Abstract
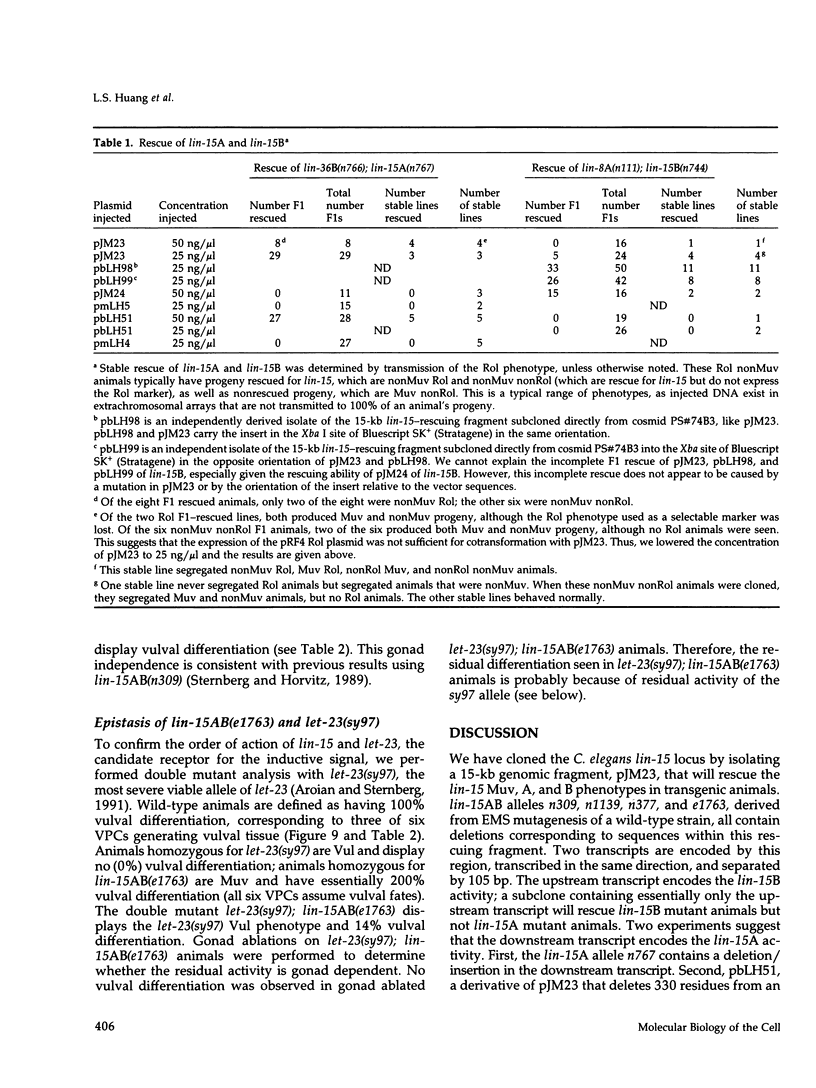
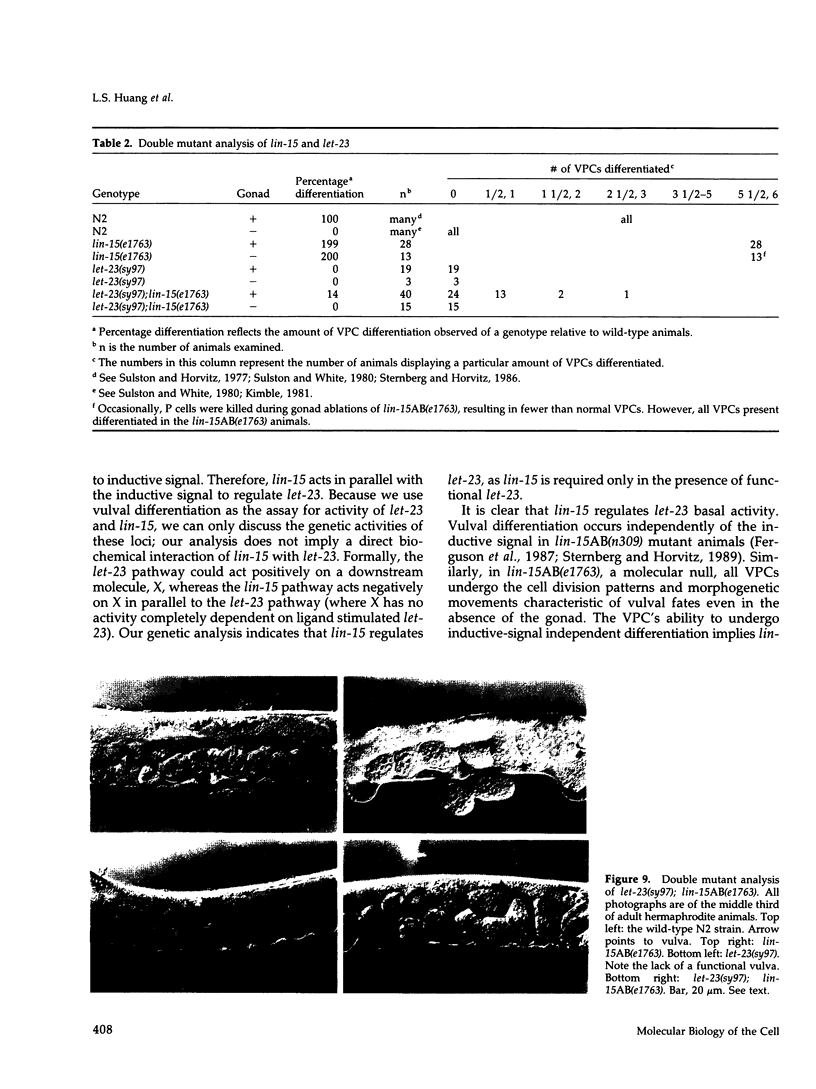
During Caenorhabditis elegans vulval development, an inductive signal from the anchor cell stimulates three of the six vulval precursor cells (VPCs) to adopt vulval rather than nonvulval epidermal fates. Genes necessary for this induction include the lin-3 growth factor, the let-23 receptor tyrosine kinase, and let-60 ras. lin-15 is a negative regulator of this inductive pathway. In lin-15 mutant animals, all six VPCs adopt vulval fates, even in the absence of inductive signal. Previous genetic studies suggested that lin-15 is a complex locus with two independently mutable activities, A and B. We have cloned the lin-15 locus by germline transformation and find that it encodes two nonoverlapping transcripts that are transcribed in the same direction. The downstream transcript encodes the lin-15A function; the upstream transcript encodes the lin-15B function. The predicted lin-15A and lin-15B proteins are novel and hydrophilic. We have identified a molecular null allele of lin-15 and have used it to analyze the role of lin-15 in the signaling pathway. We find that lin-15 acts upstream of let-23 and in parallel to the inductive signal.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Anderson P., Brenner S. A selection for myosin heavy chain mutants in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4470–4474. doi: 10.1073/pnas.81.14.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroian R. V., Lesa G. M., Sternberg P. W. Mutations in the Caenorhabditis elegans let-23 EGFR-like gene define elements important for cell-type specificity and function. EMBO J. 1994 Jan 15;13(2):360–366. doi: 10.1002/j.1460-2075.1994.tb06269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroian R. V., Sternberg P. W. Multiple functions of let-23, a Caenorhabditis elegans receptor tyrosine kinase gene required for vulval induction. Genetics. 1991 Jun;128(2):251–267. doi: 10.1093/genetics/128.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L., Horvitz H. R. A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant. Cell. 1987 Dec 24;51(6):1071–1078. doi: 10.1016/0092-8674(87)90593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U., Renfranz P. J., Hinton D. R., Rabin B. A., Benzer S. The sevenless+ protein is expressed apically in cell membranes of developing Drosophila retina; it is not restricted to cell R7. Cell. 1987 Oct 9;51(1):151–158. doi: 10.1016/0092-8674(87)90020-1. [DOI] [PubMed] [Google Scholar]
- Beitel G. J., Clark S. G., Horvitz H. R. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature. 1990 Dec 6;348(6301):503–509. doi: 10.1038/348503a0. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Thomas J. Cis and trans mRNA splicing in C. elegans. Trends Genet. 1988 Nov;4(11):305–308. doi: 10.1016/0168-9525(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol. 1981 Mar;82(2):358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- Cigarroa F. G., Coughlin J. P., Donahoe P. K., White M. F., Uitvlugt N., MacLaughlin D. T. Recombinant human müllerian inhibiting substance inhibits epidermal growth factor receptor tyrosine kinase. Growth Factors. 1989;1(2):179–191. doi: 10.3109/08977198909029127. