Summary
Dendritic cells (DCs) initiate and control the adaptive immune response against infections. However, their contributions to the anti-self adaptive immune response in autoimmune disorders like systemic lupus erythematosus are uncertain. By constitutively deleting DCs in MRL.Faslpr mice we show that they have complex roles in murine lupus. The net effect of DC deletion was to ameliorate disease. DCs were crucial for the expansion and differentiation of T cells but, surprisingly, not required for their initial activation. Correspondingly, kidney interstitial infiltrates developed in the absence of DCs, but failed to progress. DC deletion concomitantly decreased inflammatory and regulatory T cell numbers. Unexpectedly, plasmablast numbers and autoantibody concentrations depended on DCs, in contrast to total serum immunoglobulin concentrations, suggesting an effect of DCs on extrafollicular humoral responses. These findings reveal that DCs operate in unanticipated ways in murine lupus and validate them as a potential therapeutic target in autoimmunity.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with diverse clinical manifestations. Studies targeting B cells in both mice and humans have demonstrated the importance of B cells in promoting immune activation and tissue damage in lupus, partly by antibody-independent mechanisms (Chan et al., 1999a; Chan et al., 1999b; Looney, 2010). Activated T cells also play important roles, as disease is substantially reduced, though not eliminated, when T cells are absent or inhibited (Jabs et al., 1992; Jevnikar et al., 1994). In contrast, the role of dendritic cells (DCs) in the pathogenesis of SLE is largely unknown.
The contributions of DCs may be complicated, in part because there are numerous subsets of them (Geissmann et al., 2010). DCs could influence SLE in several ways: presentation of self-antigen to autoreactive T cells; secretion of proinflammatory cytokines; and promotion of B cell autoantibody production, either directly or indirectly. DCs are widely considered to be critical for initiating T cell responses in infections (Mellman and Steinman, 2001). Based on this notion, it could be assumed that they are the primary antigen presenting cells (APCs) to induce T cell autoimmunity. However, depending on their activation state DCs might also support peripheral T cell self-tolerance instead of T cell immunity (Hawiger et al., 2001). In one (Ohnmacht et al., 2009), but not another study (Birnberg et al., 2008), constitutive deletion of DCs on a non-autoimmune background elicited autoimmunity.
DCs could also boost disease by secreting inflammatory cytokines. For example, peripheral blood T cells from SLE patients produce larger amounts of IFN-γ than those from healthy individuals (Harigai et al., 2008) and genetic deletion of IFN-γ or its receptor in MRL.Faslpr mice ameliorates disease (Balomenos et al., 1998; Schwarting et al., 1998). Activated cDCs secrete IL-12-p70 which elicits the production of IFN-γ by NK and T cells and promotes the differentiation of naïve T helper cells into Th1 effectors. Furthermore, type I IFNs produced by pDCs lead to cDC maturation and lower the activation threshold for toll-like receptor agonists.
Aside from priming of CD4+ T cells that in turn promote the anti-self B cell response, DCs can also affect the autoreactive B cell response directly. DCs possess a non-degradative antigen uptake pathway that facilitates interaction of the B cell receptor with whole antigen on the DC surface (Qi et al., 2006; Bergtold et al., 2005). The importance of this mechanism has yet to be elucidated, but the finding implies enhancement of humoral responses by direct DC-B cell interactions. Moreover, DCs are important sources of B cell activating factor of the TNF-family (BAFF) and a proliferation-inducing ligand (APRIL), which are implicated in promoting B cell survival and plasmablast differentiation, as well as regulating self-tolerance by influencing survival of anergic B cells (Treml et al., 2009).
The relative importance of DCs compared to other APCs in generating anti-self T cell immunity cannot necessarily be inferred from their dominance in inducing anti-pathogen T cell immunity because of potential roles of B cells as APCs. While classically it was thought that B cells mainly contribute to lupus expression by production of pathogenic autoantibodies, multiple studies in mice and patients have suggested that APC function of B cells is critical in promoting disease. When B cells are depleted genetically, T cell activation is inhibited in the murine models of lupus MRL-MpJ-Faslpr (called MRL.Faslpr hereafter) and Fas-intact MRL (Chan et al., 1999b). This function of B cells is antibody-independent, as T cell activation is mainly intact in MRL.Faslpr mice that lack soluble antibodies but have B cells (Chan et al., 1999a). Most notably, B cell-deficient lupus-prone mice have virtually no residual disease, such as nephritis, vasculitis or dermatitis.
Given the functions of DCs in both promoting T cell immunity as well as establishing T cell tolerance, along with the uncertain importance of their APC function relative to B cells in activating autoreactive T cells, it is hard to predict how DCs influence disease. However, some clues that DCs are key players in autoimmunity come from studies of hyperactive or unregulated DCs in C57BL/6 mice. The adoptive transfer of bone marrow-derived DCs deficient for suppressor of cytokine signaling-1 (SOCS1) (Hanada et al., 2003) or DCs bearing the Sle3 susceptibility locus (Zhu et al., 2005) into C57BL/6 mice led to marked production of anti-nuclear antibodies (ANA) when coupled with LPS co-administration. C57BL/6 mice with a DC-specific deletion of Fas showed ANA generation and liver lesions (Stranges et al., 2007). However, although these studies have provided insights, they demonstrate the potential of DCs to cause disease when they are intentionally and selectively dysregulated, rather than what their non-redundant functions are in a polygenic, spontaneous autoimmune disease setting. The latter requires a subtractive approach by deleting DCs in a murine polygenic model of lupus.
Here, we sought to delete DCs in the MRL.Faslpr model of lupus. Several systems for DC deletion are available, each with different advantages (recently reviewed in Bar-On and Jung, 2010). We chose a constitutive deletion approach as most appropriate for the chronic, progressive nature of lupus to answer three related questions: (1) What is the overall effect of DC function in lupus? (2) At what stages of lupus pathogenesis are DCs required? (3) In what way do DCs affect the spontaneous T and B cell responses in lupus?
Results
DC depletion in lupus-prone mice
We generated DC-deficient mice (called CD11c:DTA hereafter) on the MRL.Faslpr lupus-prone genetic background. Analogous to the situation in human SLE, multiple genes with disease-associated alleles are expressed in a variety of cell lineages and act in concert to produce disease in MRL mice (Cheung et al., 2009). The Faslpr allele further contributes by accelerating and accentuating disease progression. We then compared CD11c:DTA mice with their littermate controls. Deletion efficiency of DCs was assessed by FACS analysis (Figure S1). In CD11c:DTA mice over 92% of cDCs and 86% of pDCs were depleted in the spleen (Table S1). In mesenteric lymph nodes, bone marrow and thymus (only cDCs) depletion had about the same efficiency (Table S1). Inspection of FACS plots (Figure S1) revealed that in CD11c:DTA mice, remaining events were on the border of the drawn gates and thus many of them may represent background rather than bona fide residual DCs.
