoxicity, the docetaxel was held. If chemotherapy was held >3 weeks, patients were removed from protocol treatment. For thromboembolic events, once anticoagulated, patients were allowed to continue on protocol with discontinuation of bevacizumab and estramustine. For proteinuria >2+ on dipstick, a 24-hour urine protein measurement was required, and if it showed >2000 mg/day of protein, bevacizumab was held.
Disease progression and response were based on the PSA working group Consensus Criteria15 and RECIST criteria were used for patients with measurable disease. Bone scan progression was defined as any new lesions.
Statistical Design and Data Analysis
The primary endpoint of this study was time to progression. Progression-free survival (PFS) was defined as the interval between treatment initiation and the date of progression or death, whichever occurred first. Sample size computation was based on the primary endpoint. The null hypothesis was that the median time to progression would be less than or equal to 11 months and the alternative hypothesis was that the median time to progression would be at least as great as 16 months (45% increase). The target sample size was 79 assuming an ineligibility rate of 10%. The normal approximation to the exact exponential test was used for sample size computation and the following assumptions were made: a) type I error rate=0.05, b) power=89% (type II error rate=11%), c) accrual rate of 4 patients/month over a 18-month accrual period), d) a 24-month follow-up period, and e) the time to progression follows an exponential distribution. Under the alternative hypothesis, 55 progression events were expected at the end of the trial.
Toxicity was an important secondary endpoint with a hypothesized null hypothesis that the acceptable toxicity probability was ≤0.80 versus the alternative hypothesis that acceptable toxicity probability was ≥0.90. Unacceptable toxicity was defined as death, grade 4 febrile neutropenia or any serious grade 3 or grade 4 toxicity, excluding nausea, vomiting, alopecia or hypersensitivity. For the purpose of this study, a “serious” toxicity was defined as cardiac, thrombosis/embolism, or CNS hemorrhage/bleeding. Toxicity was monitored using a three-stage design after 15, 35 and 72 patients were enrolled and received one full cycle of therapy. This design had a type I error rate of 0.10 and a power of 89% based on binomial simulations. The decision rules for continuation of accrual required at least 11 out of 15 patients, at least 27 out of 35 patients and at least 62 out of 72 did not experience unacceptable toxicity.
Other endpoints considered were PSA progression-free survival (PSA PFS), overall survival, duration of objective response and duration of PSA decline. PFS was defined as the interval between treatment initiation and the date of any disease progression (bone, PSA PFS, soft tissue), or clinical deterioration, or death, whichever occurred first. PSA PFS was defined as time to first biochemical progression using the PSA consensus criteria or death, whichever occurred first. Overall survival was defined as interval between treatment initiation and the date of death. Duration of response was defined as the date of the first CR or PR to the date that the patient had disease progression. Duration of PSA response was interval between date from the first 50% decline in PSA to the date when the patient met the criteria for disease progression. The 95% confidence interval for the objective response rate was computed based on the binomial distribution. Overall survival, objective PFS, PSA PFS and PFS distributions were estimated using the Kaplan-Meier product-limit method.16
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every three years to review source documents. The auditors verify compliance with federal regulations and protocol requirements in a sample of protocols at each institution. On-site review of medical records was done on a subgroup of 39 patients (49%) of the 79 patients under this study. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality assurance included careful review of data by CALGB Statistical Center staff and by the study chairperson. CALGB statisticians performed all statistical analyses.
RESULTS
Patient Characteristics
Seventy-nine patients were enrolled between October 2001 and November 2002; 77 patients were eligible and assessable for treatment outcome (one patient did not meet eligibility criteria and one patient was registered but never received therapy). The median age was 69, with a median PSA of 123 ng/ml (range 0.1-2231 ng/ml) (Table 1). The median time since diagnosis was 4 years, with an overall range of 0-17 years. Eighty-six percent of the patients had bone disease, with 19% having visceral involvement. Overall 51% of the patients had measurable disease as defined by RECIST.
Table 1.
