Abstract
BACKGROUND AND PURPOSE
Shikonin exhibits a wide range of anti-inflammatory actions. Here, we assessed its effects on maturation of murine bone marrow-derived dendritic cells (BM-DCs) and on allergic reactions in a murine model of asthma.
EXPERIMENTAL APPROACH
Cultured murine BM-DCs were used to investigate the effects of shikonin on expression of cell surface markers and their stimulation of T-cell proliferation and cytokine production. The therapeutic potential of shikonin was evaluated in a model of allergic airway disease.
KEY RESULTS
Shikonin dose-dependently inhibited expression of major histocompatibility complex class II, CD80, CD86, CCR7 and OX40L on BM-DCs, induced by a mixture of ovalbumin (OVA; 100 µg·mL−1) and thymic stromal lymphopoietin (TSLP; 20 ng·mL−1). Shikonin-treated BM-DCs were poor stimulators of CD4+ T lymphocyte and induced lower levels of interleukin (IL)-4, IL-5, IL-13 and tumour necrosis factor (TNF)-α release by responding T-cells. After intratracheal instillation of shikonin in OVA-immunized mice, OVA challenge induced lower IL-4, IL-5, IL-13, TNF-α and eotaxin release in bronchial alveolar lavage fluid, lower IL-4 and IL-5 production in lung cells and mediastinal lymph node cells and attenuated OVA-induced lung eosinophilia and airway hyperresponsiveness.
CONCLUSION AND IMPLICATIONS
Shikonin effectively suppressed OVA + TSLP-induced BM-DC maturation in vitro and inhibited allergic inflammation and airway hyperresponsiveness in a murine model of asthma, showing good potential as a treatment for allergic asthma. Also, our model provides a novel platform for screening drugs for allergic diseases.
Keywords: shikonin, dendritic cells, asthma, allergic inflammation, airway hyperresponsiveness
Introduction
Shikonin and its derivatives are analogues of naphthoquinone pigments, the major components of root extracts of a Chinese medicinal herb (Lithospermum erythrorhizon), and are ingredients of several folk medicines (Chen et al., 2002). L. erythrorhizon roots have been claimed to be beneficial for burns, anal ulcers, haemorrhoids, infected crusts, bedsores, external wounds and oozing dermatitis (Papageorgiou et al., 1999). Shikonin was first isolated in its acetate form from the roots of L. erythrorhizon by Majima and Kuroda (1922). A diversity of pharmacological actions of this compound have been reported, such as inhibition of vascular permeability and acute oedema induced by histamine by topical application of shikonin (Hayashi, 1977), inhibition of cyclooxygenase-2 transcription through down-regulation of extracellular signal-regulated kinase 1/2 and activation protein-1 activities (Subbaramaiah et al., 2001), inhibition of leukotriene B4 (LTB4) biosynthesis (Wang et al., 1994), suppression of mast cell degranulation (Wang et al., 1995) and protection of the vasculature by inhibition of the neutrophil respiratory burst (Kawakami et al., 1996), and blocking CCL5 (RANTES) and CCL4 (MIP-1α) binding to human monocytes and chemokine binding to the CC chemokine receptor, CCR1 (Chen et al., 2001; receptor nomenclature follows Alexander et al., 2009). Additionally, shikonin has shown anti-cancer effects, and clinical trials in China showed that shikonin, which inhibited the growth of lung cancer and improved immune function, was effective in the later stages of lung cancer (Guo et al., 1991; Hisa et al., 1998). Shikonin is also involved in wound healing (Ozaki et al., 1994) and inhibition of platelet activation (Chang et al., 1993); it also has antimicrobial effects (Ueba et al., 1993; Yamasaki et al., 1993). Although a broad range of biological and pharmacological activities of shikonin have been reported, the possible effects of shikonin on allergic inflammation have not been determined.
Allergic asthma is characterized clinically by hypersecretion of mucus, chronic inflammation of the airways and airway hyperresponsiveness. Studies in asthmatic humans and animal models of asthma have suggested that CD4+ T helper 2 (TH2) lymphocytes play a crucial role in allergic asthma (Chung and Barnes, 1999; Wills-karp, 1999). In addition to TH2 cell effects, dendritic cells (DCs) are the predominant antigen-presenting cells in the lung; inflammatory cytokine-associated eosinophilic airway inflammation, goblet cell hyperplasia, and bronchial hyperreactivity can be completely prevented in the absence of DCs (Lambrecht et al., 1998). Previous studies have shown that DCs play key roles both in TH2 priming and maintaining allergic airway inflammation (Constant et al., 2002). The ability of DCs to polarize TH responses is highly dependent on exogenous factors (Banchereau et al., 2000), and a number of molecules have been identified that can convert DCs into TH2-polarizing antigen-presenting cells, including prostaglandin E2 (Kalinski et al., 1997) and thymic stromal lymphopoietin (TSLP) (Ito et al., 2005).
In this study, to investigate possible therapeutic effects of shikonin on allergic asthma, we used ovalbumin (OVA) combined with TSLP to convert bone marrow-derived DCs (BM-DCs) into TH2-polarizing DCs. We analysed DC maturation and DC-stimulated TH2 differentiation and activation in vitro. We also evaluated the in vivo effects on TH2 cytokine expression in bronchoalveolar lavage fluid, airway inflammation, and airway hyperresponsiveness using OVA-immunized BALB/c mice.
Methods
Preparation of BM-DCs
All animal care and experimental procedures were approved by the Animal Committee of China Medical University. The mice were housed in temperature-controlled rooms with a 12 h light/12 h dark cycle and were given food and water ad libitum. The preparation of BM-DCs was modified from a previously described method (Inaba et al., 1992; Suen et al., 2001). Briefly, bone marrow cells from the femurs and tibias of female BALB/c mice (3–6 weeks old, 13–17 g from the National Laboratory Animal Center, Taiwan) were first depleted of red cells with lysis buffer. Approximately 106 cells were placed in 24-well plates in 1 mL of Rose Park Memorial Institution (RPMI) 1640 medium supplemented with 5% fetal bovine serum, recombinant murine granulocyte macrophage colony-stimulating factor (GM-CSF) (500 U·mL−1), interleukin (IL)-4 (1000 U·mL−1) (Pepro Tech, Inc., Rocky Hill, NJ, USA), 4 mM l-glutamine, 25 mM HEPES (pH 7.2), 50 µM 2-mercaptoethanol, 0.25 µg·mL−1 amphotericin, 100 U·mL−1 penicillin and 100 µg·mL−1 streptomycin. Every other day, fresh medium containing GM-CSF and IL-4 were replaced and non-adherent cells were transferred to a new plate to decrease the contamination by macrophages. On day 6 of culture, non-adherent cells (BM-DCs) were collected and treated with different chemical compounds for 48 h. Then, BM-DCs were analysed by flow cytometry to examine their surface marker expression, and used for other experiments.
Immunofluorescence staining and flow cytometry
BM-DCs (5–10 × 105 cells) were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD11c, anti-I-A/I-E and OX-40L, or phycoerythrin (PE)-conjugated anti-CD80, anti-CD86, anti-CD11c and anti-CCR7 (eBioscience, Inc., San Diego, CA, USA) at 4°C for 30 min.
The cells were washed and suspended in 0.5 mL of phospate buffered saline (PBS) with 0.1% sodium azide, and analysed by flow cytometry (FACScan, Becton Dickinson, Mountain View, CA, USA). A total of 2 × 105 cells were counted, and the frequency of each cell surface marker was determined using commercial software. Control cells were suspended in medium only. The flow cytometer was regularly calibrated with CaliBRITE beads (Becton Dickinson).
Cytotoxicity assay
BM-DCs were pretreated with different concentrations of shikonin for 10 min and cultured with or without OVA (100 µg·mL−1) combined with TSLP (20 ng·mL−1) for 48 h. At this time, the number of viable cells was determined using Trypan blue staining (Sugiura et al., 2007). In apoptosis assays, cells were stained with FITC-labelled Annexin V and propidium iodide according to the manufacturer's instructions (Becton Dickinson); cell apoptosis was analysed by flow cytometry.