The effect of constitutive DC depletion on central T cell tolerance is controversial (Birnberg et al., 2008; Ohnmacht et al., 2009). In our model, negative selection of T cells was unimpaired as determined by normal deletion of T cell receptor Vβ5- and Vβ11-bearing T cell subsets (data not shown). Metallophilic and marginal zone macrophages were not deleted in CD11c:DTA mice (Figure S1) in contrast to what has been reported for the inducible CD11c-DTR system (Probst et al., 2005). Pre-autoimmune 5 wk old CD11c:DTA mice showed an expansion of F4/80+ macrophages (spleen) and neutrophils (spleen and axillary lymph nodes), which was previously described in constitutively DC-deficient mice on non-autoimmune backgrounds (Birnberg et al., 2008; Ohnmacht et al., 2009) (Figure S1). Together, these findings indicate that the CD11c:DTA system efficiently deletes DCs on the MRL.Faslpr background without affecting thymic negative selection of T cells.
DCs promote glomerular and interstitial nephritis
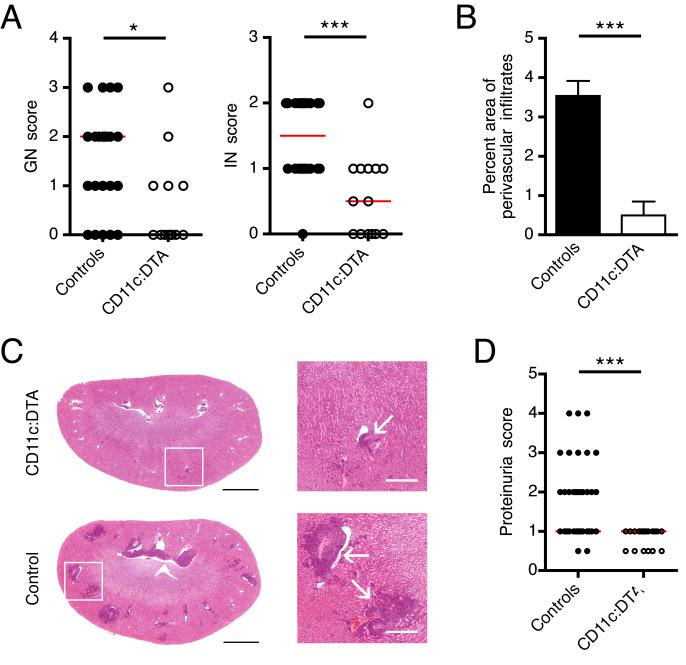
Renal involvement becomes clinically apparent in 50% of SLE patients. In most MRL.Faslpr mice it is present at 16 weeks of age. To assess whether DCs contribute to nephritis, we scored severity of glomerular (GN) and interstitial nephritis (IN) in the peritubular and perivascular region. In all locations inflammation was markedly reduced (Figure 1A). In particular we found a dramatic decrease in the number and size of perivascular infiltrates, which we separately quantitated by calculating the percentage area of the infiltrates (Figure 1B and 1C). We next tested whether the histopathological differences between CD11c:DTA and control mice reflected kidney function. Indeed, DC-deficient mice had markedly less proteinuria than littermate controls, reaching only a maximum score of 1+ (Figure 1D). These results indicate that DCs contribute to GN and IN in lupus.
Figure 1. Ameliorated lupus nephritis in DC-deficient mice.
(A) Glomerular and interstitial (peritubular and perivascular) renal disease were scored from 0 to 4 for control and CD11c:DTA mice. Each dot represents an individual mouse. Horizontal lines represent the median.
(B) The area of perivascular infiltrates expressed as a percentage of the total kidney section area is plotted for control (n = 24) and CD11c:DTA (n = 14) mice. Data are represented as mean ± SEM.
(C) Representative H&E stained kidneys sections (left, scale bar = 2 mm) and magnified details of white bordered area of both kidney sections (right, scale bar = 500 μm). Arrows indicate perivascular infiltrates.
(D) Proteinuria for control and CD11c:DTA mice. Each dot represents an individual mouse. Horizontal lines represent the median.
Statistics were calculated by two-tailed Mann-Whitney U test. *p < 0.05; ***p < 0.001.
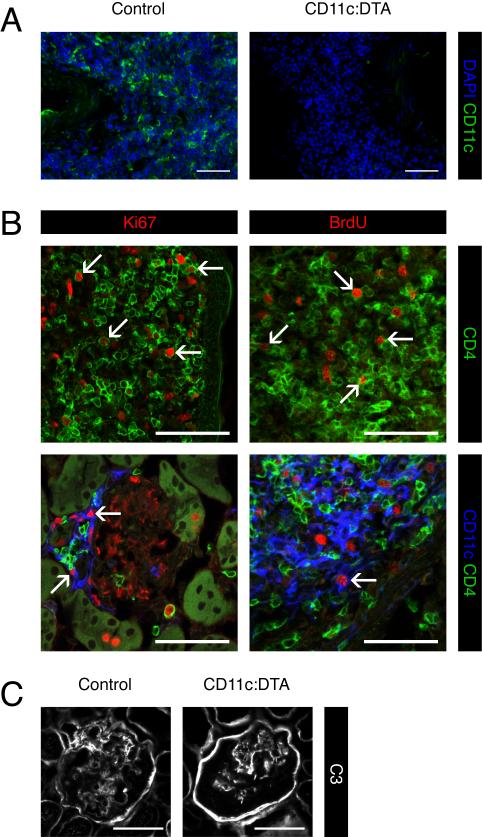
However, many DC-deficient mice still developed milder forms of GN and IN. This suggested that either depletion of DCs was incomplete in the kidneys or that DCs regulate the extent rather than the occurrence of nephritis. To address the first possibility, we used immunofluorescence microscopy to detect DCs in interstitial infiltrates. In control mice a sizable proportion of the cells in interstitial infiltrates were CD11c+ (Figure 2A). We hardly found any CD11c+ cells in the residual infiltrates in CD11c:DTA mice, suggesting that DC deletion in the kidneys was effective (Figure 2A). Beside DCs, T cells are a major constituent of kidney infiltrates in MRL.Faslpr mice and human lupus patients. We asked whether local proliferation of T cells contributed to the development of infiltrates. Indeed, many CD4+ T cells in the interstitial and periglomerular infiltrates were dividing, as demonstrated by Ki67 staining and 5-bromo-2-deoxyuridine (BrdU) staining after a 2 hr BrdU single-time pulse (Figure 2B). We could also detect proliferating CD11c+ cells (Figure 2B) consistent with the notion that DCs can undergo a limited number of divisions in non-lymphoid tissues. Thus, DCs are instrumental in the marked expansion of kidney lesions, but are not required for their establishment. Infiltrate expansion involves local proliferation of T cells in addition to their recruitment.