Demographics
| Demographics | Range | |
|---|---|---|
| Median Age | 69 | 48-88 |
| Race | ||
| Caucasian | 84% | |
| Black | 9% | |
| Other or unknown | 7% | |
| Yrs since Diagnosis-Medians | 4 | 0-17 |
| Performance Status | ||
| 0-1 | 91% | |
| 2 | 7% | |
| Laboratory Values | ||
| Median Hemoglobin g/dl | 12.7 | 8.1-14.8 |
| Interquartile Range | 11.7-13.5 | |
| PSA | 123 | 0.1-2231 |
| Interquartile Range | 50-289 | |
| Alkaline Phosphatase IU/dl | 182 | 41-5250 |
| Interquartile Range | 98-361 | |
| Extent of Disease | ||
| Measurable Disease-Total | 51% | |
| Lymph Nodes | 41% | |
| Visceral disease | 19% | |
| Soft tissue disease | 18% | |
| Bone disease | 86% | |
Study Treatment
The median number of cycles of therapy administered was 8 (range 1-30). Of the 77 evaluable patients, 24 (31%) came off therapy due to disease progression, 15 (19%) stopped due to protocol defined toxicity, and 35 (45%) for physician or patient decision, but not required by protocol. Three (4%) patients died while on therapy. Patients that stopped therapy based on physician and patient decision (n=35), did not have protocol defined disease progression but opted for a “treatment” break. Patients after such a break were not allowed to resume protocol therapy, but could restart docetaxel based therapies with the agreement of their physicians. Data detailing subsequent treatments were not prospectively collected.
Dose reductions in one or more of the three drugs, in one or more cycles, occurred in 37/77 (48%) of the patients. The common reasons for reducing the dose of treatment included: hematologic toxicity (12%), GI toxicity (6%), CNS toxicity (5%) and other (27%) such as fatigue and depression. Some patients had more than one simultatneous requirement for dose reduction.
Clinical Outcomes
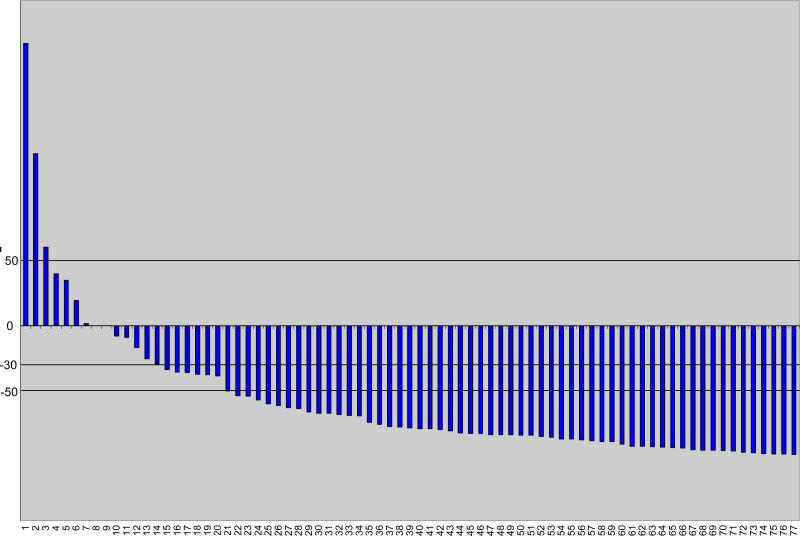
Fifty eight of 77 patients (75%, 95% confidence interval(CI) of 64%-84%) had a ≥50% post-therapy decline in PSA that was confirmed at least one month later. A waterfall plot of the PSA decline at 12 weeks after the start of therapy is shown in figure 1.
Figure 1.
Waterfall plot of PSA responses at 12 weeks after initiating thereapy
The median time to PSA response was 1.4 months. The median time to PSA progression was 9.2 months (95% CI=7.5-10.9 months). The median duration of PSA decline was 8.5 months (95% CI=6.1-9.8). Twenty three of 39 men with measurable disease (59%, 95% CI=42%-74%) has a partial response by RECIST criteria and the median duration of measurable disease response was 21.5 months (95%CI=14.6-26.7 months).