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992 Mar 26;356(6367):340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R. Genes involved in two Caenorhabditis elegans cell-signaling pathways. Cold Spring Harb Symp Quant Biol. 1992;57:363–373. doi: 10.1101/sqb.1992.057.01.041. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Later embryogenesis: regulatory circuitry in morphogenetic fields. Development. 1993 Jul;118(3):665–690. doi: 10.1242/dev.118.3.665. [DOI] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Dibb N. J., Brown D. M., Karn J., Moerman D. G., Bolten S. L., Waterston R. H. Sequence analysis of mutations that affect the synthesis, assembly and enzymatic activity of the unc-54 myosin heavy chain of Caenorhabditis elegans. J Mol Biol. 1985 Jun 25;183(4):543–551. doi: 10.1016/0022-2836(85)90170-6. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl W. J., Johnson D. E., Williams L. T. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985 May;110(1):17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R. The multivulva phenotype of certain Caenorhabditis elegans mutants results from defects in two functionally redundant pathways. Genetics. 1989 Sep;123(1):109–121. doi: 10.1093/genetics/123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Sternberg P. W., Horvitz H. R. A genetic pathway for the specification of the vulval cell lineages of Caenorhabditis elegans. Nature. 1987 Mar 19;326(6110):259–267. doi: 10.1038/326259a0. [DOI] [PubMed] [Google Scholar]
- Fire A. Integrative transformation of Caenorhabditis elegans. EMBO J. 1986 Oct;5(10):2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M., Klämbt C., Goodman C. S., Rubin G. M. The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell. 1992 Jun 12;69(6):963–975. doi: 10.1016/0092-8674(92)90615-j. [DOI] [PubMed] [Google Scholar]
- Greenwald I. S., Horvitz H. R. Dominant suppressors of a muscle mutant define an essential gene of Caenorhabditis elegans. Genetics. 1982 Jun;101(2):211–225. doi: 10.1093/genetics/101.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. S., Horvitz H. R. unc-93(e1500): A behavioral mutant of Caenorhabditis elegans that defines a gene with a wild-type null phenotype. Genetics. 1980 Sep;96(1):147–164. doi: 10.1093/genetics/96.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. The generation of diversity and pattern in animal development. Cell. 1992 Jan 24;68(2):185–199. doi: 10.1016/0092-8674(92)90465-o. [DOI] [PubMed] [Google Scholar]
- Hafen E., Basler K., Edstroem J. E., Rubin G. M. Sevenless, a cell-specific homeotic gene of Drosophila, encodes a putative transmembrane receptor with a tyrosine kinase domain. Science. 1987 Apr 3;236(4797):55–63. doi: 10.1126/science.2882603. [DOI] [PubMed] [Google Scholar]
- Han M., Aroian R. V., Sternberg P. W. The let-60 locus controls the switch between vulval and nonvulval cell fates in Caenorhabditis elegans. Genetics. 1990 Dec;126(4):899–913. doi: 10.1093/genetics/126.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Golden A., Han Y., Sternberg P. W. C. elegans lin-45 raf gene participates in let-60 ras-stimulated vulval differentiation. Nature. 1993 May 13;363(6425):133–140. doi: 10.1038/363133a0. [DOI] [PubMed] [Google Scholar]