Figure 2. Local proliferation of CD4+ T cells and DCs in kidney infiltrates.
(A) Staining for DAPI (blue) to locate nuclei and CD11c (green) to identify DCs on kidney cryosections. Shown are perivascular infiltrates. Data are representative for kidneys from 3 mice of each type.
(B) Confocal images of kidney infiltrates. CD4+ T cells and DCs were detected by staining for CD4 (green) and CD11c (blue) respectively. Dividing cells were identified by staining for Ki67 or BrdU after a 2 hr BrdU i.p. single-time pulse (red). The bottom left image shows a periglomerular infiltrate and the other images perivascular infiltrates. Arrows indicate examples of dividing cells. Data are representative for kidneys from 3 wild type MRL.Faslpr mice.
(C) Staining for complement C3 (white) to detect glomerular immune deposits. Data are representative for kidneys from 10 mice of each type.
Scale bars = 50 μm in all pictures.
Conventionally, immune complexes deposited in glomeruli are believed to be the primaxry mediators of GN. We used immunofluorescence microscopy to determine the amount of immune complex deposition in the glomeruli. Although GN and IN were ameliorated in the absence of DCs we did not detect any differences in complement factor C3 or IgG deposition in the glomeruli (Figure 2C and data not shown). These results imply that immune complexes per se are insufficient to cause GN and IN and that end-organ responses to the deposits are a critical factor. Collectively, these findings demonstrate that although DCs are not required for the initial development of GN and IN, they play a substantial role in the progression of nephritis.
DCs other than LCs contribute to dermatitis
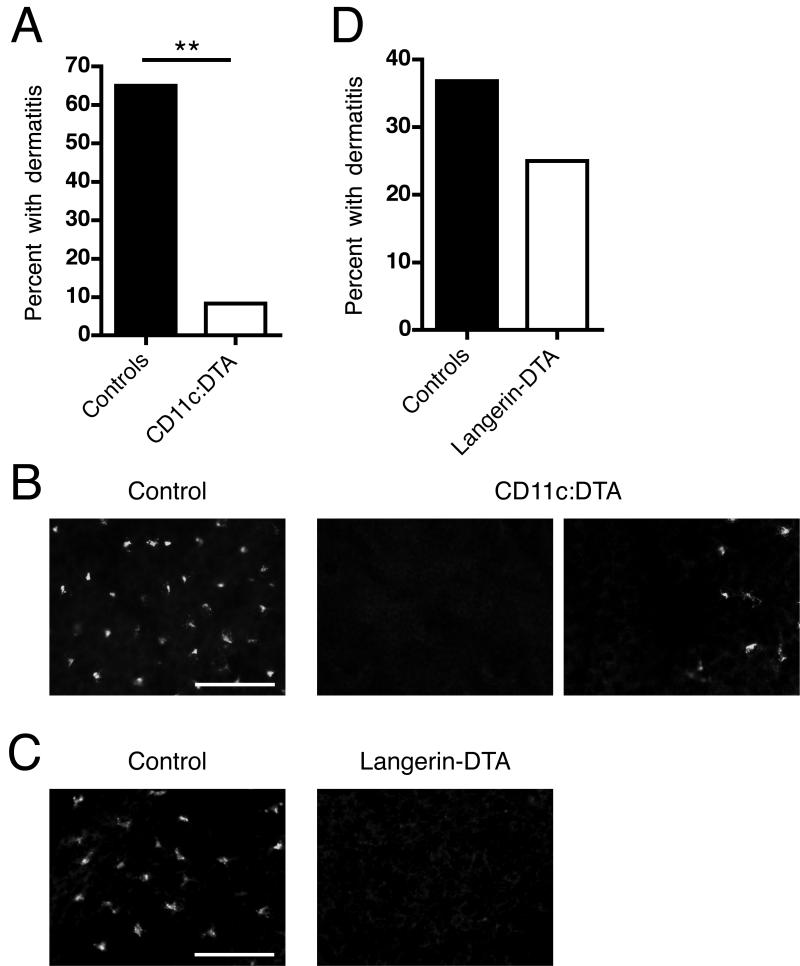
Dermatitis is a common manifestation of human SLE and among murine lupus models is uniquely found in the MRL and MRL.Faslpr strains. We assessed whether DC depletion would affect dermatitis. Because in MRL.Faslpr females develop dermatitis more frequently than males, we restricted our analysis to females. Thirteen of 20 control mice developed dermatitis but only 1 of 12 CD11c:DTA mice was affected (Figure 3A, p < 0.01), demonstrating that DCs are important for the development of dermatitis.
Figure 3. DCs other than LCs contribute to dermatitis.
(A) Percentage of female control (n = 20) and CD11c:DTA (n = 12) mice with dermatitis at 16 wk of age.
(B) Epidermal sheets from control and CD11c:DTA mice were stained for I-A/I-E (mouse MHC class II) to identify LCs (red). The images in the middle and on the right side represent 2 visual fields from the same sample to better illustrate that, rarely, small Langerhans cell clusters were detectable in CD11c:DTA mice. Data are representative of epidermal sheets from 3 mice of each type. Scale bar = 50 μm.
(C) Percentage of female control (n = 19) and Langerhans-DTA (n = 16) mice with dermatitis until death. The mean survival of Langerin-DTA mice was 145.2 days and of control mice 141.7 days.
D) Epidermal sheets from control and Langerin-DTA mice stained as in (B). Data are representative of epidermal sheets from 3 mice of each type. Scale bar = 50 μm. Statistics were calculated by Fisher’s exact test. **p < 0.01.
There are at least four DC subpopulations in the skin: LCs in the epidermis; pDCs, CD11b+ DCs and CD11b−CD103+Langerin+ DCs in the dermis (Heath and Carbone, 2009; Nestle et al., 2009). During inflammation the skin can also be infiltrated by Tip-DCs (De Trez et al., 2009). Reports differ on whether DTA expression in CD11c+ cells results in ablation of LCs (Birnberg et al., 2008; Ohnmacht et al., 2009). In our model, LCs were depleted in most areas of the epidermal sheets that we examined, with only rare small clusters of residual cells (Figure 3B). To determine whether the marked reduction in dermatitis prevalence was due to the loss of LCs alone vs other DC types, we generated MRL.Faslpr F2 mice which express DTA under the control of the Langerin promoter (Kaplan et al., 2005). In these mice, as reported on the FVB/N and C57BL/6 backgrounds (Igyarto et al., 2009; Kaplan et al., 2005), LCs are deleted (Figure 3C), but other known populations of DCs, including Langerin+ dermal DCs, are unaffected. There was no substantial difference in the prevalence of dermatitis between Langerin-DTA MRL.Faslpr F2 mice and littermate controls (Figure 3D). We conclude that LCs are dispensable but that other DC types contribute considerably to dermatitis development.