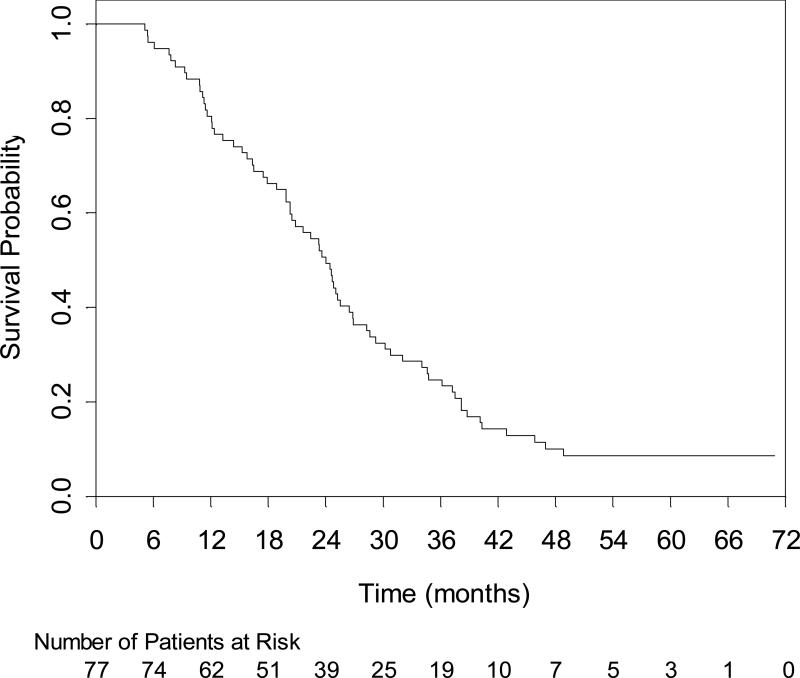
The median time to any progression for patients was 8.0 months (95% CI=5.9 to 9.5 months). Patients with measurable disease had a median time to measurable disease progression of 16.5 months (95% CI=10.9-23.2 months). 75 patients have died. Sixty eight of these patients (90.7%) have died of prostate cancer and the median follow-up time for the surviving patients is 69 months. The median overall survival time is 24 months (95% CI=20.3-26.5 months) (Figure 2).
Figure 2.
Survival curve of patients form date of enrollment
Adverse Events
Table 2 presents adverse events that are probable, possible and definitely related to treatment. Three patients died on study. One patient died of an infection without neutropenia, one patient had a mesenteric vein thrombosis, and one patient had a bowel perforation complicated by severe metabolic acidosis. Grade 3 or 4 leukopenia and neutropenia occurred in 53% and 62% of the patients, respectively, however febrile neutropenia only occurred in 3 patients (4%). Overall, severe (grade 3, 4 and 5) febrile neutropenia, infection with and without neutropenia were observed in 17 (22%) patients.
Table 2.
Adverse Events
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| N = 77 | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) |
| Leukocytes (Total WBC) | 5 (6%) | 18 (23%) | 27 (35%) | 23 (30%) | 0 |
| Neutrophils | 4 (5%) | 2(3%) | 14 (18%) | 39 (51%) | 0 |
| Platelets | 27 (35%) | 5 (6%) | 2 (3%) | 1 (1%) | 0 |
| Febrile Neutropenia | 0 (0%) | 0 (0%) | 2 (3%) | 1 (1%) | 0 |
| Documented Infections | 0 (0%) | 0 (0%) | 8 (10%) | 0 (0%) | 0 |
| Infection without neutropenia | 2 (3%) | 13 (17%) | 3 (4%) | 0 (0%) | 1 (1%) |
| Cardiac Arrhythmia | 3 (4%) | 0 (0%) | 5 (6%) | 1 (1%) | 0 |
| Edema | 20 (26%) | 10 (13%) | 3 (4%) | 0 (0%) | 0 |
| Hypertension | 8 (10%) | 1 (1%) | 3 (4%) | 1 (1%) | 0 |
| Hypotension | 0 (0%) | 2 (3%) | 2 (3%) | 0 (0%) | 0 |
| PT Prolongation | 7 (9%) | 6 (8%) | 6 (8%) | 0 (0%) | 0 |
| Fatigue | 15 (19%) | 36 (47%) | 15 (19%) | 4 (5%) | 0 |
| Anorexia | 16 (21%) | 20 (26%) | 2 (3%) | 0 (0%) | 0 |
| Colitis | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 |
| Constipation | 15 (19%) | 13 (17%) | 3 (4%) | 0 (0%) | 0 |
| Diarrhea | 21 (27%) | 15 (19%) | 5 (6%) | 0 (0%) | 0 |
| Dehydration | 2 (3%) | 4 (5%) | 2 (3%) | 0 (0%) | 0 |
| Ileus/Obstruction | 0 (0%) | 0 (0%) | 4 (5%) | 0 (0%) | 0 |
| Stomatitis/Mucositis | 11 (14%) | 16 (21%) | 3 (4%) | 0 (0%) | 0 |
| Nausea | 32 (42%) | 13 (17%) | 4 (5%) | 0 (0%) | 0 |
| Epistaxis | 32 (42%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 |
| Hemorrhage | 6 (8%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 |
| Melana/GI bleed | 0 (0%) | 0 (0%) | 2 (3%) | 0 (0%) | 0 |
| Transaminase (AST) | 5 (6%) | 3 (4%) | 3 (4%) | 0 (0%) | 0 |
| Acidosis | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Hypoalbuminemia | 20 (26%) | 10 (13%) | 3 (4%) | 0 (0%) | 0 |
| Hyperglycemia | 11 (14%) | 15 (19%) | 3 (4%) | 1 (1%) | 0 |
| Hypokalemia | 6 (8%) | 0 (0%) | 2 (3%) | 1 (1%) | 0 |
| Hyponatremia | 13 (17%) | 0 (0%) | 5 (6%) | 0 (0%) | 0 |
| CNS Ischemia | 0 (0%) | 0 (0%) | 1 (1%) | 1 (1%) | 0 |
| CNS Hemorrhage/Bleed | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 |
| Mood alteration | 8 (10%) | 3 (4%) | 3 (4%) | 0 (0%) | 0 |
| Neuropathy | 20 (26%) | 14 (18%) | 4 (5%) | 0 (0%) | 0 |
| Syncope | 0 (0%) | 0 (0%) | 4 (5%) | 0 (0%) | 0 |
