Abstract
The orexigenic effect of urocortins (Ucn 1, Ucn 2 and Ucn 3) through activation of corticotropin-releasing factor (CRF) receptors, has been well characterized after injection into the brain but not in the periphery. We examined the role of CRF receptor subtype 2 (CRF2) in the regulation of food intake using intraperitoneal (ip) injection of Ucns, the selective CRF2 antagonist, astressin2-B, and CRF2 knockout (−/−) mice. Meal structures were monitored using an automated episodic solid food intake monitoring system. Ucn 2 (3, 10 or 30 µg/kg, ip) induced a rapid in onset, long lasting and dose-dependent decrease (38%, 66% and 86% respectively at 4-h) of cumulative food intake after an overnight fast in mice. Ucn 3 anorexic effect was 10-times less potent. Astressin2-B (30 or 100 µg/kg) injected ip, but not intracerebroventricularly, blocked the inhibitory effect of ip Ucn 1 and Ucn 2 (10 µg/kg). Fasted CRF2−/− mice did not respond to ip Ucn 1 (10 µg/kg). Meal microstructure analysis of the 4-h re-feeding response to an overnight fast showed that Ucn 2 (10 µg/kg, ip) decreased meal size and duration, but increased meal frequency. In mice fed ad libitum, Ucn 2 (30 µg/kg) injected ip before the dark phase decreased the 4-h nocturnal meal size and duration without influencing meal frequency while the 10 µg/kg dose had no effect. These data indicate that Ucns, through peripheral CRF2 receptor-mediated induction of satiation, inhibit the eating response to a fast more potently than the physiological nocturnal feeding in mice.
Keywords: urocortin, corticotropin-releasing factor receptor, food intake, astressin2-B, meal pattern, mouse
1. Introduction
Urocortins (Ucns), including Ucn 1, Ucn 2 and Ucn 3, belong to the mammalian corticotropin-releasing factor (CRF) family [28,36]. Ucn 1 displays a high affinity to both CRF receptor types 1 and 2 (CRF1 and CRF2), Ucn 2 binds with high affinity to CRF2 but poorly to CRF1 [8,13] and Ucn 3 is the most selective endogenous CRF2 agonist but has lower binding affinity to CRF2 than Ucn 2 [11,13,16]. The distribution of Ucns in the central nervous system is more restricted than that of CRF, while the peptides are widely expressed in the periphery, including the cardiovascular system, gastrointestinal tract, pancreas and endocrine glands in rodents [8]. Moreover, functional studies indicate that Ucns may have a more significant role in the periphery than in the brain, as indicated by the powerful effects of peripheral administration of Ucns on cardiovascular, gastrointestinal, reproductive and immune functions and energy balance [8].
In particular, convergent studies showed that an acute intraperitoneal (ip) injection of Ucn 1 was more potent than CRF, leptin or cholecystokin-8 sulfate (CCK-8S) to induce a sustained inhibition of feeding response to a fast in lean mice [1,35,37]. Likewise, chronic subcutaneous infusion of Ucn 1 for three days suppressed daily food intake in mice [31]. In these studies, Ucn 1 injected peripherally displays a similar potency as intracerebroventricular (icv) injection [6,24,31]. We also previously demonstrated a synergistic interaction between ip injection of Ucn 1 and CCK-8S to reduce the feeding response to a fast and gastric emptying of a non-nutrient solution in mice [10]. Only a few studies have assessed the food intake alterations induced by other members of the urocortin family. Ucn 2 and, to a smaller extent Ucn 3, inhibit food intake in the light phase after a fast and in the dark phase in mice [10,35]. So far, the specific involvement of peripheral CRF2 in the peripherally administered Ucns-induced suppression of food intake is largely unexplored. Our previous study showed that selective CRF1 antagonists did not alter the ip Ucn 1-induced suppression of food intake in fasted mice while the first generation of selective CRF2 antagonist, antisauvagine-30 [30], resulted in a partial reversal, at a dose that completely suppressed ip Ucn 1-induced inhibition of gastric emptying [37]. Moreover, while meal pattern analysis is of primary importance to assess mechanisms regulating eating behavior [9], the underlying food intake microstructure induced by peripheral activation of CRF2 receptors remained unknown.
In the present study, we first established the dose-related effects of Ucn 2 and Ucn 3 injected ip on the feeding response to an overnight fast in mice. The role of peripheral CRF2 receptors in mediating ip Ucn 1 and Ucn 2 anorexigenic effects was investigated using peripheral or icv injection of the potent and long acting selective CRF2 antagonist, astressin2-B [29], and CRF2 knockout (−/−) mice. To determine whether peripheral CRF2 agonists may influence food intake through changes in gastric transit of a solid meal or behavior, the rate of gastric emptying of a standard chow meal as well as locomotor activity were assessed in ip Ucn 2-injected fasted/refed mice. Since recent studies indicate that CRF2−/− mice have increased dark phase meal size [33], we examined the influence of CRF2 receptor activation by ip Ucn 2 on meal onset, size, duration and frequency using an automated continuous food intake monitoring system, a device recently developed for mice (BioDaq, Research Diets, Inc., New Brunswick, NJ). Both light phase food intake in overnight fasted mice and nocturnal feeding in freely fed mice were monitored and analyzed.
2. Methods
2.1. Animals
Adult male C57BL/6 (7–10 weeks-old, body weight 21–30 g, Harlan, San Diego, CA) and male CRF2−/− mice (Oregon Health & Science University, Portland, OR) were used. CRF2−/− mice and their littermates were generated as previously described [5] and backcrossed onto a C57BL/6J background for eight generations. The mice were group-housed (4/cage) and fed ad libitum with standard rodent chow (Prolab RMH 2500; PMI Nutrition International, Inc., Brentwood, MO, USA) and water under controlled temperature (21–23°C) and light conditions (6:00 AM – 6:00 PM). All experiments, except otherwise stated, started around 9:00 AM in mice fasted overnight. NIH guidelines were followed in all experimental procedures that were undertaken under the auspices of an OLAW Assurance of Compliance (A3002-01) and performed according to approved Animal Components of Research Protocols (IACUC Committee of the VA Greater Los Angeles Healthcare System, # 99-06–820 and 99–127-07).
2.2. Peptides
In initial binding studies, human Ucn 2, compared to mouse Ucn 2, and mouse Ucn 3, compared to human Ucn 3, were shown to have higher binding affinity to both CRF2 receptor isoforms in membrane of mouse CRF2β stably transfected cells [16]. Therefore, the Ucn peptides used were human Ucn 2, mouse Ucn 3 and mouse Ucn 1, as well as the selective CRF2 antagonist, astressin2-B [29]. All peptides were synthesized as previously described [29] at the Clayton Foundation Laboratories (Salk Institute, La Jolla, CA). The peptides in powder form were stored at −80 °C, and dissolved in sterile distilled water immediately before use. Sulfated CCK-8S (Bachem, Torrance, CA) was stored at −80 °C as stock solution (1 µg/µl saline) and diluted in saline before use.
2.3. Intracerebroventricular injections
The icv injections were performed as previously described [20]. Mice were acutely anaesthetized with isoflurane (Ethrane, Anaquest, Madison, WI), the head was carefully hand-restrained on a gauze and the injection site localized by visualizing an equilateral triangle between the eyes and the back of the head with the apex of the triangle being the injection site with the least resistance. The injection was performed manually using a 25 µl Hamilton syringe fitted with a 30 gauge needle that was shortened by adding a sleeve made from peristaltic pump tubing to obtain a needle length of 4 mm. After the procedure which lasted approximately 1 min, mice recovered within 3–4 min and were monitored in their home cages afterwards.
2.4. Measures of food intake and gastric emptying of solid meal in fasted mice
Food intake of a solid nutrient meal was measured as detailed in our previous studies in mice [37]. Animals were housed singly during the overnight fast and then, at 9:00 AM, given pre-weighed normal rodent chow for the duration of the experimental period. Food intake was calculated as the difference between the food weights before and after the feeding period, corrected for spillage. Cumulative food intake was calculated by addition of the values at the different time periods. For food intake experiments, mice were used 3 times, by a Latin Square design, with a 6–7 days recovery period between the experiments.