Impaired IgG and IgM autoantibody formation in DC-deficient mice
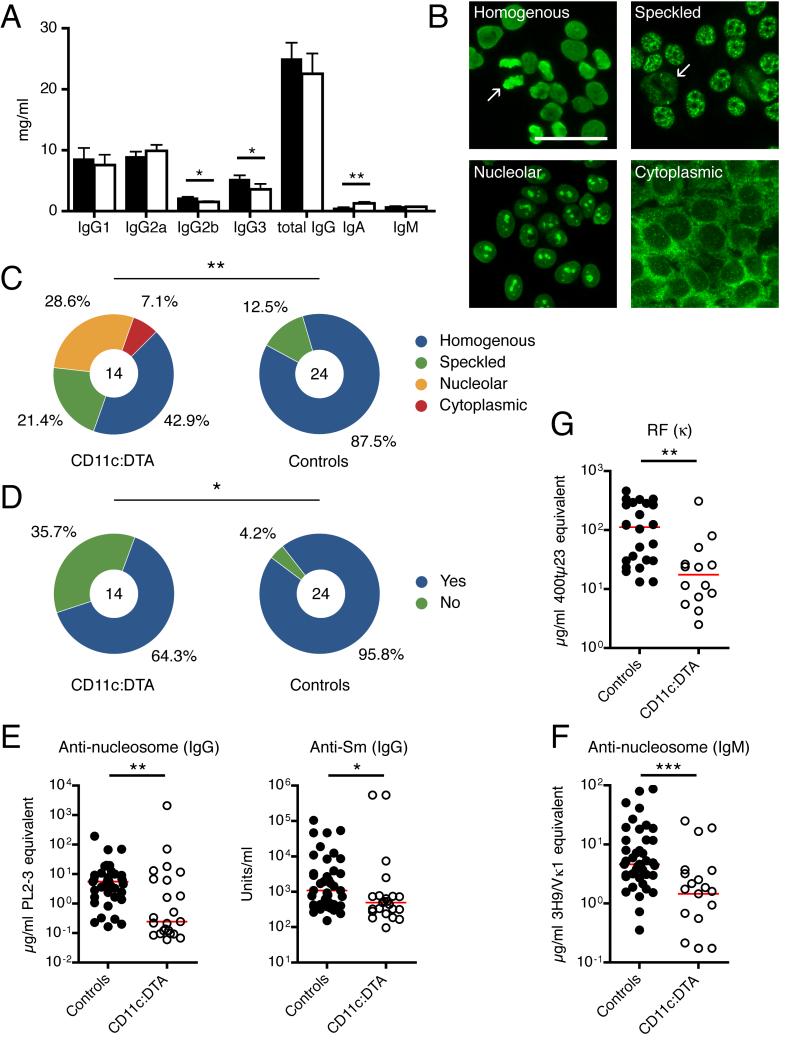
To understand the mechanisms by which DCs contribute to end-organ damage in lupus we asked how DC deletion affects the anti-self adaptive immune response. First we examined the adaptive humoral immune system. We observed slight drops in serum IgG2b and IgG3 concentrations and a slight increase for IgA in DC-deficient mice, but total serum IgG and IgM concentrations were unchanged (Figure 4A). Despite the similar overall IgG and IgM concentrations, we considered it possible that DCs have an effect on the production of autoantibodies, many of which derive from short-lived plasmablasts (Hoyer et al., 2004; William et al., 2002) rather than long-lived plasma cells that contribute most of the serum Ig (Slifka et al., 1998). To assess this, we first used the HEp-2 cell-based immunofluorescent microscopy assay to detect IgG anti-nuclear and anti-cytoplasmic antibodies. Typical for MRL.Faslpr mice, 21 of 24 (87.5%) sera from control mice produced a homogenous staining pattern, corresponding to anti-DNA and anti-chromatin IgG (Figure 4B and Figure 4C). Moreover, 23 of 24 (95.8%) control sera showed equatorial staining of mitotic chromatin, indicating specific chromatin IgG autoantibodies (Figure 4B and 4D). In contrast, only 6 of 14 (42.9%) sera from CD11c:DTA mice generated a homogenous staining pattern, with the emergence of additional patterns such as nucleolar and cytoplasmic that were rarely seen in DC-intact animals (Figure 4C, p < 0.01); similarly, only 9 of 14 (64.3%) CD11c:DTA sera produced IgG autoantibodies that bind mitotic chromatin (Figure 4D, p < 0.05). In a separate cohort of less backcrossed mice (CD11c-Cre MRL.Faslpr BC10 × Rosa26-eGFP-DTA MRL.Faslpr BC5), which developed a milder disease (data not shown), half of the sera from control mice demonstrated a homogenous staining pattern, but none of the sera from DC-deficient mice did so (Figure S2). These results indicate that DCs influence both autoantibody concentrations and composition.
Figure 4. Impaired generation of autoantibodies in the absence of DCs.
(A) Serum Ig isotype concentrations of control (n = 23) and CD11c:DTA (n = 14) mice. Data are represented as mean ± SEM.
(B) Illustration of Hep-2 ANA staining patterns from MRL.Faslpr mice. Arrows indicate the presence (left upper image) or the absence (right upper image) of mitotic chromatin staining. Scale bar = 50 μm.
(C and D) ANA staining pattern classified as homogenous, speckled, nucleolar or cytoplasmic (C); and mitotic chromatin staining classified as positive or negative (D), produced by sera from control and CD11c:DTA mice. The number in the circles indicate the number of total mice analyzed.
(E—G) Serum concentrations of anti-nucleosome IgG and anti-Sm IgG (E), antinucleosome IgM (F) and rheumatoid factor κ (G). Each dot represents an individual mouse. Horizontal lines represent the median.
Statistics were calculated by either Chi square analysis (C), Fisher’s exact test (D) or two-tailed Mann-Whitney U test (E—G). *p < 0.05, **p < 0.01, ***p < 0.001.
To quantitate serum IgG autoantibodies against nucleosomes and Sm protein (part of the RNA splicing complex) we performed ELISAs. Both anti-nucleosome and anti-Sm IgG were markedly decreased in the absence of DCs (Figure 4E). The reduction was more pronounced for anti-nucleosome (−95.6%) than for anti-Sm (−54.3%) IgG. To investigate whether the reduction of IgG autoantibodies was solely the result of impaired class switching we measured anti-nucleosome IgM by ELISA. We detected considerably less anti-nucleosome IgM in sera from DC-deficient mice than in sera from control mice (−68.4%) (Figure 4F). Additionally, we found rheumatoid factor concentrations, using anti-κ as the detection reagent, to be decreased 84.4% in sera from CD11c:DTA mice (Figure 4G) compared to controls. Therefore, DCs substantially promote the generation of antibodies against self-antigens, and particularly against chromatin-containing autoantigens.