- Han M., Sternberg P. W. let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell. 1990 Nov 30;63(5):921–931. doi: 10.1016/0092-8674(90)90495-z. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Automated assembly of protein blocks for database searching. Nucleic Acids Res. 1991 Dec 11;19(23):6565–6572. doi: 10.1093/nar/19.23.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. K., Albertson D. G., Brenner S. Chromosome rearrangements in Caenorhabditis elegans. Genetics. 1976 May;83(1):91–105. doi: 10.1093/genetics/83.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. K. Crossover suppressors and balanced recessive lethals in Caenorhabditis elegans. Genetics. 1978 Jan;88(1):49–65. doi: 10.1093/genetics/88.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. K., Hedgecock E. M. Limitation of the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature. 1990 Nov 8;348(6297):169–171. doi: 10.1038/348169a0. [DOI] [PubMed] [Google Scholar]
- Hill R. J., Sternberg P. W. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature. 1992 Aug 6;358(6386):470–476. doi: 10.1038/358470a0. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R., Brenner S., Hodgkin J., Herman R. K. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol Gen Genet. 1979 Sep;175(2):129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- Huang X. Y., Hirsh D. A second trans-spliced RNA leader sequence in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8640–8644. doi: 10.1073/pnas.86.22.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev Biol. 1981 Oct 30;87(2):286–300. doi: 10.1016/0012-1606(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Kretzschmar D., Brunner A., Wiersdorff V., Pflugfelder G. O., Heisenberg M., Schneuwly S. Giant lens, a gene involved in cell determination and axon guidance in the visual system of Drosophila melanogaster. EMBO J. 1992 Jul;11(7):2531–2539. doi: 10.1002/j.1460-2075.1992.tb05318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Kruiger W., Stolarsky L. S., Weber W., Evans R. M., Verma I. M., Gill G. N., Rosenfeld M. G. Expression cloning of human EGF receptor complementary DNA: gene amplification and three related messenger RNA products in A431 cells. Science. 1984 May 25;224(4651):843–848. doi: 10.1126/science.6326261. [DOI] [PubMed] [Google Scholar]
- Massagué J., Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991 Dec;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneely P. M., Herman R. K. Lethals, steriles and deficiencies in a region of the X chromosome of Caenorhabditis elegans. Genetics. 1979 May;92(1):99–115. doi: 10.1093/genetics/92.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Meyer B. J. Early aspects of Caenorhabditis elegans sex determination and dosage compensation are regulated by a zinc-finger protein. Nature. 1991 May 2;351(6321):65–68. doi: 10.1038/351065a0. [DOI] [PubMed] [Google Scholar]
- Park E. C., Horvitz H. R. C. elegans unc-105 mutations affect muscle and are suppressed by other mutations that affect muscle. Genetics. 1986 Aug;113(4):853–867. doi: 10.1093/genetics/113.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. V., Clifford R. J., Schüpbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989 Mar 24;56(6):1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Rose A. M., Baillie D. L., Candido E. P., Beckenbach K. A., Nelson D. The linkage mapping of cloned restriction fragment length differences in Caenorabditis elegans. Mol Gen Genet. 1982;188(2):286–291. doi: 10.1007/BF00332689. [DOI] [PubMed] [Google Scholar]