In separate groups, gastric emptying was determined at 2 h after a 1-h re-feeding in mice fasted overnight as in our previous studies [20]. After the 1-h re-feeding period, food and water were removed and the treatments applied as appropriate. Mice were euthanized 2 h later by cervical dislocation and the stomach was removed and weighed. Then, the stomach was opened, its content washed out with tap water, and the gastric wall dried and weighed. The amount of food (g) retained in the stomach was quantified as the difference between the total weight of the stomach with the content and the weight of the gastric wall. The gastric emptying during the experimental period was calculated according to the following equation: gastric emptying (%) = (wet weight of content recovered from the stomach/weight of food intake during 1-h re-feeding) × 100.
2.5. Locomotor activity
Locomotor activity was measured using a similar method as that described previously [2]. Overnight fasted mice were placed in individual Plexiglas cages (10 × 15 × 25 cm) with the bottom divided into 15 equal squares and with access to water and pre-weighed food. Locomotor activity was monitored by visual examination of the total number of squares crossed by the animals during the observation time. Behavior was monitored by an investigator who was blinded to the treatments.
2.6. Automated monitoring of meal microstructures
The microstructure analysis of feeding behavior was conducted using the BioDAQ episodic Food Intake Monitor for mice (BioDAQ, Research Diets, Inc., New Brunswick, NJ), which allows continuous monitoring of meal patterns in undisturbed mice with minimal human interference as recently described [32]. Mice were accustomed for one week to single housing and to access the standard rodent diet (AIN-93M, Research Diets, Inc.) through the feeding hopper attached to regular housing cage with contains environmental enrichment and bedding material. In these studies, we used the AIN-93M balanced rodent diet that causes less spillage to assure more accurate measurements. Water was provided ad libitum from regular water bottles. Mice usually habituated to the new environment within 3–4 days and showed normal food intake and regular body weight gain. On the day of the experiment, feeding activity was monitored continuously for a period of 24 h and data were analyzed.
All definitions were based on the manufacturer’s recommendations as detailed previously [32]. The BioDAQ system weighs the hopper with food (±0.01 g) second by second and algorithmically detects 'not eating' as weight stable and 'eating' as weight unstable. Feeding bouts (changes in stable weight before and after a bout) are recorded as feeding bout vectors with a start time, duration, and amount consumed. Bouts are separated by an inter-bout interval. Meals consist of one or more bouts separated by an inter-meal interval. The inter-bout interval, inter-meal interval and minimum meal amount are user definable. We defined the inter-bout interval of 5 sec, inter-meal interval of 5 min and minimum meal amount of 0.02 g. Thus, food intake was considered as one meal when the feeding bouts occurred within 5 min of the previous response and their sum was equal to or greater than 0.02 g. If bouts of feeding were longer that 5 min apart, they were considered as a new meal. Meal structures included the number of meals (meal frequency), meal size, meal duration, inter-meal interval (time difference between the end of one meal and the initiation of the next meal) and total time spent eating (time in min or % that mice spent in feeding bouts or meals). These parameters were calculated by the software provided by the manufacturer (BioDAQ Monitoring Software 2.2.02). The satiety ratio was calculated as the average inter-meal interval divided by the average meal size, and the rate of ingestion was expressed as meal eating rate in mg/min.
2.7. Experimental protocols
All the experiments were started around 9:00 AM in overnight fasted mice, except otherwise stated,
2.7.1. Effect of ip Ucn 1, 2 and 3 on food intake
Overnight fasted mice received ip injections of either vehicle (5 ml/kg), Ucn 1 (3 µg/kg), Ucn 2 (3, 10 or 30 µg/kg) or Ucn 3 (30, 100 and 300 µg/kg) and were given free access to pre-weighed chow afterwards. Then, food intake was measured at 0.5, 1, 2, 3, 4, 5, 6 and 7 h post injection. The doses used were based on our previous dose-response studies in mice [37].
2.7.2. Effect of selective CRF2 antagonist, astressin2-B injected ip or icv on ip Ucn 1- and Ucn 2-induced inhibition of food intake
Overnight fasted mice received ip injections of vehicle or astressin2-B (30 or 100 µg/kg), and 10 min later, of vehicle, Ucn 1 or Ucn 2 (10 µg/kg) and were then given free access to pre-weighed chow food. Food intake was measured at 0.5, 1, 2 and 4 h post injections. The doses of ip astressin2-B were based on the effective dose blocking ip Ucn 1-induced delayed gastric emptying in mice [29].
To test the selectivity of astressin2-B, overnight fasted mice were injected ip with CCK-8S (10 µg/kg) or vehicle 10 min after astressin2-B (100 µg/kg) and then given free access to pre-weighed chow food. Food intake was measured at 0.5, 1, 2 and 4 h post injections. In another study, to assess whether peripheral blockade of CRF2 receptors alone will influence food intake under non maximally stimulated conditions, astressin2-B (100 µg/kg), or vehicle was injected ip in non-fasted mice and food intake was measured at 0.5, 1, 2 and 4 h post injections.
To examine site specificity of CRF2 antagonist, overnight fasted mice were first injected icv with astressin2-B (0.75 µg/mouse =30 µg/kg) or vehicle (5 µl/mouse) and 10 min later, received an ip injection of either vehicle or Ucn 2 (10 µg/kg). Then, food intake was monitored as described above for a period of 2 h.
2.7.3. Effect of ip Ucn 1 on food intake in CRF2 deficient mice
CRF2−/− and wild type mice fasted overnight were injected ip with Ucn 1 at 10 µg/kg or vehicle. Then, mice had access to pre-weighed chow and food intake was measured at 0.5, 1, 2 and 4 h post injection.
2.7.4. Effect of CRF2 antagonist, astressin2-B injected ip on ip Ucn 2-induced inhibition of gastric emptying
After an overnight fast, mice had access to pre-weighed standard chow for 1 h. Then, food and water were removed, astressin2-B (10 µg/kg) or vehicle was injected ip and 15 min later Ucn 2 (3 µg/kg) or vehicle ip. The doses of astressin2-B and Ucn 2 were based on our previous studies [29]. Gastric emptying was determined 2 h later as described above.
2.7.5. Locomotor activity after ip injection of Ucn 1 and Ucn 2
After an overnight fast, mice were injected ip with Ucn 1 (3 µg/kg), Ucn 2 (10 µg/kg) or vehicle (5 ml/kg) and locomotor activity was monitored for 2 h as described above. The cumulative 2-h food intake (2 h) was assessed at the end of the experiment.
2.7.6. Effect of ip Ucn 2 on meal microstructure
Two sets of experiments were performed. In the first one, mice were fasted overnight, and injected ip with Ucn 2 (10 µg/kg) or vehicle (5 ml/kg). In the other experiment, mice fed ad libitum were injected ip immediately before the onset of the dark phase (6:00 PM) with Ucn 2 (10 or 30 µg/kg) or vehicle (5 ml/kg). The microstructure of ingestive behavior was monitored continuously thereafter for 24 h using the BioDAQ episodic Food Intake Monitor under undisturbed conditions.
2.8. Statistical analysis
All values are reported as mean ± SEM. One way ANOVA followed by the Tukey post hoc test was used to assess differences between groups in food intake, meal structures, and gastric emptying experiments. Genotype and peptide effects on food intake in CRF2−/− mice were analyzed by two-way ANOVA. The coefficiency of locomotor activity and food intake was analyzed by Spearman’s rank order correlation analysis. Differences were considered significant when the p value was < 0.05.