Plasmablast numbers and class switching are decreased in the absence of DCs
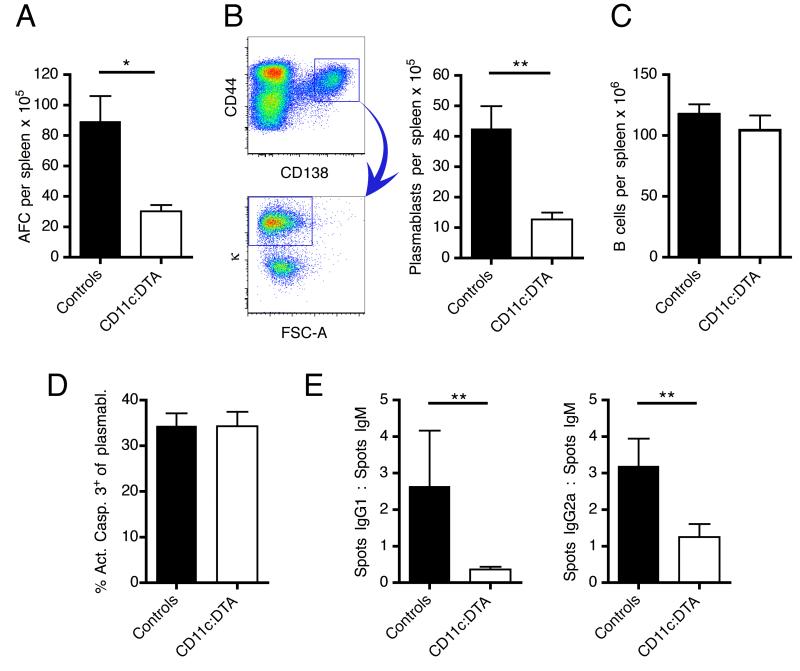
Short-lived plasmablasts are a major source of autoantibodies in MRL.Faslpr mice and in other lupus mouse models (Hoyer et al., 2004; William et al., 2002). They originate from B cell responses at extrafollicular loci, whereas long-lived plasma cells derive from germinal centers. Consistent with the decrease in autoantibody concentrations, the number of splenic antibody forming cells was substantially reduced in DC-deficient mice, as assessed by ELISpot assays (Figure 5A). We obtained corresponding results by flow cytometry, identifying plasmablasts as CD138+CD44+intracellular-kappahi cells (Figure 5B). Importantly, there was no difference in B cell numbers between DC-deficient and control mice (Figure 5C), suggesting that DC deletion does not impair B cell homeostasis. The apoptosis rates of plasmablasts were similar in both groups (Figure 5D). It is therefore most likely that the reduced numbers of plasmablasts in CD11c:DTA mice result from impaired plasmablast generation. The unaltered total serum IgG and IgM concentrations indicate that long-lived plasma cells, which mainly reside in the bone marrow, were unaffected by the deletion of DCs.
Figure 5. Reduced plasmablast numbers and class switching in CD11c:DTA mice.
(A) Number of κ light chain antibody forming cells (AFC) per spleen determined by ELISpots. n = 14 for control mice and n = 13 for CD11c:DTA mice.
(B) Numbers of plasmablasts (CD138+CD44+intracellular-kappahi, after gating on EMA− TCRβ− cells) per spleen in control (n = 6) and CD11c:DTA (n = 6) mice. The pseudocolor plots on the left side illustrate the last 2 gating steps.
(C) Numbers of B cells (CD22hiCD19hi) per spleen in control (n = 24) and CD11c:DTA (n = 14) mice.
(D) Apoptosis of plasmablasts assessed by staining for active caspase-3.
(E) ELISpot assays for IgG1, IgG2a and IgM secreting cells in the spleen were performed. Numbers of IgG1 and IgG2a spots were normalized to numbers of IgM spots to reflect the selective effect on switching that DC deletion might have. n = 14 for control mice and n = 13 for CD11c:DTA mice.
Data are represented as mean ± SEM. Statistics were calculated by two-tailed Mann-Whitney U test. *p < 0.05, **p < 0.01.
Notably, there was a ~23-fold difference for anti-nucleosome IgG but only a ~3-fold difference for anti-nucleosome IgM between CD11c:DTA and control mice (Figure 4E and 4F). A possible explanation for this was that polyreactive IgM antibodies caused more background in the IgM specific ELISA, partly obscuring the impact of DC deletion on IgM anti-nucleosome formation. Alternatively, it was also possible that there is a selective effect of DCs on class switching. ELISpots specific for IgG1, IgG2a and IgM antibody forming cells revealed that indeed class switching, as assessed by the ratio of IgG1:IgM and IgG2a:IgM spots, was partly blocked in splenic plasmablasts (Figure 5E) in CD11c:DTA mice. Taken together, these results demonstrate that DC deletion impairs plasmablast generation along with class switching. As a consequence, serum IgM, and even more so IgG, autoantibody concentrations are reduced.
DCs are critical for T cell expansion but not activation
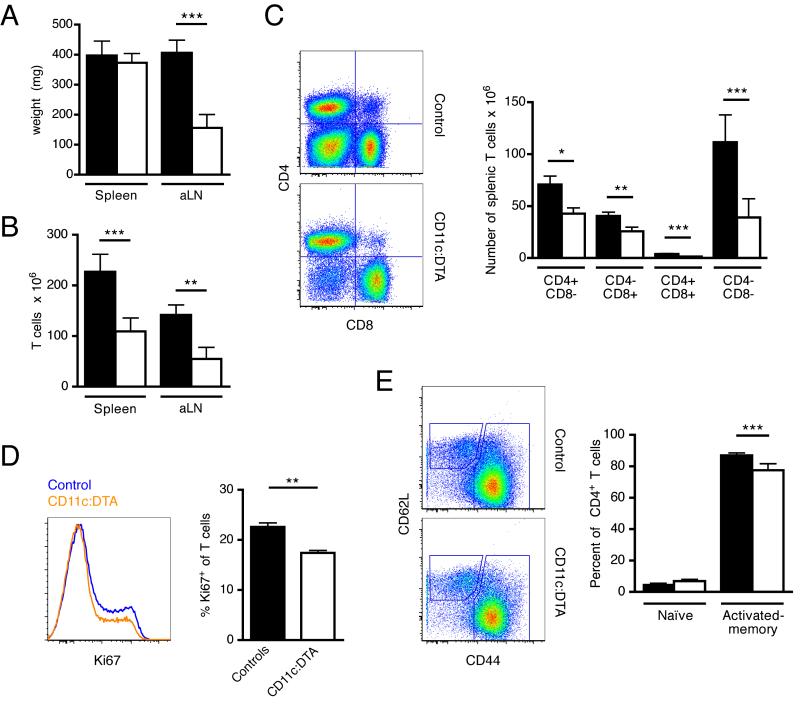
Next, we investigated the effect of DC deletion on T cell immunity in lupus. At 16 weeks of age MRL.Faslpr mice develop a severe lymphadenopathy and splenomegaly caused by the accumulation of T cells. Those T cells in large part express the B cell marker B220 and are negative for the coreceptors CD4 and CD8. Double negative T cells are thought to be derived from activated CD8+ T cells which in lpr mice cannot be removed in a Fas-FasL dependent fashion (Mehal and Crispe, 1998). They are considered to be mainly inert. Axillary lymph node weight was drastically reduced in the absence of DCs (Figure 6A). Spleen weight was about the same in DC-deficient and control mice (Figure 6A). On a non-autoimmune background, DC-deficient mice have a higher average spleen weight than littermate controls because constitutive deletion of DCs leads to the expansion of macrophages and neutrophils (Birnberg et al., 2008; Ohnmacht et al., 2009). Thus, a similar spleen weight in CD11c:DTA and control MRL.Faslpr mice likely reflected a balance