Quantitative real-time polymerase chain reaction
RNA was converted into complementary DNA and then quantified by quantitative real-time polymerase chain reaction analysis using ABI PRISM 7900 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The signal in this study was generated by the binding of the fluorophore SYBR Green (Applied Biosystems) to double-stranded DNA. The partial cycle giving a statistically significant increase in OX-40L product was determined (threshold cycle; Ct) and normalized to the Ct for β-actin. OX-40L and β-actin were amplified using SYBR Green PCR Master Mix (Applied Biosystems). The primers of OX-40L are sense strand 5′-GTCTGCCTGCAACTCTCTTCCT-3′ and antisense strand 5′-CTCCTCTGAGTCTTTG-GATTGGA-3′; the primers of β-actin are sense strand 5′- ACTGCCGCATCCTCTTCCT-3′ and antisense strand 5′-ACCGCTCGTTGCCAATAGTG-3′.
Experimental protocol for asthma model
Female BALB/c mice and DO11.10 TCR transgenic (Tg) mice aged 6–8 weeks were obtained from the Animal Center of the College of Medicine, National Taiwan University. Mice were sensitized by i.p. injection of 50 µg of OVA emulsified with 2 mg of aluminium hydroxide (AlumImuject; Pierce Chemical, Rockford, IL, USA) in a total volume of 200 µL, boosted with 25 µg of OVA emulsified with 2 mg of aluminium hydroxide, and challenged three times with OVA (100 µg in a total volume of 40 µL) by intranasal (i.n.) administrations on consecutive days (see scheme in Figure 4A). Shikonin was delivered to anaesthetized animals (with diethyl ether) intratracheally once daily on days 35–39 (five treatments). There were 8–10 mice per group. Mice were killed with CO2.
Figure 4.
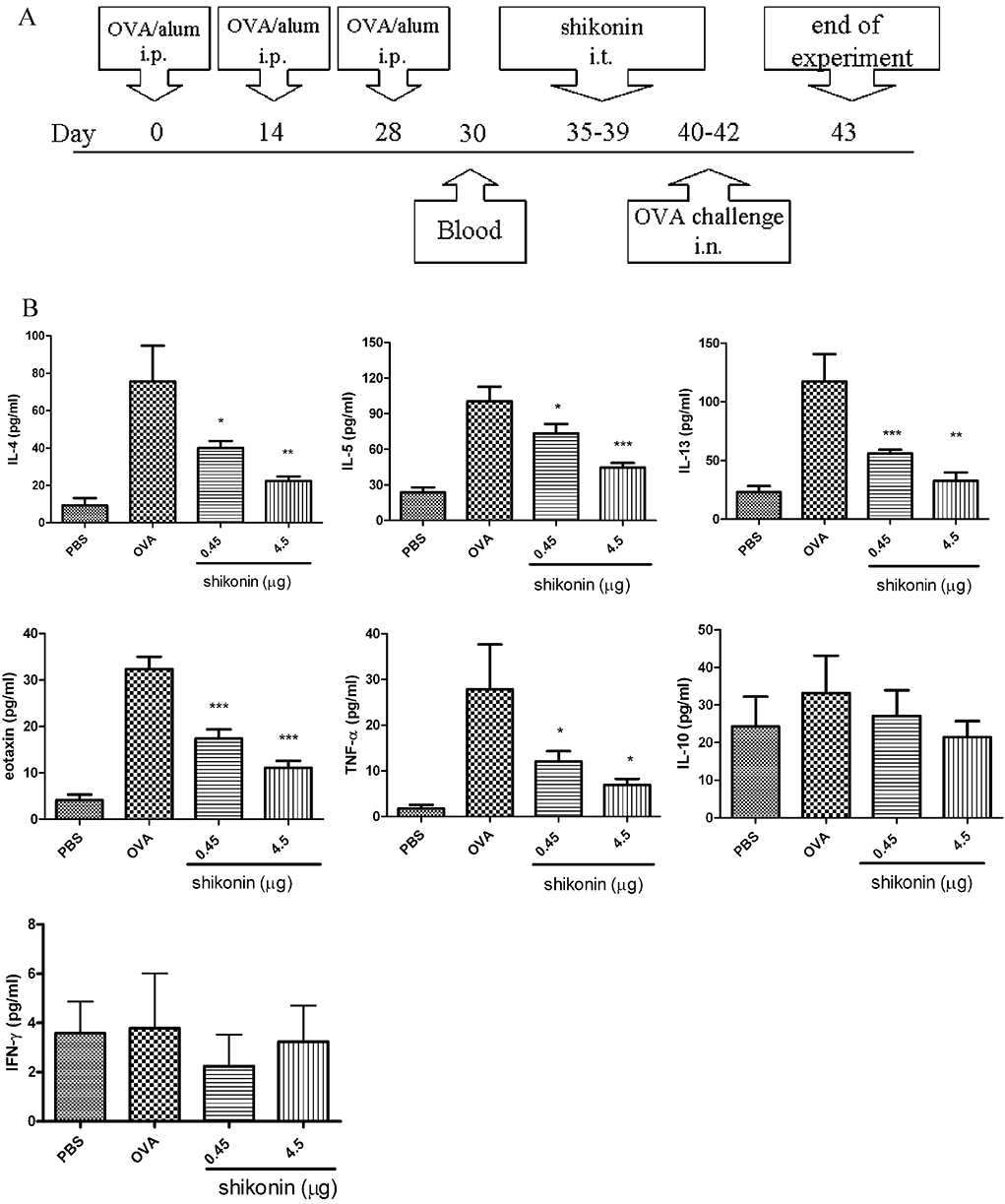
Suppression of cytokine levels in BALF after administration of shikonin in a murine model of asthma. (A) Brief scheme of animal sensitization and challenge. (B) Cytokine levels in BALF were analysed by enzyme-linked immunosorbent assay. Data were expressed as mean ± SEM (n = 8–10). The experimental groups consisted of a PBS control group (PBS sensitized and challenged) and the other groups sensitized and challenged with ovalbumin (OVA) (OVA groups). Shikonin treatment is shown as the daily dose (µg) given i.t. for 5 days. Data were expressed as mean ± SEM (n = 8–10). *P < 0.05; **P < 0.01; ***P < 0.001 different from the OVA group without shikonin.; i.n., intranasal; i.t., intratracheal.
Mixed lymphocyte reaction
Day 6 BM-DCs from BALB/c mice were treated with different concentrations of shikonin for 10 min and cultured with or without OVA (100 µg·mL−1) combined with TSLP (20 ng·mL−1) for 48 h. On day 8, BM-DCs were treated with mitomycin C (50 µg·mL−1) for 30 min and washed three times with Hank's balanced salt solution (HBSS), and then cells were collected for the following proliferation assay. Spleen CD4+ T-cells were isolated from OVA-immunized mice (for the protocol, see Figure 4A) and purified by positive selection using anti-CD4+ microbeads and the MiniMACs system according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA, USA). The purity of T-cells was analysed by flow cytometry (CD4+ T-cells: >95%). Freshly isolated CD4+ T-cells (2 × 105 cells) were co-cultured with BM-DCs at a 1:10 DC/T-cell ratio in the presence or absence of OVA (5 µg·mL−1) medium in round-bottomed 96-well microtiter plates. After 4 days, mixed cell cultures were pulsed with 5-bromo-2-deoxyuridine (BrdU) for measurement of cell proliferation. Briefly, 2 nmol of BrdU was added to each well, and the plate was incubated at 37°C for 8 h. The amount of BrdU incorporated into the T-cells was measured using the anti-BrdU monoclonal antibody in the enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Roche Diagnostics GmbH, Roche Applied Science, Germany). In siRNA-transfected BM-DC experiment, the numbers of CD4+ T-cells is lower (1 × 105 cells), and cells were co-cultured with 1/10-fold of BM-DCs (1 × 104 cells). In some experiments, freshly isolated DO11.10 spleen CD4+ T-cells were co-cultured with shikonin-treated BM-DCs for 4 days, and cell proliferation and cytokine production were detected as previously described.
Cytokine assays
Cell culture supernatants were collected 48 h after different drug treatments and stored at −20°C before analysis by ELISA according to the manufacturer's instructions. Standards were prepared from recombinant mouse interferon (IFN)-γ, IL-4, IL-5, tumour necrosis factor (TNF)-α, IL-13, IL-10, and eotaxin (CCL11) (R&D Systems, Minneapolis, MN, USA).