3 Results
3.1. Ucn 2 and Ucn 3 injected ip inhibit food intake response to a fast in mice
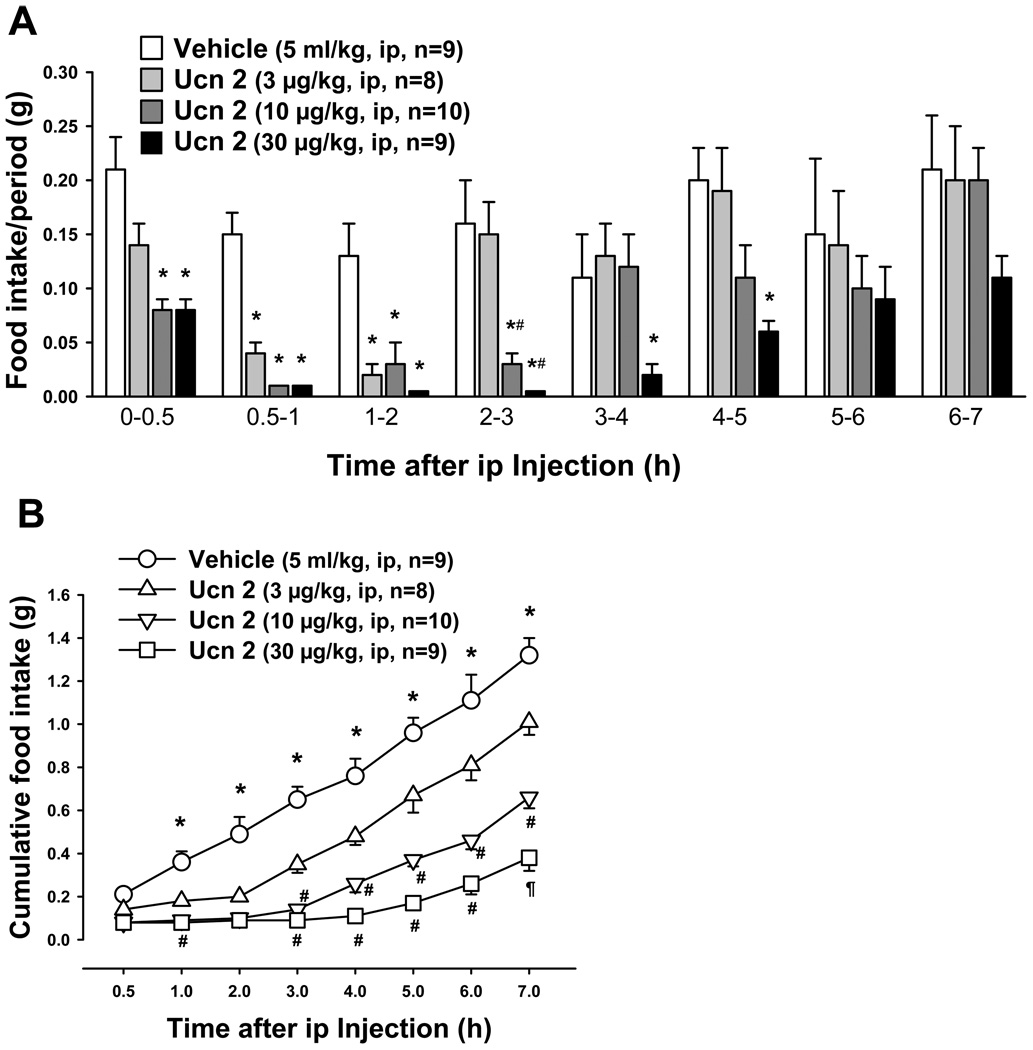
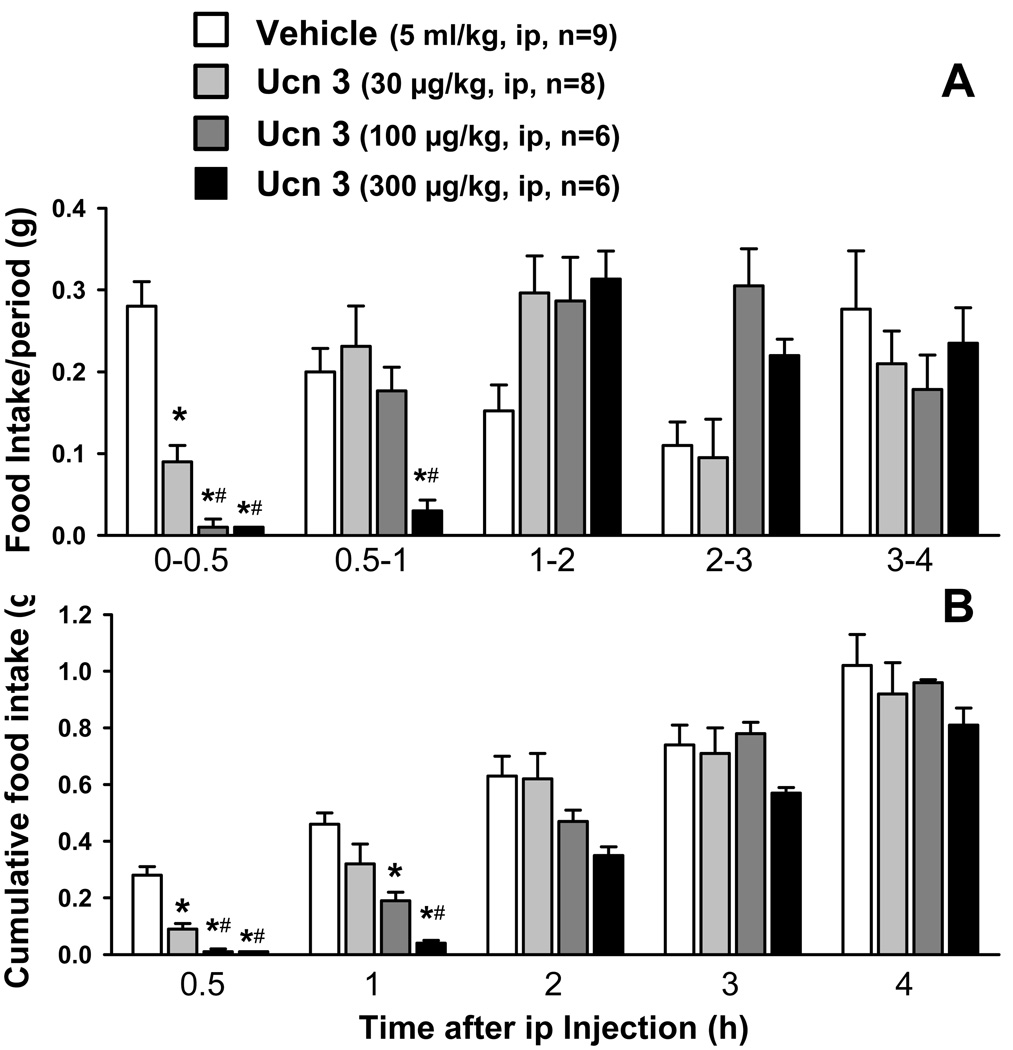
Intraperitoneal injection of Ucn 2 during the light phase dose-dependently reduced the food intake response to an overnight fast in mice (Fig. 1). The time course study showed that both the duration (Fig. 1A) and magnitude (Fig. 1B) of the food intake inhibition were dose-dependently influenced by ip Ucn 2. The reduction of food intake induced by ip Ucn 2 at 3, 10 and 30 µg/kg was observed within the first 30 min, reached a maximal inhibition (78.6%, 85.7% and 92.9%) during the 0.5 to 2 h and lasted for 2 h, 3 h and 5 h respectively as monitored for each period (Fig. 1A). When expressed as cumulative food intake, the anorexic effect remained significant during the whole 7-h experimental period (Fig 1B). Ucn 1 (3 µg/kg, ip) had a similar inhibitory effect on food intake in fasted mice as Ucn 2 (3 µg/kg, ip) tested in the same experimental conditions (g/2 h: Ucn 1: 0.16 ± 0.03, n=8, Ucn 2: 0.20 ± 0.02, n=11 vs. vehicle: 0.49 ± 0.08, n=9; p < 0.001). However, higher doses of Ucn 3 (30, 100 and 300 µ/kg) were required to inhibit food intake in fasted mice (Fig. 2). The reduction of re-feeding response to a fast also occurred at 0.5 h reaching 67.8%, 96.4% and 96.4% respectively with a maximal response occurring during the first hour post injection (54.2%, 64.6% and 91.7% respectively; Fig. 2A). The cumulative food intake showed a significant reduction only until 2 h after ip injection at the highest dose (300 µg/kg; Fig. 2B).
Fig. 1.