between increased numbers of myeloid cells and reduced T cell accumulation in DC-deficient mice. Indeed, FACS analysis confirmed lower T cell numbers in the axillary lymph nodes and spleen in mice lacking DCs (Figure 6B). Among the CD4+, CD8+, CD4+CD8+ and double negative T cell subsets, the double negative population exhibited the greatest relative and absolute decrease (Figure 6C). The dampened T cell expansion in the absence of DCs was associated with a smaller fraction of proliferating T cells, as shown by Ki67 staining (Figure 6D). Surprisingly, the effect of DC deletion on spontaneous CD4+ T cell activation was very modest (Figure 6E). Activated - memory CD4+ T cells as a percentage of total CD4+ T cells were only slightly decreased in 16 wk old CD11c:DTA mice compared to littermate controls. There was no appreciable difference for naïve CD4+ T cells between both groups (Figure 6E), being very low in both cases. In mice at an early stage of disease (12 wk) there was a small increase in naïve CD4+ T cells in DC-deficient animals, but about 70% of CD4+ T cells still had an activated-memory phenotype (Figure S3). These observations are consistent with efficient activation of naïve T cells even in the absence of DCs. Thus, T cell expansion but not initial activation substantially depends on DCs in MRL.Faslpr mice.
Figure 6. DCs are critical for T cell expansion but not activation.
(A) Weight of spleens and the two largest axillary lymph nodes of control (black bars) and CD11c:DTA (white bars) mice.
(B—C) Cell numbers per spleen of control (black bars) and CD11c:DTA (white bars) mice for total T cells (B) or the T cell subsets CD4+, CD8+, CD4+CD8+ and double negative (C). Panels on the left show sample flow cytometric data.
(D) T cell proliferation determined by Ki67 staining. The histogram on the left shows representative examples of Ki67 staining of gated EMA−TCRβ+ cells.
(E) CD44 and CD62L staining of CD4+ T cells of 16 wk old control (black bars) and CD11c:DTA (white bars) mice to identify naïve (CD44loCD62Lhi) and activated-memory (CD44hi) subpopulations. Representative flow cytometric data on gated CD4+ T cells is shown on the left.
Data in bar graphs are represented as mean ± SEM. (A—C) n = 24 for control mice and n = 14 for CD11c:DTA mice. (D—F) n = 12 for control mice and n = 9 for CD11c:DTA mice. Statistics were calculated by two-tailed Mann-Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001.
Reduced Treg proliferation and survival in the absence of DCs
Several groups have reported decreased numbers and or inhibitory function of Tregs in SLE patients, although not with unanimity (Horwitz, 2008). Treg numbers are reported to increase in SLE patients upon treatment with corticosteroids, plasmapheresis and rituximab (Horwitz, 2008). On the basis of these findings, ameliorated disease in CD11c:DTA mice suggested that there might be higher Treg numbers in DC-deficient animals than in controls. However, it has recently been proposed that DCs control the homeostatic proliferation of Tregs (Darrasse-Jèze et al., 2009). In that model decreased DC numbers led to decreased Treg numbers, although another study found that DC depletion had no influence on number and function of Tregs in the steady state (Birnberg et al., 2008). Hence, the effect of DC deletion on Tregs in murine lupus was uncertain.
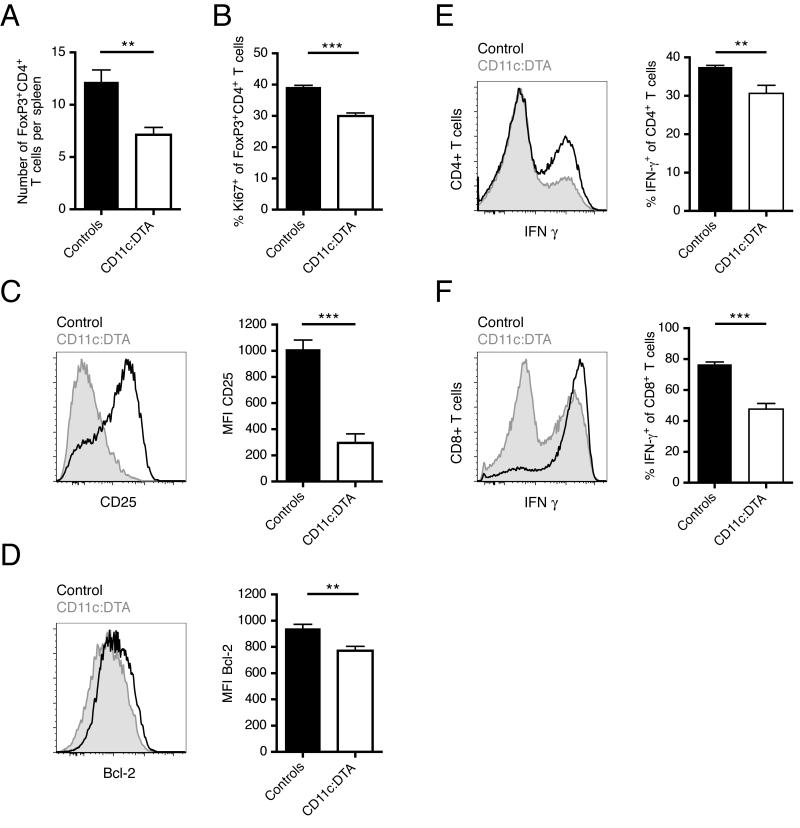
Numbers of Tregs (CD4+FoxP3+) in the spleen were markedly decreased in DC-deficient mice (Figure 7A). Moreover, Ki67 staining revealed that Tregs proliferated less in the absence of DCs (Figure 7B). This is consistent with a model in which DCs stimulate Treg proliferation, either directly or indirectly. We further observed that expression of the IL-2 receptor constituent CD25 was reduced on CD4+FoxP3+ T cells derived from DC-deficient mice (Figure 7C). IL-2 is critically important for the development, expansion and survival of natural and induced Tregs (Malek, 2008). To examine whether IL-2 receptor signaling might be reduced in Tregs from DC-deficient mice, we examined the expression of the IL-2 induced anti-apoptotic protein Bcl-2. We found that Bcl-2 was indeed downregulated in Tregs of CD11c:DTA mice (Figure 7D). Together, these observations indicate that DCs induce Treg proliferation and support their survival under inflammatory conditions.