Bronchoalveolar lavage and lung histology
Bronchoalveolar lavage, using 1 mL of HBSS instilled with a syringe, provided bronchoalveolar lavage fluid (BALF); cells were collected by gentle aspiration of the HBSS three times and then centrifuged (Miyabara et al., 1998). Differential cell numbers were counted from cytology preparations. Cell slides were prepared using a cytospin and Liu staining. A total of 300 cells were counted under a light microscope. Supernatants of BALF were assayed by ELISA. Lungs were fixed with 10% neutral phosphate-buffered formalin, and sections were prepared and stained with haematoxylin/eosin (H&E) and periodic acid-Schiff (PAS) to quantify the number of infiltrating inflammatory cells and mucus production by microscopy. Airway inflammation was quantified by the number of inflammatory cells in subepithelial and subendothelial area, and expressed as the number of inflammatory cells per subepithelial and subendothelial area (mm2) in lung sections. The number of PAS-positive and PAS-negative bronchial epithelial cells was determined in individual airways. Results were expressed as the percentage of PAS-positive cells per bronchiole.
Measurement of airway resistance in anaesthetized mice
Airway resistance was assessed as the increase in pulmonary resistance after challenge with aerosolized methacholine (MCh) in anaesthetized mice using a modification of the techniques described by Glaab et al. (2004). Mice were anaesthetized with 70–90 mg·kg−1 pentobarbital sodium (Sigma, St. Louis, MO, USA), tracheotomized and mechanically ventilated at a rate of 150 breaths per minute, with a tidal volume of 0.3 mL and a positive end-expiratory pressure of 3–4 cm H2O with a computer-controlled small animal ventilator (Harvard Rodent Ventilator, model 683, South Natick, MA, USA). PE-50 tubing was inserted into the oesophagus to the level of the thorax and coupled to a pressure transducer (LDS GOULD, Valley View, OH, USA). Flow was measured by electronic differentiation of the volume signal. Pressure, flow and volume changes were recorded. Pulmonary resistance was calculated using a software program (Model PNM-PCT100W, LDS PONEMAH Physiology Platform, LDS GOULD). MCh aerosol was generated with an in-line nebulizer and given directly through the ventilator. Results were expressed as the pulmonary resistance (RL) of three independent experiments.
OVA-specific and total serum antibody assay
ELISA determined total sera and anti-OVA immunoglobulin (Ig) E, IgG1 and IgG2a antibody titers. Briefly, 96-well microtiter plates were coated with OVA (1 µg per well) or predetermined concentrations of anti-mouse IgE, IgG1 or IgG2a (Pharmingen, San Diego, CA, USA) in NaHCO3 buffer (pH 9.6). After overnight incubation at 4°C, the plates were washed and blocked with 3% bovine serum albumin (BSA) in PBS for 2 h at room temperature. Serum samples were diluted and added to each well overnight at 4°C. Plates were then washed. Either biotin-conjugated anti-mouse IgE, IgG1 or IgG2a (0.5 mg·mL−1, Pharmingen) diluted in 3% BSA-PBS buffer (1:500) was added for 45 min at room temperature. Avidin-conjugated horseradish peroxidase (1:5000, Pierce Biotechnology, Rockford, IL, USA) was then added for another 30 min at room temperature. The reaction was developed by peroxidase substrate, 3,3′,5,5′-tetramethylbenzidine (KPL, Gaithersburg, MD, USA) and then stopped with 2N H2SO4. Absorbance was determined at 450 nm in a microplate reader. Antibody levels were compared to standard serum, and IgG1, IgE and IgG2a concentrations in standard serum were arbitrarily assigned 1 ELISA unit.
Preparation of lymph node cells
Mediastinal lymph nodes were harvested and pooled from each group at the end of the experiment (day 43). Single-cell suspensions were obtained by mechanical disruption. Cells were stimulated in vitro with 10 µg·mL−1 OVA or 1 µg·mL−1 CD3 combined with 1 µg·mL−1 CD28 for 72 h. The cell medium was collected for cytokine analysis.
Lung mononucleocyte preparation
After death, lungs were perfused with 10 mL PBS through the right ventricle. Lungs were then removed and pooled from each group at the end of the experiment (day 43). After removal from animals, lungs were cut into small pieces, and single-cell suspensions were obtained by a stainless steel cell dissociation sieve. Debris was removed by using a cell strainer (100 µm, BD Biosciences, Franklin Lakes, NJ, USA). Then, mononucleocytes were isolated by Ficoll-Plaque Plus according the manufacturer's instructions (GE Healthcare, Sweden). Cells were stimulated in vitro with 10 µg·mL−1 OVA or 1 µg·mL−1 CD3 combined with 1 µg·mL−1 CD28 for 72 h. The cell medium was collected for cytokine analysis.
Statistical analysis
Results are given as means ± SEM (n≥ 3). Group comparisons were performed by one-way anova followed by Newman–Keuls post hoc test. P < 0.05 was considered significant.
Materials