Dose response and time course of food intake inhibition induced by intraperitoneal Ucn 2 in overnight fasted mice. A: food intake/period. B: cumulative food intake. Data are mean ± SEM. In A: * p < 0.05 vs. vehicle; # p < 0.05 vs Ucn 2 (3 µg/kg). In B: * p < 0.05 vs. Ucn 2 at different doses; # p < 0.05 vs Ucn 2 (3 µg/kg) ; ¶ p < 0.05 vs hUcn 2 at 3 and 10 µg/kg.
Fig. 2.
Dose response and time-course of food intake inhibition induced by intraperitoneal Ucn 3 in overnight fasted mice. A: food intake/period. B: cumulative food intake. Data are mean ± SEM. * p < 0.05 vs. vehicle, # p < 0.05 vs Ucn 3 at 30 µg/kg.
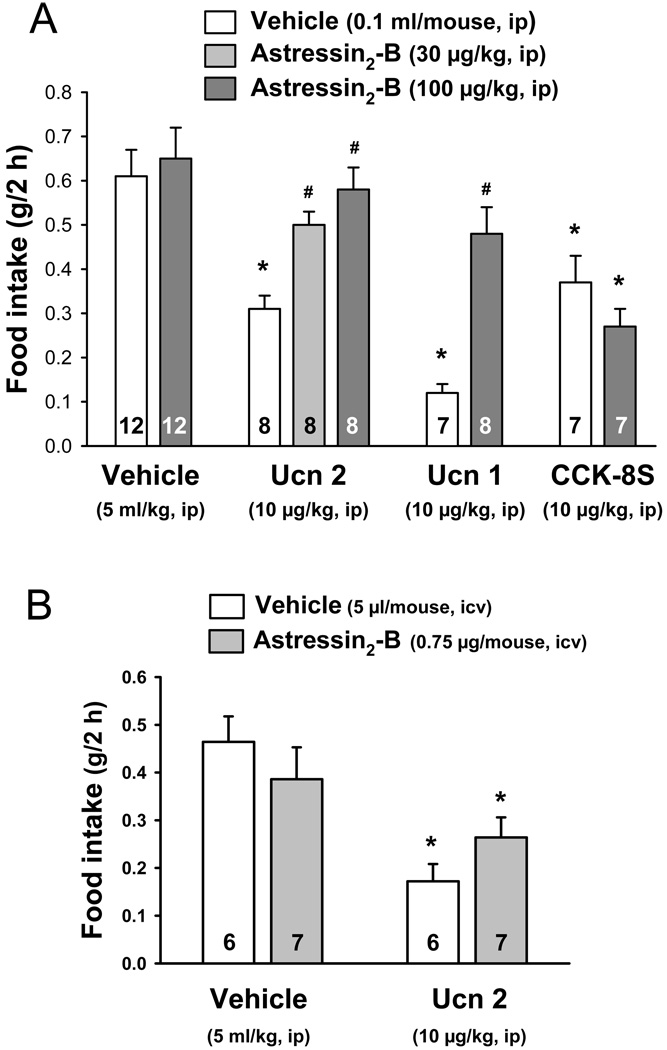
3.2. The CRF2 antagonist, astressin2-B, injected ip unlike icv, blocks ip Ucn 1- and Ucn 2-induced inhibition of food intake in fasted mice
Astressin2-B (30 and 100 µg/kg, ip) dose-dependently blocked ip Ucn 2 (10 µg/kg, ip)-induced reduction of the 2-h food intake (0.50 ± 0.03 and 0.58 ± 0.05 vs. 0.31 ± 0.03 g, p < 0.001 Fig. 3A) and also reversed the inhibitory effect of ip Ucn 1 (10 µg/kg) on 2-h food intake after a fast (0.48 ± 0.06 vs. 0.12 ± 0.02 g, p < 0.001; Fig. 3A). A higher dose of astressin2-B (200 µg/kg), had a similar reversal of Ucn 1-reduced food intake (data not shown). CCK-8S (10 µg/kg) injected ip induced a similar reduction of 2-h food intake after an overnight fast as ip Ucn 2 (10 µg/kg) (Fig. 3A). However, astressin2-B (100 µg/kg, ip) pretreatment did not influence CCK-8S effect (0.27 ± 0.04 vs. ip saline + CCK-8S 0.37 ± 0.06 g/2h, p > 0.05; Fig. 3A). Astressin2-B (100 µg/kg) injected ip before ip vehicle modified neither the 2-h food intake response to an overnight fast in mice compared to vehicles alone (0.65 ± 0.07 vs. 0.61 ± 0.06 g, p > 0.05; Fig. 3A) nor the food intake in non-fasted mice up to 4 h post injection compared with vehicle (0.22 ± 0.06 vs. 0.27 ± 0.07 g/4 h, n=6 and 7; p > 0.05).
Fig. 3.
Blockade of intraperitoneal Ucn 1 and Ucn 2 anorexigenic effect by the CRF2 antagonist, astressin2-B, after intraperitoneal (A) but not intracerebroventricular administration (B) in overnight fasted mice. Astressin2-B did not have effect on a peripheral non CRF receptor-binding peptide, CCK-8S–inhibited food intake (A). Data are mean ± SEM. Animals per group are indicated on each bar. * p < 0.05, vs. vehicle + vehicle. # p < 0.05 vs. vehicle + Ucn 1 or Ucn 2.
Next, we investigated whether central CRF2 plays a role in the peripheral Ucn 2 action. Astressin2-B injected icv (30 µg/kg) did not block the 63% decrease in food intake induced by ip injection of Ucn 2 (10 µg/kg) in mice pretreated with icv vehicle although there was a tendency to have higher values compared with icv vehicle + Ucn 2 (0.26 ± 0.04 vs 0.17 ± 0.04 g/2h; Fig. 3B). Astressin2-B injected icv alone did not influence the feeding response to a fast compared with vehicle (p > 0.05; Fig. 3B).
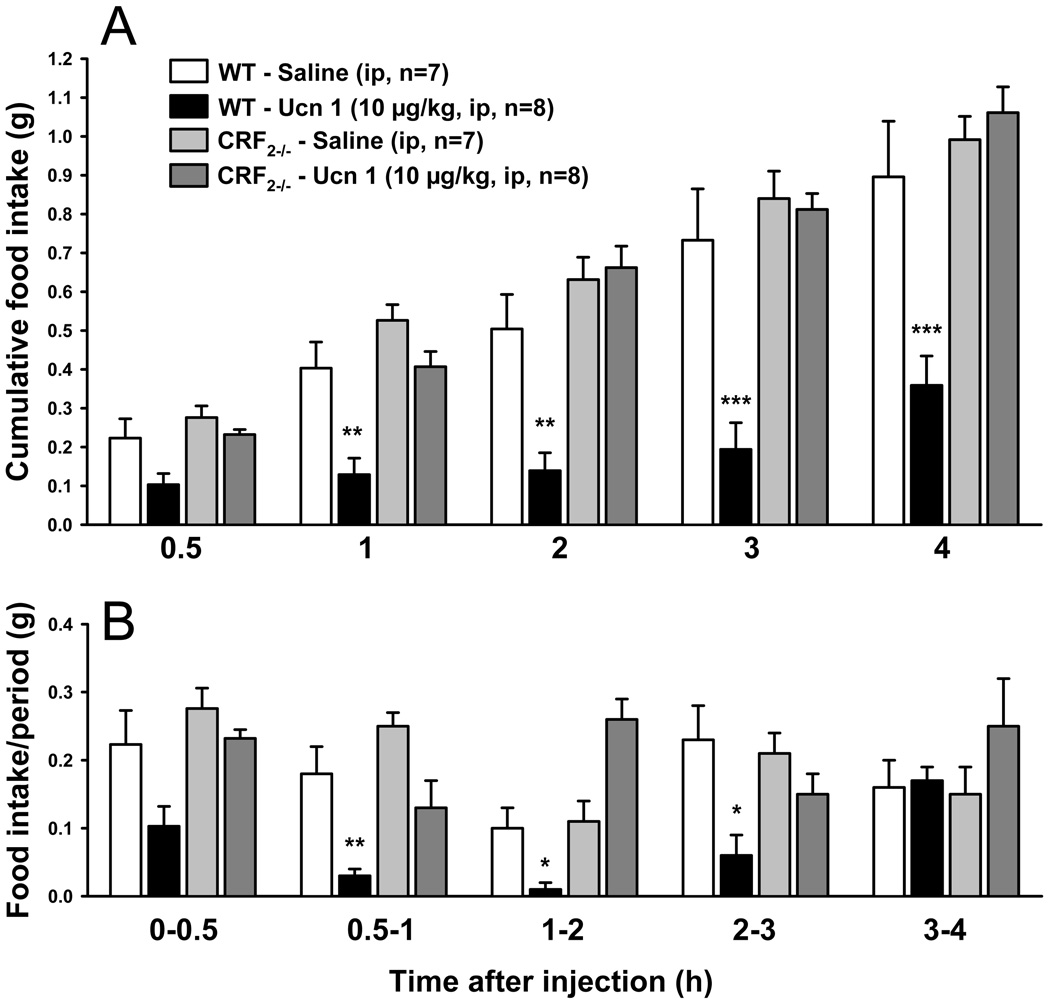
3.3. Ucn 1 injected ip no longer inhibits food intake in fasted CRF2-deficient mice
There was no significant difference in body weight between CRF2−/− mice and their wild type littermates (31.1 ± 0.6 vs. 32.5 ± 1.4 g, p > 0.05). After an overnight fast, mice of both genotypes injected ip with vehicle had a similar re-feeding food intake at any time point checked (Fig. 4). Compared to vehicle, Ucn 1 (10 µg/kg) injected ip reduced the cumulative food intake in the wild type littermates up to 4 h post injection (0.36 ± 0.08 vs. 0.90 ± 0.14 g, p < 0.001), whereas CRF2−/− mice did not show any inhibition of food intake in response to ip Ucn 1 (1.06 ± 0.07 vs. 0.99 ± 0.06 g, p > 0.05; genotype × treatment, F(1,27) = 11.8, p = 0.02; Fig. 4).