Figure 7. DCs drive differentiation of CD4+ and CD8+ T cells into IFN-γ producing effectors and support for Treg proliferation and survival.
(A) Numbers of CD4+FoxP3+ T cells per spleen.
(B) Proliferation of CD4+FoxP3+ T cells determined by Ki67 staining.
(C) CD25 expression of CD4+FoxP3+ T cells.
(D) Expression of intracellular Bcl-2 of CD4+FoxP3+ T cells.
(E—F) Intracellular IFN-γ staining of PMA/ionomycin stimulated splenocytes gated on CD4+ (E) or CD8+ (F) T cells.
The histograms in (C—F) show representative flow cytometric data from control (black line) and CD11c:DTA (gray shaded) mice. Data in bar graphs are represented as mean ± SEM. n = 12 for control mice and n = 9 for CD11c:DTA mice. Statistics were calculated by two-tailed Mann-Whitney U test. **p < 0.01, ***p < 0.001.
Spontaneous differentiation of CD4+ and CD8+ T cells into IFN-γ producing effectors is DC-dependent
In human SLE peripheral blood T cells secrete large amounts of IFN-γ and have higher mRNA expression of the Th1 transcription factor T-bet (Harigai et al., 2008). In MRL.Faslpr mice IFN-γ hyperproduction and increased Th1 cell numbers are a consistent finding (Prud’homme et al., 1995). Disruption of the Ifng gene in MRL.Faslpr mice ameliorates disease (Balomenos et al., 1998). We asked whether DCs not only promote T cell proliferation but also help CD4+ T cell differentiation into Th1 effectors. After stimulation of total splenocytes for 4 hours with phorbol myristate acetate (PMA) and ionomycin, we stained for intracellular IFN-γ. The frequency of IFN-γ producing cells among CD4+ T cells derived from CD11c:DTA mice was decreased compared to those from controls (Figure 7E). Moreover, a smaller fraction of CD8+ T cells from DC-deficient animals produced IFN-γ (Figure 7F). Thus, DCs contribute considerably to the hyperproduction of IFN-γ by promoting Th1 priming and the differentiation of CD8+ T cells into IFN-γ secreting cells.
Discussion
The cellular mechanisms that promote and regulate autoimmune disease have been the subject of intense interest and study. B cell targeted therapies ameliorate disease severity in murine and several human autoimmune disorders (Ahuja et al., 2007; Dörner et al., 2009; Yanaba et al., 2008) As antibody-independent mechanisms have gained support in murine and patient studies, antigen presentation by B cells and direct T cell-mediated tissue damage have come to the forefront of disease pathogenesis concepts. However, the role of the DC, considered the primary APC in the immune system for pathogen recognition, has yet to be evaluated in spontaneous, systemic autoimmunity. Here we have addressed this question with a genetic system that has also recently been used to evaluate induced immune responses (Ohnmacht et al., 2009). Almost all cDCs and most pDCs were deleted in CD11c:DTA MRL.Faslpr mice. A caveat of ablation systems that use the promoter of the Itgax gene is that it might be active in subpopulations of cell types that are not DCs. We did survey other potentially affected populations but did not notice any differences attributable to the expression of CD11c:DTA; however, we cannot rule out subtle effects on non-target populations.
Although DCs promoted accumulation of T cells in secondary lymphoid tissues, the proportion of T cells that maintained a naïve phenotype was small, and barely affected by the severe reduction in DCs achieved in the CD11c:DTA mice. If DCs were the primary APC in the initial activation of T cells, one would have expected a substantial naïve T cell compartment in their absence. This was not the case, in contrast to the situation in B cell deficient mice, which do have a markedly expanded naïve T cell compartment (Chan et al., 1999b). It is important to note that the limited reduction in T cell activation in CD11c:DTA mice is unlikely due to residual DCs. Despite extensive depletion of cDCs, we did not see a proportional effect on maintenance of naïve T cells, whereas given the very low frequencies of DCs compared to T cells, DCs should have been the limiting factor.
In contrast, the striking reduction of tissue infiltrates and pathology implies that DCs have non-redundant role in tissue pathogenesis. Upon interaction with infectious organisms, DCs migrate to the draining lymph nodes, where they prime T cells. However, the finding that T cells proliferate in situ in inflamed kidneys leads us to hypothesize that DCs might also promote the local expansion of T cells that were previously activated presumably in secondary lymphoid tissues. This notion is supported by reports showing that herpes simplex virus specific memory CD8+ T cells are stimulated and expanded in sensory ganglia by DCs upon reinfection (Wakim et al., 2008). In this pathogen response, local antigen presentation by DCs, leading to reactivation of T cells, similarly plays an important role. The presence of small renal infiltrates in the absence of DCs indicates that although DCs are critical for the expansion of kidney infiltrates they are not required to initiate them. Residual infiltrates in CD11c:DTA mice cannot reasonably be attributed to incomplete DC deletion. Had there been “leakiness” that contributed to residual pathology and T cell activation, then one would have expected these renal infiltrates to contain DCs, which they did not.
The quality of differentiation of T cells in secondary lymphoid tissues was specifically affected by the presence of DCs. In CD11c:DTA mice CD4+ T cell activation generated less IFN-γ producing Th1 and CD8+ T cells. These roles of DCs are important, as there is a substantial IFN-γ component to disease in MRL.Faslpr mice, as well as in other murine lupus models and in humans (Balomenos et al., 1998; Harigai et al., 2008; Schwarting et al., 1998). In addition to IFN-γ, type I IFNs (IFN-α/β) are thought to be critical in SLE (Banchereau and Pascual, 2006). One important source of type I IFNs is pDCs. Because pDCs are also substantially deleted in CD11c:DTA mice, it is not readily possible to distinguish their roles from cDCs. We did attempt to selectively deplete pDCs using the anti-BST-2 antibody (Blasius et al., 2006). This was effective in BALB/c but not MRL.Faslpr mice (data not shown), presumably owing to global defects in FcγR-mediated cellular depletion in lupus-prone mice (Ahuja et al., 2007). Hence, the determination of specific pDC effects will have to await genetic approaches for selective and long-term pDC depletion, which are not yet available.
We found that, in agreement with one (Darrasse-Jèze et al., 2009), but not two other, studies (Birnberg et al., 2008; Ohnmacht et al., 2009), Treg expansion and maintenance of phenotype was DC dependent. Whereas, on a non-autoimmune background, acute loss of DCs led to decreased numbers of Tregs but increased numbers of Th1 and Th17 cells, there was instead a concomitant drop in both regulatory and inflammatory (Th1) T cell numbers in lupus prone mice. This emphasizes the importance of overall context in the net effect of DCs, which can affect multiple limbs of the immune system. The reduction in Tregs was a result of decreased proliferation and survival.