Shikonin (Figure 1A) was purchased from EMD Chemical, Inc. (Darmstadt, Germany). OVA (grade V) was purchased from Sigma Chemical Co. RPMI 1640 medium, HBSS, penicillin, streptomycin, l-glutamine and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA, USA). TSLP was purchased from R&D Systems, Inc. siRNAs (for OX-40L and negative siRNA, designed and chemically modified to not known target genes in human, mouse and rat cells) were purchased from Dharmacon RNAi Technology (Thermo Fisher Scientific, Lafayette, CO, USA).
Figure 1.
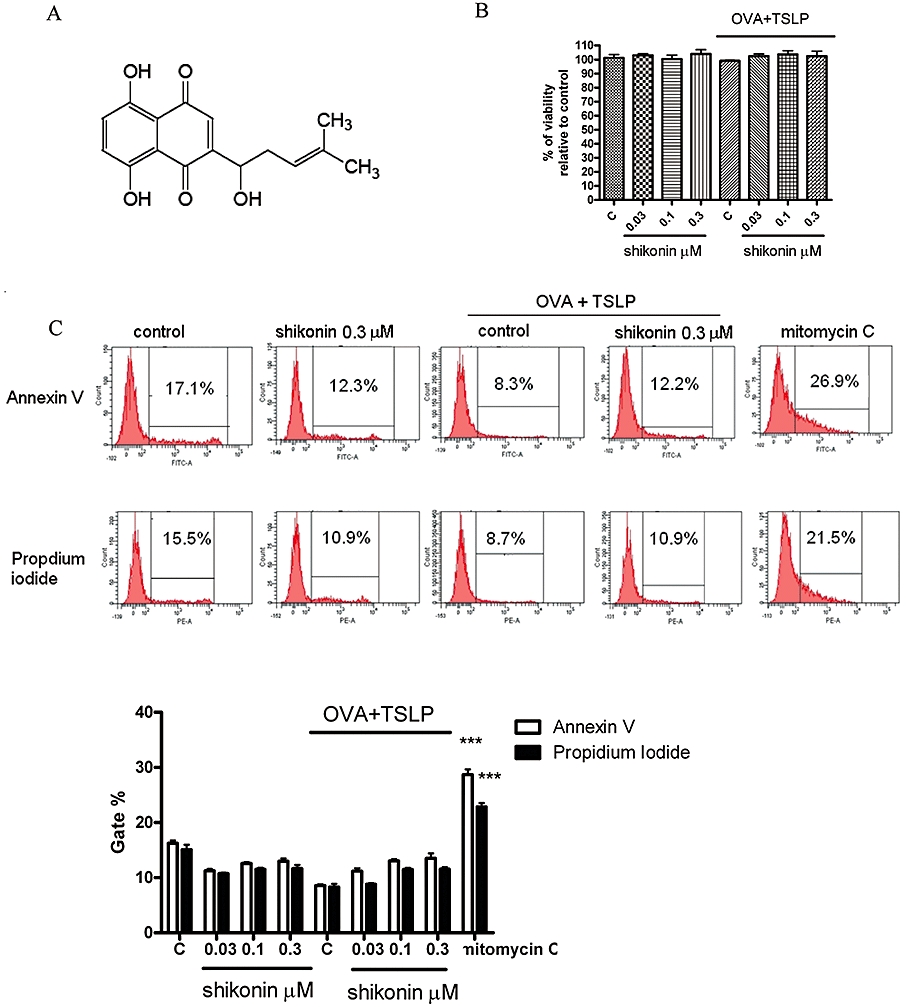
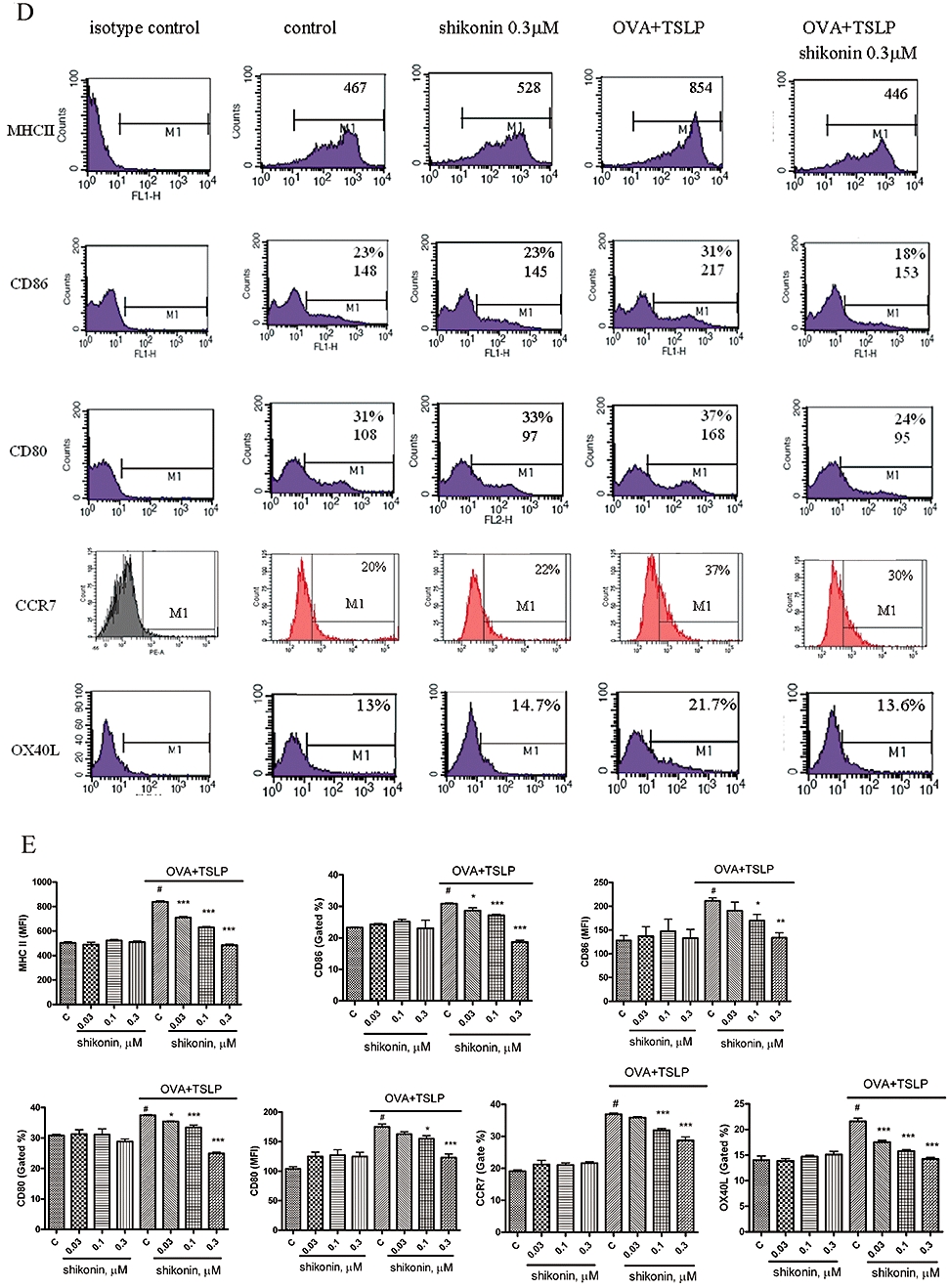
Shikonin inhibited the expression of surface markers on murine bone marrow-derived dendritic cells (BM-DCs). (A) Chemical structure of shikonin. (B) BM-DCs were prepared as described in Methods. BM-DCs were treated with shikonin for 10 min, then cultured with or without 100 µg·mL−1 ovalbumin (OVA) combined with 20 ng·mL−1 thymic stromal lymphopoietin (TSLP) for 48 h; cell viability was detected by Trypan blue exclusion. (C) Cells were stained with fluorescein isothiocyanate-labelled Annexin V and propidium iodide, and cell apoptosis was analysed by flow cytometry as described in Methods. BM-DCs treated with 25 µg·mL−1 mitomycin C for 6 h were used as a positive control. Histograms represent quantification of apoptosis % by gated region, which was analysed by flow cytometry. Data were expressed as mean ± SEM (n = 3). ***P < 0.001, compared with the control group (no treatment; C). (D) BM-DCs were treated with shikonin for 10 min and then cultured with or without 100 µg·mL−1 OVA combined with 20 ng·mL−1 TSLP for 48 h. Expression of surface markers was analysed by flow cytometry. The values shown in the flow cytometry profiles are the gated % and the mean fluorescence intensity indexes. Cells were gated on CD11c, and the incidence of CD11c+ cells expressing the antigen of interest is indicated within each histogram. (E) Histograms represent quantification of surface marker expression analysed by flow cytometry. Data were expressed as mean ± SEM (n = 3–6). #P < 0.001, compared to the control, without OVA + TSLP, group. *P < 0.05; **P < 0.01; ***P < 0.001, compared to the control, with OVA + TSLP, group. MHC, major histocompatibility complex.
Results
Shikonin decreases OVA + TSLP-induced BM-DC surface marker expression
First, we evaluated possible cytotoxic effects of shikonin on BM-DCs. After treatments with shikonin at 0.03, 0.1 and 0.3 µM for 48 h, DCs did not show any necrosis as shown by Trypan blue exclusion assay (Figure 1B) or apoptosis by Annexin V and propidium iodide staining assays (Figure 1C). Next, we investigated surface marker expression. DCs treated with different concentrations of shikonin did not show any apparent changes for major histocompatibility complex (MHC) class II, CD80, CD86 or CCR7 expression (Figure 1D and E).
Because OVA is an allergen and TSLP released by epithelial cells can induce DC activation that is involved with TH2 cell differentiation (Simecka, 1998), we used a mixture of OVA and TSLP (OVA + TSLP) to drive DCs to become TH2-polarizing antigen-presenting cells. Shikonin (0.03, 0.1 and 0.3 µM) dose-dependently inhibited the OVA + TSLP-induced expression of MHC class II, CD80, CD86 and CCR7 (Figure 1E). Because TSLP-activated DCs can induce an inflammatory TH2 response via the OX40 ligand (OX40L) (Ito et al., 2005), we also evaluated OX40L expression on BM-DCs. After treatments with shikonin (0.03, 0.1 and 0.3 µM), OX40L expression on BM-DC did not show any apparent differences compared with the control group (Figure 1D and E). After treatment with OVA + TSLP, OX40L expression on BM-DC was significantly increased and shikonin dose-dependently inhibited this OVA + TSLP-induced OX40L expression (Figure 1E).
Shikonin-treated BM-DCs are poor stimulators of CD4 T lymphocytes and induce lower levels of cytokine release by responding T-cells
Next, we investigated the ability of shikonin-treated BM-DCs to inhibit the proliferation of OVA-immunized BALB/c spleen CD4+ T-cells. CD4+ T-cells treated with phorbol myristate acetate (5 ng·mL−1) and ionomycin (1 µM) were used as a positive control and cell proliferation monitored by BrdU incorporation (Figure 2A). OVA + TSLP-treated DCs were twofold to 2.5-fold more efficient stimulators of CD4+ T-cells, compared to untreated DCs (Figure 2A) and also induced increased production of IL-4, IL-5, IL-13 and TNF-α (Figure 2B). Shikonin dose-dependently decreased CD4+ T proliferation, and IL-4, IL-5, IL-13 and TNF-α production was stimulated by OVA + TSLP-treated DCs. However, in the OVA + TSLP-treated DC group, OVA (5 µg·mL−1) treatment did not stimulate IL-4, IL-5, IL-13 and TNF-α production. In this experiment, we did not detect any IFN-γ production among different treatment groups. We also compared the levels of cell proliferation and cytokine production in CD4+ T-cells isolated from spleens of DO11.10 mice, which were co-cultured with shikonin-treated BM-DCs (Figure 2C). We found that the trend of CD4+ T-cell proliferation and cytokine production was similar to CD4+ T-cells isolated from OVA-immunized mice, but that the inhibition of OVA + TSLP-induced cell proliferation and IL-4 and IL-5 production after shikonin treatment was less than that observed in CD4+ T-cells, isolated from OVA-immunized mice.
Figure 2.