Fig. 4.
Urocortin 1 injected intraperitoneally inhibits food intake in fasted wild type mice (WT) but not in CRF2−/− litermates. A: cumulative; and B: per period food intake. Data are mean ± SEM. * p < 0.05 vs. vehicle.
3.4. The CRF2 antagonist, astressin2-B, prevents ip urocortin 2-induced inhibition of gastric emptying of a solid meal in fasted mice
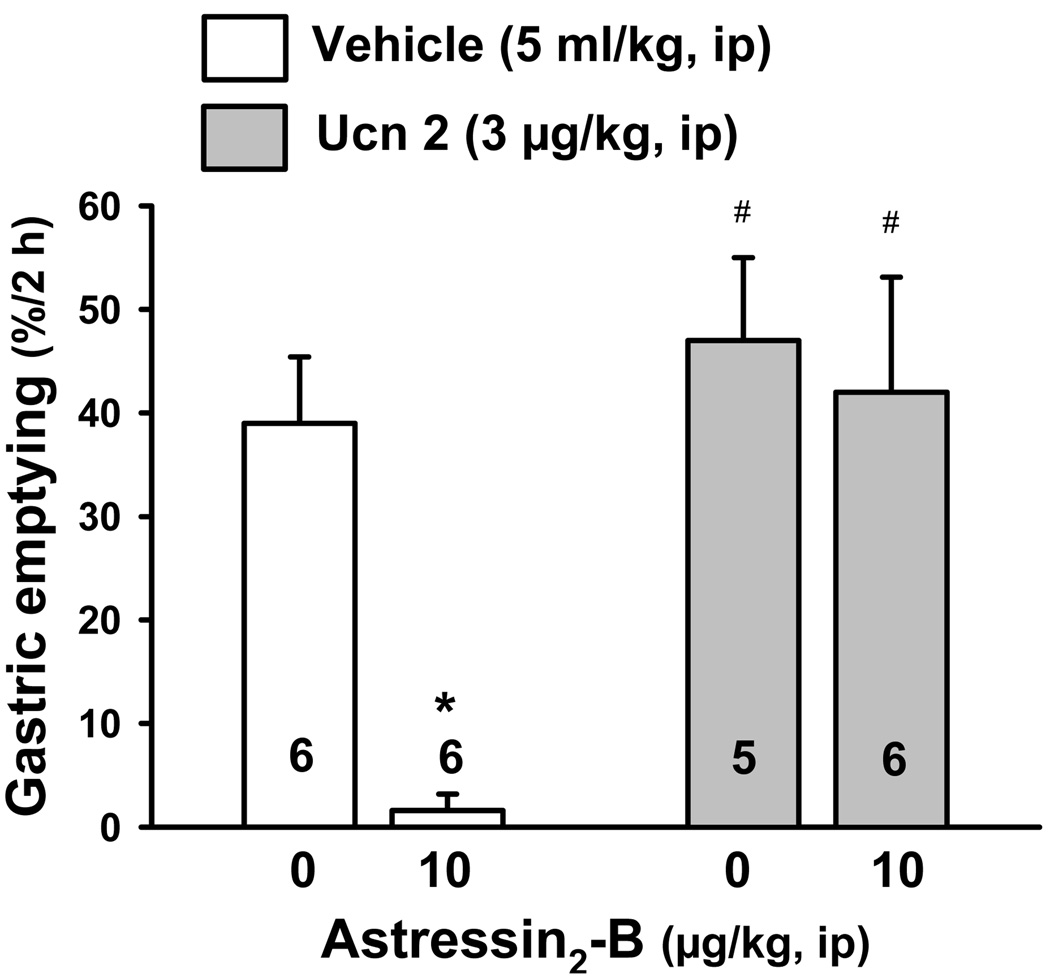
Gastric emptying was suppressed by 96% after ip injection of Ucn 2 (3 µg/kg) compared to vehicle (1.6 ± 1.6 vs. 39.0 ± 6.4 %/2 h; p < 0.05) in mice fasted overnight and re-fed with standard chow for 1 h before ip injection. Astressin2-B (10 µg/kg, ip) completely prevented ip Ucn 2-induced delay in gastric emptying (47.0 ± 8.0 %/2 h) while by itself, astressin2-B did not influence gastric emptying (42.0 ± 11.1 %/2 h; Fig. 5).
Fig. 5.
The CRF2 antagonist, astressin2-B, injected intraperitoneally blocked intraperitoneral Ucn 2-induced inhibition of gastric emptying of a solid meal in mice. Mice were food-deprived overnight and exposed to food for 1 h, then food and water were removed and vehicle or astressin2-B were injected ip followed by ip vehicle or Ucn 2 and gastric emptying was monitored 2 h later. Data are mean ± SEM. Animals per group are indicated on each bar. * p < 0.05 vs. all other experimental groups.
3.5. Ucn 1 and 2 do not alter the 2-h post injection locomotor activity while decreasing food intake in fasted mice during the 2 h re-feeding period
Ucn 1 (3 µg/kg) injected ip did not significantly alter the overall locomotor activity during the 2-h re-feeding period after a fast, as monitored by the number of squares crossed (Ucn 1: 300 ± 52 vs. vehicle: 460 ± 52, n = 6–7/group, p > 0.05) except during the 30–45 min period post injection (13 ± 9, n = 6 vs. 86 ± 14; p < 0.05), but not at any other time points. Ucn 2 (10 µg/kg, ip), did not significantly change locomotor activity at any time point compared to vehicle (2 h: 354 ± 41 vs vehicle: 460 ± 52, n = 7/group, p > 0.05). The 2-h cumulative food intake monitored simultaneously was reduced by both Ucn 1 and Ucn 2 compared to vehicle (0.18 ± 0.03 and 0.27 ± 0.03 respectively vs. vehicle 0.54 ± 0.04 g, p < 0.05). There was no correlation between locomotor activity and the 2-h food intake after either ip Ucn 1 or Ucn 2.