| Bone pain | 1 (1%) | 8 (10%) | 4 (5%) | 0 (0%) | 0 |
| Neuropathic pain | 1 (1%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 |
| Pain | 7 (9%) | 16 (21%) | 11 (14%) | 0 (0%) | 0 |
| Cough | 11 (14%) | 1 (1%) | 3 (4%) | 0 (0%) | 0 |
| Dyspnea | 0 (0%) | 13 (17%) | 3 (4%) | 3 (4%) | 0 |
| Proteinuria | 11 (14%) | 5 (6%) | 1 (1%) | 0 (0%) | 0 |
| Thrombosis/Embolism | 0 (0%) | 0 (0%) | 4 (5%) | 2 (3%) | 1 (1%) |
Fatigue was common with 25% having severe grade 3 or 4 fatigue that required dose reduction in 4 cases, delaying therapy in 2 cases and in at least 6 cases therapy was discontinued. The median onset of the severe fatigue was at 7 cycles. Seven patients (9%) had thromboembolic complications. Four patients (5%) developed deep venous thrombosis and three patients (4%) had pulmonary emboli which resulted in death of one patient. One patient had a grade 4 cerebral hemorrhage in the left parietal lobe associated with mild aphasia that partially recovered. Two patients had a grade 3 or 4 cerebral vascular accident and one was associated with grade 3 hyponatremia with resolution of mental status changes with treatment.
Low grade epistaxis occurred in 42% of the patients, one patient had grade 3 epistaxis and two patients had grade 3 gastrointestinal bleed that were self limited. Mild hypertension (grade 1 and 2) was seen in 11% of the patients and more severe (grade 3 or 4 hypertension) was observed in 5% of the cases without serious complications. Low grade proteinuria was seen in 20% of the patients but grade 3 or 4 proteinuria was only observed in one patient after 22 cycles of therapy that improved after discontinuing bevacizumab.
DISCUSSION
The addition of bevacizumab to standard chemotherapy has significantly improved the survival in colon cancer patients and data suggests that bevacizumab may play a similar role in patients with CRPC. To further extend the regimen of docetaxel and estramustine tested by the CALGB17, bevacizumab was added to evaluate the safety and clinical activity of this combination. While studies now suggest that the addition of estramustine may add little clinical benefit to Docetaxel, this study was designed and implemented prior to release of this data. 2,3
The combination of estramustine, docetaxel and bevacizumab has encouraging anti-tumor activity with 75% of the patients having a 50% or more post-therapy PSA decline and 59% of patients with measurable disease achieving a complete or partial response. This is very favorable when compared the results that have been observed in many docetaxel based trials (Table 3). Nevertheless, progression free survival, which was the primary endpoint of the study was 8.1 months and did not meet the specified study endpoint of 11 months. However, the 11 month PFS was an ambitious goal and evolving information now question whether PFS is an adequate endpoint in a non-randomized study.18 In addition, it should be noted that most patients stopped therapy not for progression, but due to need for a break from treatment, which would shorten an endpoint such as progression free survival. Similarly, overall survival in a non-randomized study is difficult to interpret. However a median survival of 24 months (95% CI—20.3-26.5 mo) which was observed with these patients surpassed the survival that was observed in sequential clinical trials in a similar population by the CALGB by adding carboplatin, or exisulind to the docetaxel and estramustine backbone.5,6
Table 3.