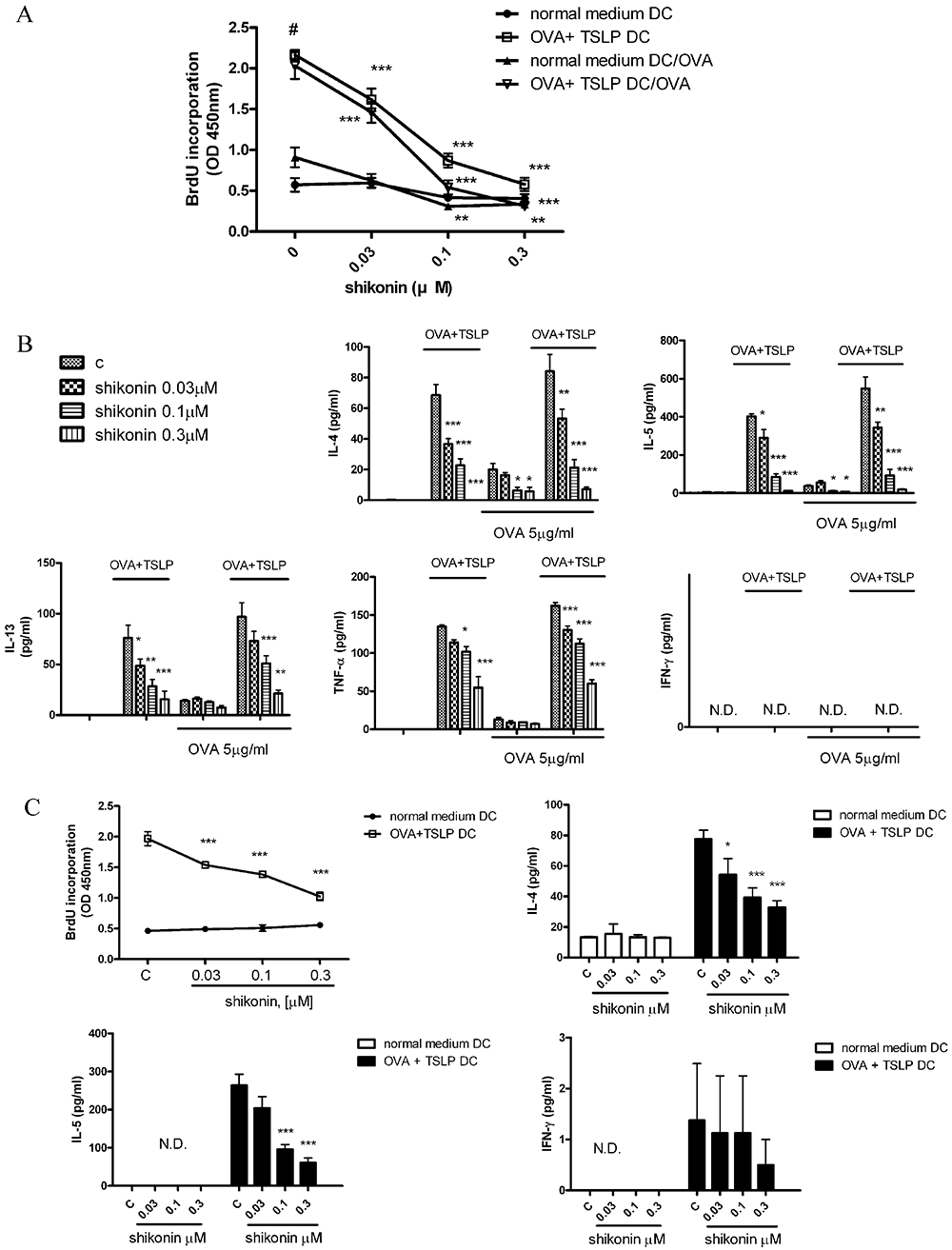
Shikonin dose-dependently inhibited CD4+ T-cell responses. (A) On day 6 of culture, bone marrow-derived dendritic cells (BM-DCs) were treated with different concentrations of shikonin for 10 min and then cultured with or without ovalbumin (OVA) (100 µg·mL−1) + thymic stromal lymphopoietin (TSLP) (20 ng·mL−1) for 48 h. On day 8, BM-DCs were collected and treated with 50 µg·mL−1 mitomycin C for 30 min. To assay proliferation, fresh spleen CD4+ T-cells isolated from OVA-immunized mice (see Figure 4A, for the protocol) were co-cultured with different treatments of BM-DCs at a 1:10 DC/T-cell ratio, in the presence or absence of 10 µg·mL−1 OVA for 4 days. Proliferation of CD4+ T-cells was measured by uptake of BrdU with an ELISA kit, as described in Methods. Normal-medium DC groups represent DCs cultured in RPMI medium, with or without shikonin. OVA + TSLP DC groups represent DCs, treated with or without shikonin and then cultured in the RPMI supplement with OVA + TSLP. Normal medium DC/OVA or OVA + TSLP/OVA represent differently treated DCs cultured with T-cells in the presence of 10 µg·mL−1 OVA. (B) Cytokine-secreting pattern of OVA-immunized CD4+ T-cells after stimulation with shikonin-treated BM-DCs. Shikonin-treated BM-DCs pulsed with or without OVA + TSLP were collected and treated with 50 µg·mL−1 mitomycin C for 30 min. BM-DCs were co-cultured at a 1:10 ratio with OVA-immunized CD4+ T-cells in the presence or absence of 10 µg·mL−1 OVA. After 4 days, supernatants were collected, and levels of cytokine production were analysed by enzyme-linked immunosorbent assay (ELISA). Data were expressed as mean ± SEM (n = 5). *P < 0.05; **P < 0.01; ***P < 0.001, compared with the OVA + TSLP control group. (C) Shikonin dose-dependently inhibited responses of CD4+ T-cells from spleens of DO11.10 mice. BM-DCs were treated with different concentrations of shikonin for 10 min and then cultured with or without 100 µg·mL−1 OVA combined with 20 ng·mL−1 TSLP for 48 h. Spleen CD4+ T-cells freshly isolated from D011.10 mice were co-cultured at a 1:10 ratio with different treatments of BM-DCs. CD4+ T-cell proliferation was measured by uptake of BrdU with an ELISA kit, as described in Methods. Supernatants were analysed for cytokine production by ELISA. Data were expressed as mean ± SEM (n = 5). #P < 0.001, compared to the control, without OVA + TSLP, group. *P < 0.05; **P < 0.01; ***P < 0.001, compared with the OVA + TSLP control group. N.D., not detected.
To clarify the contribution of OX40L to the inhibition of cytokine release in CD4+ T-cells by shikonin in BM-DCs, we used RNA interference assays to knockdown OX40L expression. After transfection of OX40L siRNA in BM-DCs for 72 h, OX40L mRNA expression was significantly knocked down in control and OVA + TSLP groups (Figure 3A). BM-DCs were transfected with OX40L siRNA for 72 h and co-cultured with CD4+ T-cells from OVA-immunized mice for 4 days. IL-4 and IL-5 expression was significantly inhibited in all OX40L siRNA-transfected groups, indicating that the IL-4 and IL-5 production induced by OVA + TSLP was mainly dependent on OX40L. Knockdown of OX40L expression in BM-DCs attenuated the stimulatory effects of TH2 cells. Therefore, in co-cultures of CD4+ T-cells and BM-DCs with knockdown of OX40L, stimulation with OVA + TSLP induced relatively low levels of IL-4 and IL-5 secretion (Figure 3B). This reduced cytokine output was still sensitive to inhibition by shikonin (Figure 3B).
Figure 3.