3.6. Effect of Ucn 2 injected ip on meal pattern in overnight fasted mice and nocturnal feeding in freely fed mice
The food intake monitoring in overnight fasted mice started at 9AM during the light phase, after ip injection. Ucn 2 (10 µg/kg, ip) reduced cumulative food intake up to 24 h post-injection compared with vehicle (4.11 ± 0.11 vs. 4.49 ± 0.08 g, p < 0.05). When the time was divided in 4-h periods, the reduction of food intake occurred in the first 4 h post-injection (0.98 ± 0.10 vs. 1.52 ± 0.12 g in vehicle, p < 0.01), whereas no significant differences were observed thereafter (data not shown). The microstructure analyses of the ingestive behavior were therefore limited to the first 4-h period post ip injection. Ucn 2 reduced meal size and duration by 70.7% and 69.7% respectively, and the percentage of time spent for eating by 32.2%, but increased the meal frequency by 44.0% with a 55.3% of decrease in inter-meal intervals and no significant change of feeding bouts compared to vehicle (Table 1). The latency to the first meal was similar in Ucn 2- and vehicle-treated mice, while the duration of the first meal was 82.2% shortened by Ucn 2 (Table 1). The meal eating rate was not significantly altered while the satiety ratio was significantly increased during the first 4 h after ip Ucn 2 compared to vehicle (Table 1).
Table 1.
Effect of ip Ucn 2 on 4-h meal pattern of light phase feeding in overnight fasted/re-fed mice or dark phase feeding in ad libitum fed mice
| Parameter | Overnight fasted/re-fed in light phase |
Fedad libitumin dark phase | ||||
|---|---|---|---|---|---|---|
| Vehicle | Ucn 2 (10 µg/kg) |
Vehicle | Ucn 2 (10 µg/kg) |
Vehicle | Ucn 2 (30 µg/kg) |
|
| Meal frequency (number/4h) |
2.38 ± 0.56 | 4.25 ± 0.56* | 4.86 ± 0.74 | 4.86 ± 0.67 | 4.86 ± 0.67 | 4.13 ± 0.29 |
| Meal size (g/meal) | 0.82 ± 0.12 | 0.24 ± 0.03*** | 0.14 ± 0.01 | 0.13 ± 0.02 | 0.17 ± 0.01 | 0.10 ± 0.01*** |
| Meal duration (min) |
74.49 ± 14.88 | 22.56 ± 2.93** | 16.67 ± 2.70 | 14.86 ± 1.94 | 16.84 ± 1.20 | 11.32 ± 0.77** |
| Feeding bouts (number/4h) |
25.88 ± 2.24 | 21.38 ± 2.50 | 22.14 ± 5.82 | 26.00 ± 6.42 | 22.57 ± 2.75 | 18.75 ± 1.96 |
| Total meal time (min/4h) |
130.18 ± 6.53 | 88.24 ± 9.34** | 84.85 ± 19.38 | 72.50 ± 13.28 | 77.91 ± 6.66 | 45.80 ± 3.29*** |
| Inter-meal interval (min) |
126.54 ± 23.85 | 56.45 ± 6.91* | 29.66 ± 6.30 | 33.96 ± 4.22 | 41.34 ± 6.32 | 40.23 ± 3.65 |
| Time spent in meals (%/4h) |
54.24 ± 2.72 | 36.76 ± 3.89** | 35.35 ± 8.08 | 30.21 ± 5.54 | 32.46 ± 2.78 | 19.08 ± 1.37*** |
| Latency to 1st meal (min) |
4.29 ± 0.81 | 5.38 ± 1.15 | 33.67 ± 13.36 | 44.70 ± 19.31 | 14.38 ± 8.14 | 68.34 ± 16.17* |
| Duration 1st meal (min) |
87.38 ± 12.93 | 15.56 ± 3.33*** | 11.94 ± 3.00 | 11.58 ± 3.54 | 16.69 ± 3.19 | 8.48 ± 1.89* |
| Eating rate 1st meal (mg/min) |
7.27 ± 1.59 | 4.77 ± 0.82 | 3.61 ± 1.28 | 3.31 ± 0.88 | 2.69 ± 0.65 | 1.74 ± 0.61 |
| Eating rate/4h (mg/min) |
6.61 ± 1.48 | 4.01 ± 0.49 | 3.63 ± 0.56 | 3.08 ± 0.17 | 3.32 ± 0.42 | 2.33 ± 0.17* |
| Satiety ratio (min/g) |
156.53 ± 14.20 | 239.54 ± 29.70* | 253.80 ± 74.03 | 282.09 ± 31.62 | 244.01 ± 33.79 | 419.12 ± 30.32** |
Each group had vehicle controls tested at the same time of Ucn 2 and also injected ip. Data are mean ± SEM; n=7–8 mice/group.
p < 0.05,
< 0.01,
< 0.001 vs. vehicle, respectively.
When assessed during the dark phase, Ucn 2 (10 µg/kg, ip) did not change cumulative food intake compared to ip vehicle, neither during the first hour (0.1 ± 0.1 vs. 0.1 ± 0.0 g, n = 8/group, p > 0.05) nor during the 24 h period after ip peptide injection (2.6 ± 0.1 vs. 2.6 ± 0.3 g, p > 0.05), as well as when the data were analyzed for the 2–3 h and 4–6 h periods post ip injection (data not shown). The analyses of meal structures did not show significant changes in any of the parameters analyzed (Table 1). However, at 30 µg/kg, ip Ucn 2 induced a significant reduction of the 24-h cumulative dark phase food intake in non-fasted mice compared to vehicle (2.4 ± 0.1 vs. 3.0 ± 0.2 g, n = 8/group; p < 0.001). When the time course of the response was analyzed, Ucn 2 significantly reduced the nocturnal cumulative food ingestion during the first 4 h post injection (0.4 ± 0.0 vs. 0.8 ± 0.1 g, p < 0.001), whereas no effects were observed during the remaining 24-h measurement time span (data not shown). The Ucn 2 action was immediate, since the latency to the first meal was 4.8 times longer and the duration of the first meal was reduced to half compared to ip vehicle. During the first 4 h, the meal size and duration as well as percentage of time spent eating were significantly reduced by 41.2%, 30.6% and 41.2% respectively, whereas meal frequency and inter-meal intervals did not differ between Ucn 2- and vehicle-treated animals (Table 1). The satiety ratio of the first 4 h was increased while the meal eating rate was decreased by Ucn 2 compared to vehicle (Table 1).
4. Discussion
This study provides novel insight to the characteristics of peripherally injected Ucns to curtail the feeding response to an overnight fast in mice which is mediated by activation of peripheral CRF2 receptors and associated with alterations in ingestive behavior indicative of induction of satiation by ip Ucn 2.
Ucn 2 injected ip at doses ranging from 3 to 30 µg/kg (equivalent to 0.5 to 5.6 nmol/kg based on formula weight) results in rapid in onset (within 30 min) and dose-related (33–62%) inhibition of the light phase food intake in overnight fasted mice. Ucn 2 action reached a maximal suppression (73–93%) during the 1st and 2nd h post injection and is sustained with a 24, 50 and 71 % cumulative food intake decrease still observed at 7 h post injection at 3, 10 or 30 µg/kg, respectively. A similar inhibitory effect was previously observed after ip Ucn 1 at 0.16–1.6 nmol/kg (1, 3 and 10 µg/kg) in mice under the same experimental conditions [37]. By contrast, Ucn 3 required ten times higher equimolar doses (5.6–56 nmol/kg) to induce a dose related, 68–96%, suppression of cumulative food intake at 30 min, which was no longer significant at 2 h post injection. These findings are indicative of Ucn 1 ≥ Ucn 2 >Ucn 3 order of potency for peripheral administered Ucns to inhibit food intake response to an overnight fast in mice. One previous study comparing the potency of ip Ucns using higher doses (4 and 12 nmol/kg) also showed Ucn 1 > Ucn 2 > Ucn 3 to suppress food intake after an overnight fast in mice [35]. As both 125I-Ucn 2 and 125I-Ucn 3 have been reported to be similarly stable in mice serum [14], the difference in potencies between ip Ucn 2 and Ucn 3 may have a bearing with their pharmacological characteristics. The binding affinity of mouse Ucn 3 to mouse CRF2β is decreased by a factor of 56 compared to that of Ucn 2 (IC50 14 vs. 0.25 nM) [13]. There is also a difference in biological potencies to activate CRF2 receptors, as assessed by the intracellular accumulation of cAMP in HEK-mCRF2β cells, although less prominently than that in binding affinity studies [13].