Comparison of Adverse events for Docetaxel based regimens
| Doc.\Pred. TAX-327 Tannock et al.2 | Doc.\EMP\Pred. SWOG 9906 Petrylak et al.3 | Doc\EMP\Pred.\Bev CALGB 90006 Picus et al. | |
|---|---|---|---|
| Adverse Events Grade 3,4 or 5 | |||
| Neutropenia | 32% | 12.5% | 69% |
| Febrile neutropenia | 3% | 5% | 4% |
| Infection | - | 13.6% | 16% |
| Fatigue | 5% | - | 25% |
| Pain | - | 11% | 14% |
| Thrombosis\Embolism | - | 6% | 9% |
| Hemorrhage | - | 4% | 1% |
| Death, treatment related | 0.3% | 2.4% | 3.8% |
| Clinical Outcomes | |||
| 50% PSA Decline | 32% | 12.5% | 75% |
| CR\PR –measurable disease | 12% | 17% | 59% |
| Progression Free Survival | ~8.2 months | 6.3 months | 8.0 months |
| Overall Survival | 19.2 months | 17.5 months | 24 months |
Bevacizumab can be added to the docetaxel and estramustine backbone safely, but is associated with some increase in toxicity. Neutropenia was observed in 69% of the patients which is two to three times higher than reported in the pivotal phase III studies with docetaxel in CRPC. 2,3 Some of this frequency was due to obtaining blood counts on a weekly basis, which was not done on other trials using docetaxel. However, this high level of neutropenia did not translate into an increased proportion of patients developing febrile neutropenia.
Bevacizumab induced grade 3 or 4 hypertension was seen in 5% of the cases but was controlled. There were three treatment related deaths observed with the bevacizumab combination. Deaths were attributed to an infection without neutropenia, a mesenteric vein thrombosis, and bowel perforation. While the addition of bevacizumab appears to have contributed to the deaths of these patients, the mortality due to arterial thromboembolic events or other causes does not appear to be higher than other studies in other tumors in an elderly population treated with bevacizumab combinations.19 In addition, clearly the use of estramustine may have contributed to some of these vascular related events. Thrombosis in this trial is not different from other trials utilizing Estramustine.20 Overall the safety data from this study would indicate that the addition of bevacizumab to docetaxel may modestly increase the morbidity of the therapy in this elderly population and appropriate patient selection is required to maintain safety when using bevacizumab.
In summary, this phase II trial has demonstrated that the addition of VEGF blockade with bevacizumab to a docetaxel based regimen for the treatment of patients with metastatic CRPC is feasible, well tolerated and may provide clinical benefit. The encouraging results observed with this regimen have formed the basis for an intergroup, phase III, double blind placebo controlled trial in castrate resistant prostate cancer that is comparing docetaxel and prednisone with or without bevacizumab with a primary endpoint of overall survival. This study is fully accrued, and the data is maturing.
ACKNOWLEDGEMENTS
The research for CALGB 90006 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman), and to the CALGB Statistical Center (Stephen George, PhD, CA 33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Cancer and Leukemia Group B Statistical Office, Durham, NC, (Stephen George, Ph.D., supported by CA33601)
Christiana Care Health Services, Inc., CCOP, Wilmington, DE (Stephen S. Grubbs, M.D., supported by CA45418)
Dana Farber Cancer Institute, Boston, MA (Eric P. Winer, M.D., supported by CA 32291)
Mount Sinai Medical Center, Miami, FL (Rogerio Lilenbaum, M.D.)
North Shore University Hospital, Manhasset, NY (Daniel R. Budman, M.D., supported by CA35279)
The Ohio State University Medical Center, Columbus, OH (Clara D. Bloomfield, M.D., supported by CA 77658)
Rhode Island Hospital, Providence, RI (William Sikov, M.D.)