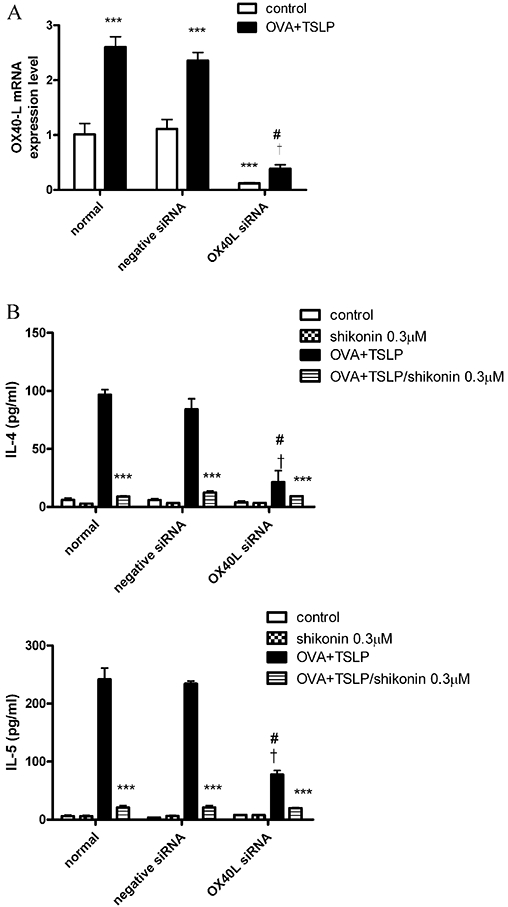
Inhibition of interleukin (IL)-4 and IL-5 production in T-cells by knockdown of OX40L siRNA. (A) Bone marrow-derived dendritic cells (BM-DCs) were transfected with 20 nM OX40L siRNA and negative siRNA by Lipofectamine 2000 for 72 h, and OX40L mRNA expression was detected by real-time PCR as described in Methods. (B) BM-DCs were transfected with 20 nM OX40L siRNA and negative siRNA for 72 h, treated with 0.3 µM shikonin for 10 min, and then cultured with or without ovalbumin (OVA) + thymic stromal lymphopoietin (TSLP) for 48 h. Freshly isolated spleen CD4+ T-cells isolated from OVA-immunized mice (see protocol in Figure 4A) were co-cultured with different treatments of BM-DCs for 4 days. Supernatants were analysed for cytokine production by ELISA, as described in Methods. Data were expressed as mean ± SEM (n = 3). ***P < 0.001, compared with the OVA + TSLP group, respectively. #P < 0.001, compared with normal OVA + TSLP group; †P < 0.001, compared with the negative siRNA OVA + TSLP group.
Intratracheal instillation of shikonin decreases bronchial alveolar lavage fluid, lung cell and mediastinal lymph node cell TH2 cytokine levels in a murine model of asthma
We examined the effects of shikonin in a murine model of asthma. Mice were sensitized to OVA and challenged with i.n. OVA droplet aspiration, as shown in Figure 4A. Mice were treated once a day with shikonin on days 35–39 (five treatments) (Figure 4A). One day after the final challenge, we measured cytokine contents in the BALF.
Mice sensitized and challenged with OVA (OVA groups) showed increases in IL-4, IL-5, IL-13, TNF-α and eotaxin release in BALF compared to control, PBS-sensitized and challenged mice (PBS groups; Figure 4B). After treatment with shikonin, IL-4, IL-5, IL-13, TNF-α and eotaxin levels in BALF were dose-dependently decreased. However, IFN-γ and IL-10 levels in BALF were not affected either by challenge with OVA or by treatment with shikonin.
We also investigated whether shikonin inhibited T lymphocyte activation in lung and mediastinal lymph nodes. As shown in Supporting Information Figure S1 (see Supporting Information), the number of lung and mediastinal lymph node cells were increased in OVA-immunized mice (positive group). After mice were treated with shikonin, the number of lung and mediastinal lymph node cells was significantly decreased, as compared with the positive group (Supporting Information Figure S1A). As shown in Supporting Information Figure S1B and S1C, treatment with a mixture of CD3 and CD28 (CD3 + CD28) induced IL-4, IL-5, and IFN-γ production in lung cells and mediastinal lymph node cells, isolated from OVA-immunized mice. Shikonin inhibited this induced IL-4 and IL-5 production but not that of IFN-γ. Note that in OVA-treated lung cell and mediastinal lymph node cell groups, levels of IL-4 and IL-5 production were lower than those in mice treated with (CD3 + CD28).
Inhibition of allergen-induced airway inflammation by shikonin
To investigate the effects of shikonin on airway inflammation, we analysed the cellular composition in BALF at 48 h after the last three OVA challenges (days 42–44). In PBS-sensitized and challenged mice (PBS group), no obvious inflammatory cells were noted in BALF. However, after sensitization and challenge with OVA (OVA group), the numbers of macrophages and eosinophils in BALF were significantly increased (Figure 5A). After intratracheal instillation of shikonin, challenge of the OVA group induced less eosinophils in BALF, although macrophage numbers were not affected (Figure 5A).
Figure 5.
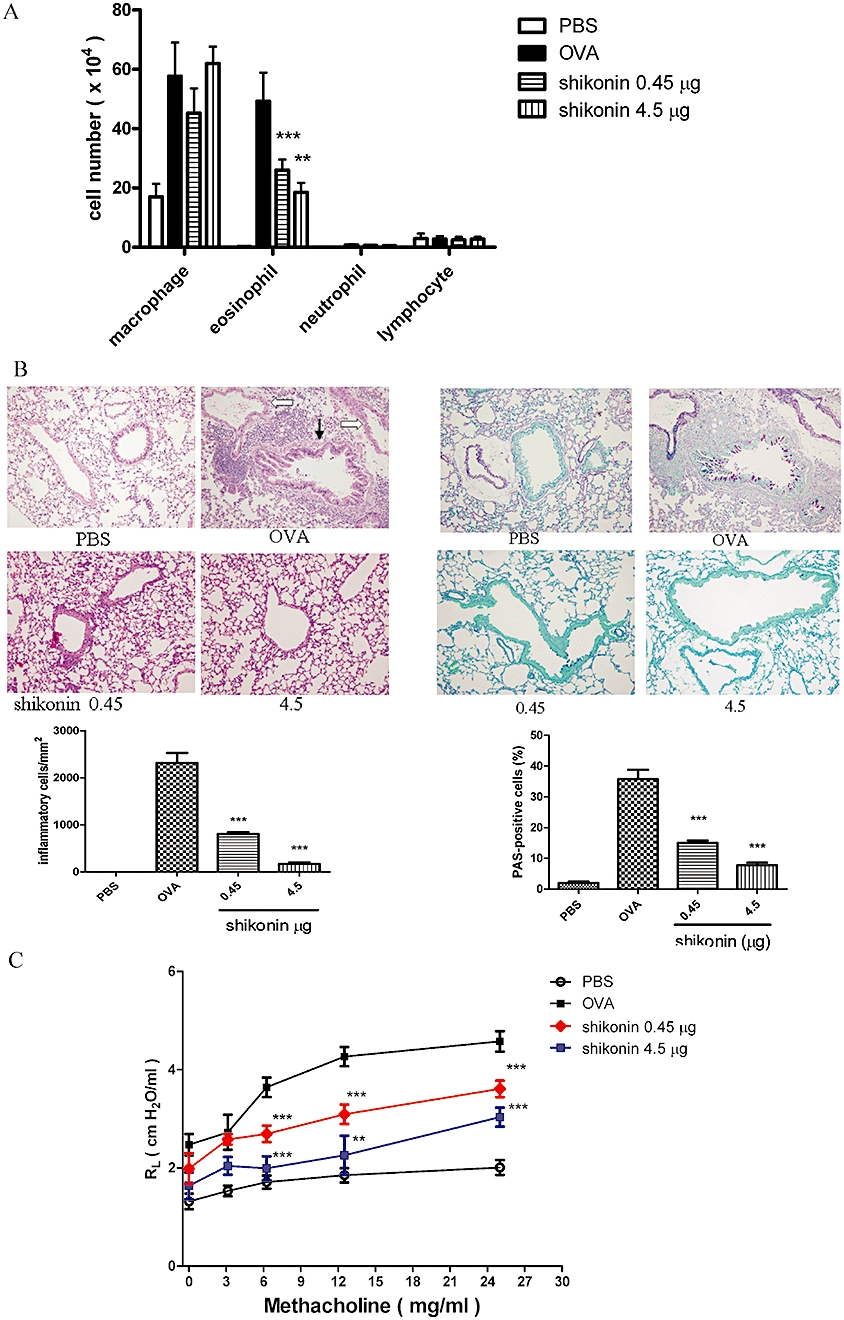
Airway inflammation and hyperresponsiveness inhibited by shikonin. Profile of cells in BALF and histological analysis of lung tissue in mice after shikonin treatment, shown as the daily dose (µg) given i.t. for 5 days. (A) Total cell counts were determined on 1 mL BALF and differential cell counts were assessed by Liu staining. Data were expressed as mean ± SEM (n ≥ 10) a*P < 0.05, different from the ovalbumin (OVA) group; b*P < 0.05 different from the PBS group. (B) Lung sections were stained with haematoxylin/eosin (H&E) for measurement of inflammatory cells and periodic acid-Schiff (PAS) for measurement of mucus production around the airways. Data revealed a different extent of cellular infiltration of the peri-airway region. Original magnification, ×200. Quantification of infiltration was by counting the number of inflammatory cells per mm2 of subepithelial and subendothelial area. Mucus-producing cells were quantified as the percentage of PAS-positive cells per bronchiole. Data were expressed as mean ± SEM (n = 5). ***P < 0.001, compared with the OVA group. In the H&E-stained lung section of the OVA group, the bronchiole is indicated with a black arrow and the vessel is indicated with a white arrow. (C) Airway resistance as measured by invasive body plethysmography. Data were expressed as mean ± SEM of the value of pulmonary resistance (RL) (n = 5). **P < 0.01; ***P < 0.001, versus the OVA group.