- Schejter E. D., Shilo B. Z. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989 Mar 24;56(6):1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Spieth J., Brooke G., Kuersten S., Lea K., Blumenthal T. Operons in C. elegans: polycistronic mRNA precursors are processed by trans-splicing of SL2 to downstream coding regions. Cell. 1993 May 7;73(3):521–532. doi: 10.1016/0092-8674(93)90139-h. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Nüsslein-Volhard C. Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell. 1992 Dec 11;71(6):987–1001. doi: 10.1016/0092-8674(92)90394-r. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens L. M., Nüsslein-Volhard C. The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature. 1989 Apr 6;338(6215):478–483. doi: 10.1038/338478a0. [DOI] [PubMed] [Google Scholar]
- Sternberg P. W., Horvitz H. R. Gonadal cell lineages of the nematode Panagrellus redivivus and implications for evolution by the modification of cell lineage. Dev Biol. 1981 Nov;88(1):147–166. doi: 10.1016/0012-1606(81)90226-8. [DOI] [PubMed] [Google Scholar]
- Sternberg P. W., Horvitz H. R. Pattern formation during vulval development in C. elegans. Cell. 1986 Mar 14;44(5):761–772. doi: 10.1016/0092-8674(86)90842-1. [DOI] [PubMed] [Google Scholar]
- Sternberg P. W., Horvitz H. R. The combined action of two intercellular signaling pathways specifies three cell fates during vulval induction in C. elegans. Cell. 1989 Aug 25;58(4):679–693. doi: 10.1016/0092-8674(89)90103-7. [DOI] [PubMed] [Google Scholar]
- Sternberg P. W. Intercellular signaling and signal transduction in C. elegans. Annu Rev Genet. 1993;27:497–521. doi: 10.1146/annurev.ge.27.120193.002433. [DOI] [PubMed] [Google Scholar]
- Sternberg P. W. Lateral inhibition during vulval induction in Caenorhabditis elegans. Nature. 1988 Oct 6;335(6190):551–554. doi: 10.1038/335551a0. [DOI] [PubMed] [Google Scholar]
- Strathmann M., Hamilton B. A., Mayeda C. A., Simon M. I., Meyerowitz E. M., Palazzolo M. J. Transposon-facilitated DNA sequencing. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1247–1250. doi: 10.1073/pnas.88.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977 Mar;56(1):110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., White J. G. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 1980 Aug;78(2):577–597. doi: 10.1016/0012-1606(80)90353-x. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Stern M. J., Horvitz H. R. Cell interactions coordinate the development of the C. elegans egg-laying system. Cell. 1990 Sep 21;62(6):1041–1052. doi: 10.1016/0092-8674(90)90382-o. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Bowtell D. D., Hafen E., Rubin G. M. Localization of the sevenless protein, a putative receptor for positional information, in the eye imaginal disc of Drosophila. Cell. 1987 Oct 9;51(1):143–150. doi: 10.1016/0092-8674(87)90019-5. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Van Vactor D. L., Jr, Cagan R. L., Krämer H., Zipursky S. L. Induction in the developing compound eye of Drosophila: multiple mechanisms restrict R7 induction to a single retinal precursor cell. Cell. 1991 Dec 20;67(6):1145–1155. doi: 10.1016/0092-8674(91)90291-6. [DOI] [PubMed] [Google Scholar]
- Villeneuve A. M., Meyer B. J. The role of sdc-1 in the sex determination and dosage compensation decisions in Caenorhabditis elegans. Genetics. 1990 Jan;124(1):91–114. doi: 10.1093/genetics/124.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]