The respective role of brain CRF1 and CRF2 receptors in mediating the early and late components of the anorexigenic effects of central injection of CRF and Ucns has been extensively characterized in rodents [7,8,41,42]. However, less is known about CRF receptor subtype(s) involved in the peripheral action of Ucns to influence food intake [37]. Regarding the CRF1/CRF2 receptor agonist, Ucn 1 [16], we previously ruled out the involvement of CRF1 receptors in its peripheral effects since the CRF1-selective antagonists CP-154,526 and DPM904 did not prevent ip Ucn 1-induced reduction of feeding response to an overnight fast in mice [37]. However CRF2 receptors seemed to be only partially involved in ip Ucn 1 inhibitory effect on the basis that the CRF2-selective antagonist, antisauvagine-30 [30], injected ip at a maximal effective dose in other functional bioassays achieved only a 35% reversal of the anorexic effect of Ucn 1 [37]. In the present study, the more potent and longer acting CRF2 selective peptide antagonist, astressin2-B [29], developed after antisauvagine-30 [30], injected ip reversed ip Ucn 1-induced inhibition of food intake response to a fast by 74% at an antagonist:agonist (molar) ratio of 11:1 and ip Ucn 2 by 60% and 93% at antagonist:agonist ratios of 3:1 and 10:1, respectively. The specificity of astressin2-B antagonist action toward urocortin peptides interacting with CRF2 receptors is shown by the lack of peptide effect on ip CCK-8S–induced reduction of food intake after a fast. Moreover, CRF2−/− mice do not respond to ip Ucn 1, while their wild type littermates injected ip with Ucn 1 had a reduced food consumption after an overnight fast. As Ucn 3 is the most specific agonist known for CRF2 receptors, with no detectable binding to CRF1 receptors (nM IC50: >2000 vs. 350 for Ucn 2 and 1.6 for Ucn 1) [13], it can be inferred that the anorexic effect of Ucn 3 is also CRF2 receptor mediated. Collectively data obtained using genetic deletion of CRF2 receptor, as in CRF2−/− mice, acute peripheral pharmacologic blockade of CRF2 receptor with a potent CRF2 antagonist, astressin2-B, and the selective CRF2 agonists, Ucn 2 and Ucn 3, provide strong support for a primary role of CRF2 receptors in ip Ucns-induced suppression of feeding response to a fast in mice.
The central and peripheral distribution of CRF2 receptors [4,17,26,27], along with the evidence that Ucn 2 displays one of the highest lipophilicity among peptides and can cross the blood brain barrier at a moderate rate in an intact form by passive diffusion upon intravenous injection in mice [14] may support the idea that ip Ucn 2 can act at peripheral and/or central sites. However, convergent data support that CRF2-mediated anorexigenic effect of Ucns is initiated in the periphery. We showed that blockade of brain receptors by astressin2-B injected into the lateral brain ventricle did not prevent ip Ucn 2-induced reduction of food intake while a similar dose injected ip was effective to antagonize ip Ucn 2 anorexigenic effect. The tendency to have higher values of food intake after ip Ucn 2 in icv astressin2-B pretreated mice compared with icv vehicle may be related to peripheral leakage of the peptide at such a dose as reported previously [19,22]. In addition, peripheral Ucn 1 does not cross the blood brain barrier in mice [14]. Lastly, there are differential time courses in the onset of food intake suppression which is rapid after ip Ucn 2 and Ucn 3 (present study) and delayed by 3–6 hours after central injection, as reproducibly established in mice and rats [7,12,23,28,41]. The location(s) at which Ucn 2 interacts with peripheral CRF2 receptors remain to be defined. CRF2 receptors are expressed in cell bodies of the nodose ganglia and vagal afferents, as shown by binding studies using a CRF2 antagonist [15], and Ucn 2 activates rat gastric vagal afferent fibers in vitro [10]. This pathway is well established to contribute signaling to the brain to influence food intake [3] and may be a likely substrate related to ip Ucns-induced altered feeding response to a fast.
Alterations in locomotor activity may influence the feeding pattern. In the present study, Ucn 1 and Ucn 2 injected ip at a dose resulting in 67% and 50% inhibition of 2-h cumulative food intake response to an overnight fast respectively, did not alter locomotor activity monitored simultaneously, indicating that peptide action is not linked with motor impairment. However, blockade of gastric emptying of a solid meal may contribute to the prolonged suppression of food intake after a fast, as increased in gastric volume induced by food/fluid influences the degree of gastric fullness and reduces subsequent food intake [25]. In overnight fasted mice given with access to food for 1-h before treatment, Ucn 2 induces a significant higher wet content of the stomach compared with ip vehicle 2-h after ip injection. Moreover, consistent with the lower potency of ip Ucn 3 to reduce food intake, we previously reported that a 10-times higher ip dose of Ucn 3 than Ucn 2 was required to inhibit gastric emptying of a solid meal in mice [21]. However, the interrelationship between the suppression of food intake and gastric emptying is still to be clarified. While an antagonist:agonist (astressin2-B:Ucn 2) ratio of 1:1 is efficient to completely prevent the delayed gastric emptying of a solid meal induced by ip Ucn 2, a 12:1 ratio is required to achieve full reversal of ip Ucn 2-inhibitory action on food intake as monitored 2 h post peptide injection for both parameters. Therefore, it is more likely that the delayed gastric emptying may participate only partially in the food intake reduction induced by ip Ucn 2 during the initial 2-h post injection.
Microstructure analyses of meal patterns in response to activation and/or depletion of selective pathways provide relevant insight to their underlying mechanisms of action [9,33]. Using a novel automated food intake monitoring device that allows continuous recording of standard rodent chow in undisturbed mice [32], we established that ip Ucn 2 significantly decreases food intake in response to an overnight fast during the first 4 h post injection, thus confirming the observations with standard manual methods. Microstructure analysis during this 4-h period showed that ip Ucn 2 reduces meal size, meal duration and inter-meal interval without affecting the onset to the first meal. These findings indicate that peripheral Ucn 2 facilitates the satiating value of food or the process of terminating feeding. As an increase in the latency to the first meal and a decrease in meal frequency are characteristic of treatments inducing aversion or malaise, such as lithium chloride [38], the observed absence of change in latency to the first meal and increased meal frequency after ip Ucn 2 provides indirect evidence that the Ucn 2 anorexigenic effect in fasted mice is not secondary to malaise or taste aversion.
When Ucn 2 was injected ip just before the dark phase in ad libitum fed mice, the decrease in nocturnal food intake was achieved by reducing meal size, meal duration, and time spent eating. However, inter-meal intervals were not changed and onset to the first meal was delayed. It cannot be ruled out that the increased latency to eat along with the decreased size of the first meal in non-fasted mice injected with Ucn 2 at 30 µg/kg at the beginning of the dark phase is related to a brief phase of taste aversion response and/or malaise. However, since the meal frequency is not significantly altered, this explanation is unlikely to underlie the long-lasting changes observed and rather points to a specific anorexigenic effect of Ucn 2 during the physiological feeding period in the dark phase. These data provide the first characterization of the satiation-like effects of ip Ucn 2 on meal structure of nocturnal eating or in response to a fast in mice. Previous studies analyzing meal pattern after Ucn 2 injected icv at a dose of 30 µg/kg in rats also showed a satiation-like change in meal structure during the dark phase beginning at 3 h post injection, while a 10 times lower dose had no effect [7,12]. In the present study, 30 µg/kg of Ucn 2 were required to suppress the dark phase food intake, while 10 µg/kg were efficient to suppress the feeding response to a fast. This is indicative that peripheral CRF2 activation more effectively reduces food intake when the motivation to eat is high, as shown by the 46% higher food consumption in the re-feeding response to a fast compared to dark phase feeding. It may be speculated that ip Ucn 2 interferes more readily with the processing of gut-derived signals under overnight fasting conditions [39] than with specific brain neuropeptide circuitries recruited during nighttime feeding in ad libitum fed mice [18,34,40]. In support of an interaction with gut signaling, we recently showed a potentiating interaction between ip Ucn 1 and ip CCK-8S to induce reduction of food intake in mice that was mediated through peripheral CRF2 receptors [10].