Histological examination of lung sections from the OVA group showed a large number of infiltrating inflammatory cells and increased mucus formation around the airway, compared to that in the PBS group (Figure 5B). In addition, we found that the peribronchial inflammation was more severe than the perivascular inflammation, as shown in the H&E-stained lung sections from the OVA group (Figure 5B). After treatment with shikonin, challenge of the OVA group showed clearly less inflammatory cell infiltration and mucus formation than in OVA mice without shikonin treatment.
Intratracheal delivery of shikonin suppresses the development of airway hyperresponsiveness
We investigated if shikonin could affect the development of airway hyperresponsiveness in a murine model of asthma. One day after the final challenge, airway responsiveness was assessed by pulmonary resistance using invasive body plethysmography. BALB/c mice sensitized and challenged with OVA showed increased lung resistance (RL) to MCh inhalation compared to PBS-sensitized and challenged mice (Figure 5C; OVA group and PBS group). After treatment with shikonin, RL levels were significantly inhibited compared with the untreated OVA group.
Shikonin did not affect allergen-specific and total immunoglobulin levels in serum
On day 43, mice were bled to examine serum immunoglobulin levels. Compared to the PBS group, OVA-specific and total serum IgE levels in OVA-immunized groups, with or without shikonin-treatment, were significantly increased (Figure 6). However, OVA-specific and total serum IgE levels were not altered by treating the OVA groups with shikonin at either dose (Figure 6). Also, no difference was observed in the OVA-specific and total serum IgG2a and IgG1, TH1 and TH2 response-related immunoglobulins.
Figure 6.
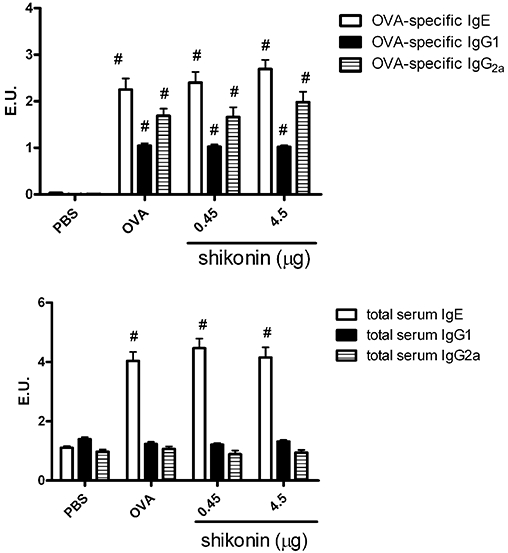
Serum levels of ovalbumin (OVA)-specific and total IgE, IgG1 and IgG2a in sensitized (OVA) and non-sensitized (PBS) mice. On day 43, blood samples were obtained, and OVA-specific and total IgE, IgG1, and IgG2a concentrations were measured by enzyme-linked immunosorbent assay (ELISA) (n = 6). Shikonin treatment is shown as the daily dose (µg) given i.t. for 5 days. No differences in serum levels of OVA-specific and total IgE, IgG1, and IgG2a were observed among the different groups of shikonin-treated mice. Data were expressed as mean ± SEM. #P < 0.05, different from the PBS group. Antibody levels were compared to standard serum, and IgG1, IgE and IgG2a concentrations in standard serum were arbitrarily assigned 1 ELISA unit (1 EU).
Discussion and conclusions
In this study, we used OVA + TSLP to activate BM-DCs into TH2-polarizing antigen-presenting cells. These cells expressed higher levels of surface markers (MHC II, CD80, CD86, CCR7 and OX40L) and stimulated OVA-immunized CD4+ T-cell proliferation and their release of IL-4, IL-5, IL-13 and TNF-α. This is the first study that has used OVA + TSLP-activated BM-DCs to provide a test system form compounds with therapeutic potential for asthma. Using DCs as a cell model to evaluate potential treatments in immune diseases has been widely applied in cancer (Ding et al., 2009), autoimmune disease (Baldwin et al., 2010) and allergic diseases (Lin et al., 2005). In this study, we found that shikonin showed therapeutic potential for allergic asthma through several mechanisms.
First, shikonin dose-dependently inhibited OVA + TSLP-induced BM-DC maturation by decreasing the cell surface expression of MHC class II molecules, the co-stimulatory molecules CD80, CD86, OX40L and CCR7. Unlike other DC stimuli, such as CD40L and toll-like receptor ligands, TSLP does not stimulate myeloid DCs to produce the polarizing cytokine IL-12 or the pro-inflammatory cytokines TNF-α, IL-1β and IL-6. TSLP triggers myeloid DCs to produce an array of different chemokines, including CCL11, IL-8 (CXCL8), thymus and activation regulated chemokine [TARC (CCL17)] and I-309 (CCL1), which are important for recruiting eosinophils, neutrophils and TH2 cells, respectively (Ito et al., 2005). OX40L was identified as a key molecule expressed by TSLP-activated DCs using microarray analyses. OX40L is expressed on DC surfaces and acts as a co-stimulatory signal for TH2 priming and memory induction. In addition, previous studies have demonstrated that DCs activated by TSLP can prime naïve CD4+ T-cells to differentiate into TH2 cells via OX40L (Ito et al., 2005) and produce chemokines that attract eosinophils, TH2 cells and neutrophils to induce innate allergic responses and cause additional adaptive allergic responses (Wang and Liu, 2009). In our study, we found that DCs, which had been pulsed with OVA + TSLP, activated CD4+ T-cells to release IL-4 and IL-5 and that this release was mainly dependent on OX40L. In addition, after knockdown of OX40L expression in DCs, IL-4 and IL-5 release in activated CD4+ T-cells was attenuated by shikonin treatment. This indicates that shikonin inhibited TH2 cytokine release in CD4+ T-cells possibly through other pathways whose elucidation needs further investigation.
In addition, we found that the levels of CD80 and CD86 expression for OVA + TSLP-treated DCs were twofold lower than 100 ng·mL−1 lipopolysaccharide-treated DCs, and there was no IL-12 released into the culture medium (data not shown). A previous study also found that allergen-fungal protease-treated DCs expressed lower levels of surface markers (CD80 and CD86) and produced less IL-12 (Lamhamedi-Cherradi et al., 2008). However, the mechanisms by which different extracellular stimuli that activate TH1-polarizing and TH2-polarizing DC maturations show different functional properties is still unknown and needs further investigation.
Second, shikonin-treated BM-DCs altered stimulation of T lymphocyte growth and differentiation. Shikonin dose-dependently inhibited OVA + TSLP-induced OVA-immunized CD4+ T-cell proliferation and TH2 cytokine release. In addition, we did not detect IFN-γ production, which could also account for the absence of IL-12 production in OVA + TSLP-stimulated BM-DCs.
Third, in a murine model of asthma, shikonin showed therapeutic potential in vivo by reducing TH2 cytokine release in BALF and lung and mediastinal lymph node cells, and by inhibiting airway inflammation and airway hyperresponsiveness. We found that shikonin did not inhibit T-cells, stimulated with (CD3 + CD28), from releasing IFN-γ in lung and mediastinal lymph node cells, which might indicate that shikonin specifically attenuates activation of TH2 cells induced by (CD3 + CD28). In addition, because serum total and OVA-specific IgE, IgG1 and IgG2a levels did not show any apparent changes after intratracheal instillation of shikonin, we suggest that intratracheal shikonin had a local effect in lung, but not a systemic effect, on allergic responses.