Syracuse Hematology-Oncology Assoc., CCOP, Syracuse, NY (Jeffrey Kirshner, M.D., supported by CA45389)
University of California at San Francisco, San Francisco, CA (Alan P. Venook, M.D., supported by CA 60138)
University of Chicago, Chicago, IL (Gini Fleming, M.D., supported by CA 41287)
University of Minnesota, Minneapolis, MN (Bruce A. Peterson, M.D., supported by CA16450)
University of Nebraska Medical Center, Omaha, NE (Anne Kessinger, M.D., supported by CA77298)
University of North Carolina at Chapel Hill, Chapel Hill, NC, (Thomas Shea, M.D., supported by CA47559)
Vermont Cancer Center, Burlington, VT (Hyman B. Muss, M.D., supported by CA77406)
Wake Forest University School of Medicine, Winsotn-Salem, NC (David D. Hurd, M.D., supported by CA47559)
Washington University School of Medicine, St. Louis, MO (Nancy L. Bartlett, M.D., supported by CA77440)
Western Pennsylvania Hospital, Pittsburgh, PA (Richard Shadduck, M.D.)
Partial funding for this study was provided by Sanofi-Aventis, US.
Footnotes
Study has been presented as an abstract at the annual meeting of ASCO in 2003
References
- 1.Crawford ED, Blumenstein BA, Goodman PJ, et al. Leuprolide with and without flutamide in advanced prostate cancer. Cancer. 1990;66:1039–1044. doi: 10.1002/cncr.1990.66.s5.1039. [DOI] [PubMed] [Google Scholar]
- 2.Tannock I, de Wit R, Berry W, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. NEJM. 2004;351:1488–90. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak D, Tangen C, Hussain M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. NEJM. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 5.Oh WK, Halabi S, Kelly WK, et al. A phase II study of estramustine, docetaxel, and carboplatin with granulocyte-colony-stimulating factor support in patients with hormone-refractory prostate carcinoma: Cancer and Leukemia Group B 99813. Cancer. 2003;98:2592–8. doi: 10.1002/cncr.11829. [DOI] [PubMed] [Google Scholar]
- 6.Dawson NA, Halabi S, Ou SS, et al. A phase II study of estramustine, docetaxel and exisulind in patients with hormone-refractory prostate cancer (HRPC): Results of Cancer and Leukemia Group B (CALGB) 90004. Clinical Genitourinary Cancer. 2008;6 doi: 10.3816/CGC.2008.n.017. in press. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endrocrinology. 1997;18:1–22. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N. Role of vascular endothelial growth factor in regulation of angiogenesis. Kidney International. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 9.Pallares J, Rojo F, Iriarte J, et al. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol Histopathol. 2006;21:857–65. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer FA, Miller LJ, Andrawis RJ. Vascular endothelial growth factor (VEGF) expression in human prostate cacner: in situ and in vitro expression of FEGG by human prostate cancer cells. Jouranl of Urology. 1997;157:2329–33. [PubMed] [Google Scholar]
- 11.Duque J, Loughlin K, Adam R. Plasma levels of vascualr endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999;45:523–27. doi: 10.1016/s0090-4295(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 12.Bok R, Halabi S, Fei D, et al. VEGF and bFGF urine levels as predictors of outcome in hormone refractory prostate cancer patients: A CALGB study. Cancer research. 2001;61:2533–2536. [PubMed] [Google Scholar]
- 13.George D, Halabi S, Shepard T, et al. Prognostic significance of plasma vascular endothelial growth factor (VEGF) levels in patients with hormone refractory prostate cancer: A CALGB study. Clinical Cancer Research. 2001;7:1932–1936. [PubMed] [Google Scholar]
- 14.Reese D, Fratesi P, Corry M. A phase II trial of humanized monoclonal anti-vascular endothelial growth factor antibody (rhu-MAb VEGF) for the treatment of androgen-indpendnent prostate cancer. Prostate. 2001;3:65–70. [Google Scholar]
- 15.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 17.Savarese DM, Halabi S, Hars V, et al. Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men ith hormone-refractoryprostate cancer: a final report of CALGB 9780. J Clin Oncol. 2001;19:2509–16. doi: 10.1200/JCO.2001.19.9.2509. [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–9. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 20.Lubiniecki GM, Berlin JA, Weinstein RB, Vaughn DJ. Thromboembolic events with estramustine phosphate-based chemotherapy in patients with hormone-refractory prostate carcinoma. Cancer. 2004;101:2755–59. doi: 10.1002/cncr.20673. [DOI] [PubMed] [Google Scholar]