The physiological role of peripheral urocortins and CRF2 receptors in the regulation of food intake is still to be defined. The present and previous studies indicate that the endogenous Ucns-CRF2 signaling pathways do not modulate the feeding response to an overnight fast as shown by the unchanged food intake in fasted mice with pharmacological blockade of CRF2 receptors with ip astressin2-B or in CRF2 receptor deficiency which is consistent with previous studies [5,33,37]. This is not related to a ceiling effect of the feeding response to a fast since astressin2-B injected ip in ad libitum fed rats did not alter the light phase food intake. In addition, we showed that ip CCK-8S–induced reduction of feeding response to a fast does not involve activation of CRF2 receptors as shown by similar CCK-8S effect in astressin2-B pretreated mice. However, peripheral CRF2 receptors may be involved in the stress-related suppression of food intake which needs to be further investigated. There is evidence in mice that restraint induces satiation-like effect on meal structure [33] which is similar to that we observed in the present study upon ip injection of Ucn 2.
In conclusion, using pharmacological tools and genetically modified animals, the present study shows that ip injection of Ucn 1 and Ucn 2, and less potently, Ucn 3, induces a rapid in onset and long lasting suppression of food intake response to an overnight fast in mice and that Ucns inhibitory action is mediated by peripheral activation of CRF2 receptors. Since there is a concomitant CRF2 mediated complete suppression of gastric emptying of a standard chow induced by ip Ucn 2, the delayed transit may contribute to the sustained reduction of re-feeding response to a fast. Meal pattern analyses indicate that ip Ucn 2 reduces food intake by inducing satiation as reflected by the reduction in meal size and meal duration both in light phase feeding in fasted mice and dark phase feeding in freely fed mice, with an increase or no change in meal frequency, respectively. Taken together, these data indicate that activation of CRF2-dependent signaling mechanisms in the periphery reduces food intake. However, the functional significance of this peripheral Ucns-CRF2 pathway, which is largely expressed in the gut [8], remains to be defined.
Acknowledgments
This work was supported by R01 NIH DK-33061, R01 NIH DDK 57238, Digestive Diseases Research Center DK 41301 (Animal Core and Administrative Supplement Grant) and Veterans Administration Research Career Scientist Award (Y.T), NIHDDK PO1 26741 (J.R.) and German Research Foundation Grants STE 1765/1-1 (A.S.), GO 1718/1-1 (M.G.). J. R. is the Dr. Frederik Paulsen Chair in Neurosciences Professor and Founder of Sentia Medical Sciences Inc. We are grateful to Mrs. Honghui Liang for her excellent technical support and we thank Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology. 1999;116:1287–1292. doi: 10.1016/s0016-5085(99)70491-9. [DOI] [PubMed] [Google Scholar]
- 2.Barrachina MD, Martinez V, Wei JY, Taché Y. Leptin-induced decrease in food intake is not associated with changes in gastric emptying in lean mice. Am. J. Physiol. 1997;272:R1007–R1011. doi: 10.1152/ajpregu.1997.272.3.R1007. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction, Neurogastroenterol. Motil. 2008;20 Suppl 1:64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzaki E, Murphy BJ, Wang L, Million M, Ohning GV, Crowe PD, Petroski R, Taché Y, Grigoriadis DE. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. J. Neurochem. 2004;88:1–11. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- 5.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 6.Cullen MJ, Ling N, Foster AC, Pelleymounter MA. Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology. 2001;142:992–999. doi: 10.1210/endo.142.3.7989. [DOI] [PubMed] [Google Scholar]
- 7.Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geary N. A new way of looking at eating. Am. J Physiol Regul. Integr. Comp Physiol. 2005;288:R1444–R1446. doi: 10.1152/ajpregu.00066.2005. [DOI] [PubMed] [Google Scholar]
- 10.Gourcerol G, Wang L, Wang YH, Million M, Taché Y. Urocortins and cholecystokinin-8 act synergistically to increase satiation in lean but not obese mice: involvement of corticotropin-releasing factor receptor-2 pathway. Endocrinology. 2007;148:6115–6123. doi: 10.1210/en.2007-0678. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 12.Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol. Exp. Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- 13.Jahn O, Tezval H, Van Werven L, Eckart K, Spiess J. Three-amino acid motifs of urocortin II and III determine their CRF receptor subtype selectivity. Neuropharmacology. 2004;47:233–242. doi: 10.1016/j.neuropharm.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Kastin AJ, Akerstrom V. Differential interactions of urocortin/corticotropin-releasing hormone peptides with the blood-brain barrier. Neuroendocrinology. 2002;75:367–374. doi: 10.1159/000059433. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence AJ, Krstew EV, Dautzenberg FM, Ruhmann A. The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br. J. Pharmacol. 2002;136:896–904. doi: 10.1038/sj.bjp.0704783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 18.Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- 19.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–1579. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 20.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J. Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J. Pharmacol. Exp. Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 22.Martins JM, Banks WA, Kastin AJ. Acute modulation of active carrier-mediated brain-to-blood transport of corticotropin-releasing hormone. Am. J. Physiol. 1997;272:E312–E319. doi: 10.1152/ajpendo.1997.272.2.E312. [DOI] [PubMed] [Google Scholar]
- 23.Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25:1703–1709. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF(2) receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am. J. Physiol. 1996;271:R766–R769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 26.Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1091–G1103. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- 27.Porcher C, Peinnequin A, Pellissier S, Meregnani J, Sinniger V, Canini F, Bonaz B. Endogenous expression and in vitro study of CRF-related peptides and CRF receptors in the rat gastric antrum. Peptides. 2006;27:1464–1475. doi: 10.1016/j.peptides.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med. Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 30.Ruhmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin- releasing factor receptor (CRFR): development of CRFR2beta-selective antisauvagine-30. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinnayah P, Blair-West JR, McBurnie MI, McKinley MJ, Oldfield BJ, Rivier J, Vale WW, Walker LL, Weisinger RS, Denton DA. The effect of urocortin on ingestive behaviours and brain Fos immunoreactivity in mice. Eur. J Neurosci. 2003;18:373–382. doi: 10.1046/j.1460-9568.2003.02760.x. [DOI] [PubMed] [Google Scholar]
- 32.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Taché Y. Activation of brain somatostatin(2) receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD, Datta R, Drago F, Vale WW, Koob GF, Zorrilla EP, Contarino A. Role of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur. J. Neurosci. 2007;26:2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, Rossi M, Ghatei M, Bloom S. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci. Lett. 2000;279:109–112. doi: 10.1016/s0304-3940(99)00955-6. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka C, Asakawa A, Ushikai M, Sakoguchi T, Amitani H, Terashi M, Cheng K, Chaolu H, Nakamura N, Inui A. Comparison of the anorexigenic activity of CRF family peptides. Biochem. Biophys. Res. Commun. 2009;390:887–891. doi: 10.1016/j.bbrc.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Martinez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am. J Physiol Regul. Integr. Comp Physiol. 2001;281:R1401–R1410. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- 38.West DB, Greenwood MR, Marshall KA, Woods SC. Lithium chloride, cholecystokinin and meal patterns: evidence that cholecystokinin suppresses meal size in rats without causing malaise. Appetite. 1987;8:221–227. doi: 10.1016/0195-6663(87)90021-3. [DOI] [PubMed] [Google Scholar]
- 39.Woods SC. Gastrointestinal Satiety Signals I. An overview of gastrointestinal signals that influence food intake. Am. J. Physiol Gastrointest. Liver Physiol. 2004;286:G7–G13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, Kalra PS, Farmerie WG, Kalra SP. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology. 1999;140:2868–2875. doi: 10.1210/endo.140.6.6789. [DOI] [PubMed] [Google Scholar]
- 41.Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol. Exp. Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]
- 42.Zorrilla EP, Taché Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol. Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]