As in vitro studies have shown shikonin to inhibit allergic reactions, in terms of inhibition of LTB4 biosynthesis (Wang et al., 1994) and suppression of mast cell degranulation (Wang et al., 1995), shikonin might suppress airway inflammation in vivo through different mechanisms. In addition, from the data presented here, it is not possible to conclude that there is a direct link between the inhibition of allergic inflammation by shikonin in vivo and the in vitro effect upon DC maturation. Further investigation is needed. A previous study found that shikonin caused necrosis and apoptosis in HL-60 and K562 cell lines (Han et al., 2009). In our study, the highest shikonin dose was 30-fold lower than that used in the study of Han et al., and we did not observe any necrotic or apoptosis effects in BM-DCs. Thus, cell apoptosis or necrosis in vitro did not contribute to the effects we observed of shikonin in allergic asthma.
In conclusion, we have described a model of BM-DCs stimulated by OVA + TSLP as an experimental system to assess compounds for effects on the development of allergic diseases. We also demonstrated that shikonin effectively suppressed OVA + TSLP-induced BM-DC maturation in vitro and inhibited allergic inflammation and airway hyperresponsiveness in a murine model of asthma. Therefore, this study provides a novel platform for screening drugs for allergic diseases and shikonin was shown to have clear potential as a drug for allergic asthma.
Acknowledgments
This study was supported by the National Science Council in Taiwan (NSC 97-2320-B-039-007-MY3) and China Medical University (CMU 96-252 and 97-283).
Glossary
Abbreviations
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- DC
dendritic cell
- i.n.
intranasal
- i.t.
intratracheal
- OVA
ovalbumin
- TSLP
thymic stromal lymphopoietin
Conflict of interest
No conflicts of interest to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Shikonin suppressed IL-4 and IL-5 production in lung cells and mediaterial lymph node cells in a murine model of asthma. (A) Lung cells and mediaterial lymph node cells were isolated from naïve or OVA-immunized mice, and cells were counted by a hemocytometer. Lung cells (B) and mediaterial lymph node cells (C) were treated with 1 µg·mL−1 CD3 + 1 µg·mL−1 CD28, or 10 µg·mL−1 OVA, for 72 h, and medium was collected for investigation of cytokine production by ELISA. Data were expressed as mean ± SEM (n = 6). #P < 0.05, compared with the respective negative group; *P < 0.05; **P < 0.01; ***P < 0.001, compared with the respective positive group. n.d., not detected.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn.) 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HM, Ito-Ihara T, Isaacs JD, Hilkens CM. TNF{alpha} blockade impairs dendritic cell survival and function in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1200–1207. doi: 10.1136/ard.2009.110502. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Chang YS, Kuo SC, Weng SH, Jan SC, Ko FN, Teng CM. Inhibition of platelet aggregation by shikonin derivatives isolated from Arnebia euchroma. Planta Med. 1993;59:401–404. doi: 10.1055/s-2006-959718. [DOI] [PubMed] [Google Scholar]
- Chen X, Oppenheim JJ, Howard OM. Shikonin, a component of antiinflammatory Chinese herbal medicine, selectively blocks chemokine binding to CC chemokine receptor-1. Int Immunopharmacol. 2001;1:229–236. doi: 10.1016/s1567-5769(00)00033-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, et al. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110:1441–1448. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Seow SV, Huang CH, Liew LM, Lim YC, Kuo IC, et al. Coadministration of the fungal immunomodulatory protein FIP-Fve and a tumour-associated antigen enhanced antitumour immunity. Immunology. 2009;128(1) Suppl:e881–e894. doi: 10.1111/j.1365-2567.2009.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaab T, Mitzner W, Braun A, Ernst H, Hohllfeld JM, Krug N, et al. Repetitive measurements of pulmonary mechanics to inhaled cholinergic challenge in spontaneously breathing mice. J Appl Physiol. 2004;97:1104–1111. doi: 10.1152/japplphysiol.01182.2003. [DOI] [PubMed] [Google Scholar]
- Guo XP, Zhang XY, Zhang SD. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Zhong Xi Yi Jie He Za Zhi. 1991;11:598–599. [PubMed] [Google Scholar]
- Han W, Xie J, Li L, Liu Z, Hu X. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis. 2009;14:674–686. doi: 10.1007/s10495-009-0334-x. [DOI] [PubMed] [Google Scholar]
- Hayashi M. Pharmacological studies of shikon and tooki.(2) pharmacological effects of the pigment components, shikonin and acetylshikonin. Nippon Yakurigaku Zasshi. 1977;73:193–203. [PubMed] [Google Scholar]
- Hisa T, Kimura Y, Takada M, Suzuki F, Takigawa M. Shikonin, an ingredient of Lithospermum erythrorhizon, inhibits angiogenesis in vivo and in vitro. Anticancer Res. 1998;18:783–790. [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deghchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski CM, Hilkens A, Snijders FG. Snijdewint and M.L. Kapsenberg, Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Adv Exp Med Biol. 1997;417:363–367. doi: 10.1007/978-1-4757-9966-8_59. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Koyama Y, Tanaka J, Ohara A, Hayakawa T, Fujimoto S. Inhibitory effect of acetylshikonin on the activation of NADPH oxidase in polymorphonuclear leukocytes in both whole cell and cell-free systems. Biol Pharm Bull. 1996;19:1266–1270. doi: 10.1248/bpb.19.1266. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–4097. [PubMed] [Google Scholar]
- Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, et al. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180:6000–6009. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-L, Liang Y-C, Lee S-S, Chiang B-L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. J Leukoc Biol. 2005;78:533–543. doi: 10.1189/jlb.0804481. [DOI] [PubMed] [Google Scholar]
- Majima R, Kuroda C. Studies on the derivatives of naphthoquinones. XIII. Acta Phytochem (Tokyo) 1922;1:43–65. [Google Scholar]
- Miyabara Y, Yanagisawa R, Shimojo N, Takano H, Lim HB, Ichinose T, et al. Murine strain differences in airway inflammation caused by diesel exhaust particles. Eur Respir J. 1998;11:291–298. doi: 10.1183/09031936.98.11020291. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Ohno A, Saito Y, Satale M. Accelerative effect of shikonin, alkammin, and acetylshikonin on the proliferation of granulation tissue in rats. Biol Pharm Bull. 1994;17:1075–1077. doi: 10.1248/bpb.17.1075. [DOI] [PubMed] [Google Scholar]
- Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin and related naphthazarin natural products. Angew Chem Int Ed Engl. 1999;38:270–300. doi: 10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev. 1998;34:235–259. doi: 10.1016/s0169-409x(98)00042-8. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Bulic P, Lin Y, Dannenberg AJ, Pasco DS. Development and use of a gene promoter-vased screem to identify novel inhibitors of cyclooxygenase-2 transcription. J Biomol Screen. 2001;6:101–110. doi: 10.1177/108705710100600206. [DOI] [PubMed] [Google Scholar]
- Suen JL, Wu CH, Chen YY, Wu WM, Chiang BL. Characterization of self-T cell response and antigenic determinants of U1A protein with bone marrow-derived dendritic cells in NZB × NZW F1 mice. Immunology. 2001;103:301–309. doi: 10.1046/j.1365-2567.2001.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, et al. Cultured lung fibroblasts from ovalbumin-challenged asthmatic mice differ functionally from normal. Am J Respir Cell Mol Biol. 2007;37:424–430. doi: 10.1165/rcmb.2007-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueba N, Otaka T, Yamazaki K, Mori H, Morimoto M. Anti-HIV-1 activities of crude drug, shikon (Lithospermi radix), in vitro. Nippon Rinsho. 1993;51(Suppl):207–212. [PubMed] [Google Scholar]
- Wang Y-H, Liu Y-J. Thymic stromal lymphopoietin, OX40-ligand, an interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Bai JY, Liu DP, Xue LM, Zhu XY. The antiinflammatory activity of shikonin and its inhibitory effect on leukotrience B4 biosynthesis. Yao Xue Xue Bao. 1994;29:161–165. [PubMed] [Google Scholar]
- Wang JP, Raung SL, Chang LC, Kuo SC. Inhibition of hind-paw edema and cutaneous vascular plasma extravasation in mice by acetylshikonin. Eur J Pharmacol. 1995;272:87–95. doi: 10.1016/0014-2999(94)00627-j. [DOI] [PubMed] [Google Scholar]
- Wills-karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Otaka T, Mori H. Screening test of crude drug extract on anti-HIV activity. Yakugaku Zasshi. 1993;113:818–824. doi: 10.1248/yakushi1947.113.11_818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