Unexpectedly, autoantibody, but not total serum Ig, concentrations were reduced in CD11c:DTA mice. We interpret this dichotomy to mean that short-lived plasmablasts, derived from an extrafollicular response, rather than long-lived plasma cells, derived from germinal centers, are DC-dependent. Consistent with this, we found that the number of splenic plasmablasts were reduced in CD11c:DTA mice. That lupus-related autoantibodies such as anti-chromatin derive mainly from short-lived plasmablasts, has been directly documented in mice (Seo et al., 2002; William et al., 2002) and inferred from their relatively rapid decay upon depletion of B cells in both mice and SLE patients (Ahuja et al., 2007; Jónsdóttir et al., 2008; Walsh and Jayne, 2007). In contrast, total serum IgG or antimicrobial antibody concentrations remain stable in SLE patients after anti-CD20 therapy (Jónsdóttir et al., 2008; Walsh and Jayne, 2007). DCs are abundant in areas of extrafollicular plasmablast generation (García De Vinuesa et al., 1999; William et al., 2002) and could provide important cytokines, including IL-6, BAFF or APRIL, which support plasmablast differentiation, isotype switch and survival (Treml et al., 2009). However, whether DCs promote plasmablast development in vivo has been questioned (Hebel et al., 2006). Corresponding with the defect in IFN-γ generation in CD11c:DTA mice, we found a class switch defect to IgG2a among short-lived plasmablasts. This suggests that DC-induced T cell differentiation enables T cells to promote class switch and possibly plasmablast generation. Such a function for T cells in extrafollicular plasmablast responses in MRL.Faslpr mice has also been suggested by Odegard et al. (Odegard et al., 2008). Moreover, DCs can present undegraded antigen to B cells, arguing for direct DC-B interactions (Qi et al., 2006; Bergtold et al., 2005). DCs have lectin receptors that take up dead cell-associated antigen, which is then directed away from the lysosomal compartment (Sancho et al., 2009). In any case, because DCs have only a quantitative effect on autoantibodies and plasmablast numbers, other cells such as macrophages and B cells must be able to compensate at least in part for DCs.
Our results extend the limited published literature on the effects of DCs on spontaneous autoimmunity. In an elegant study in which Fas was specifically deleted in DCs in C57BL/6 mice, autoimmunity ensued, demonstrating that DCs, when dysregulated, can promote autoreactivity and pathology (Stranges et al., 2007). Similarly, DC-specific deletion of SOCS-1 in DCs, which modulates a number of proinflammatory pathways, resulted in autoimmunity (Hanada et al., 2003). In these types of study, DCs are experimentally made abnormal; by definition, DCs must be upstream of all other observed pathology in such experimental setups. In our study, in the context of global abnormality, we were able to see what DCs are required for, rather than what they can do, via the ablation approach. In this case, DCs could have been either upstream or downstream of the activation of other aspects of the immune system. In this way we were able to observe unexpected roles for DCs in expanding pre-activated T cells in secondary lymphoid and probably peripheral tissues.
It is interesting that, despite that DCs promote autoimmunity in MRL.Faslpr mice and in the above-mentioned studies of dysregulated DCs on a C57BL/6 background, deletion of DCs per se in C57BL/6 mice in one case (Ohnmacht et al., 2009), but not another (Birnberg et al., 2008), led to increased autoreactivity. Differences in background genes or environmental factors (e.g. environmental organisms) might account for the contradictory results in those two studies. Another study assigned a regulatory role to pDCs in ongoing insulitis in a diabetes model (Saxena et al., 2007). This highlights the context-dependent role of DCs; clearly, we found that DCs function to promote rather than regulate disease in the setting of established predisposition to lupus. Although there is much more work to do in defining the roles of DCs in autoimmunity, including DC-T and DC-B cell interactions, our current data validate DCs as a potential new therapeutic target in autoimmunity as well as provide direction for future studies.
Material and methods
Mice
CD11c-Cre BAC transgenic C57BL/6 mice (Caton et al., 2007), and Rosa26-eGFP-DTA C57BL/6 mice (Ivanova et al., 2005) were backcrossed (13 and 9 times respectively) onto the Fas-deficient, lupus-prone MRL-MpJ-Faslpr/J strain (Jackson Laboratory). Homozygosity for the H2k haplotype and the lpr mutation was verified by PCR. The Rosa26-eGFP-DTA locus contains the gene for the diphtheria toxin α chain (DTA) that is preceded by a loxP flanked STOP cassette so that expression of the toxin is restricted to those cells expressing Cre recombinase. Both strains were then intercrossed to generate constitutively DC-deficient CD11c-Cre+Rosa26-eGFP-DTA+ mice. CD11c-Cre+, Rosa26-eGFP-DTA+ and wild type littermates were used as controls. The three types of control mice were found to be indistinguishable in all experiments performed. Langerin-DTA FVB/N mice (Kaplan et al., 2005) were crossed to MRL-MpJ-Faslpr/2J mice. F1 offspring were intercrossed to generate Langerin-DTA+ and control Langerin-DTA− Fas-deficient (Faslpr/lpr) F2 mice. For BrdU studies wild type MRL-MpJ-Faslpr mice were injected with 3 mg BrdU i.p. and sacrificed 2 hr later. All animals were maintained under specific pathogen-free (SPF) conditions and handled according to protocols approved by the Yale Institutional Animal Care and Use Committee.
Flow cytometry
Surface staining was performed in ice-cold PBS with 3% calf serum in the presence of FcR blocking antibody 2.4G2. Antibody clones used for surface staining were: anti-BST2 (927), anti-CD4 (GK1.5), anti-CD8 (TIB 105), anti-CD11b (M1/70), anti-CD11c (N418), anti-CD19 (1D3), anti-CD22 (Cy34.1), anti-CD25 (PC61), anti-CD44 (1M7), anti-CD138 (281-2), anti-F4/80 (BM8), anti-I-A/I-E (M5/114), anti-Ly6G/Ly6C (RB6-8C5) and anti-TCRβ (H57-597). Intracellular staining was performed using the BD Cytofix/Cytoperm and Perm/Wash buffers or, for intracellular FoxP3 staining, the eBioscience FoxP3 staining buffer set. For intracellular cytokine staining, 4 × 106 splenocytes were cultured for 4 hr at 37°C in 24-well plates in 2 ml culture medium containing ionomycin (750 ng/ml) and PMA (20 ng/ml). For the last 2 hr brefeldin A (10 μg/ml) was added to the cultures. Antibody clones used for intracellular staining were: anti-activated caspase 3 (C92-605), anti-Bcl-2 (3H11), anti-FoxP3 (FJK-16), anti-IFN-γ (XMG1.2), anti-kappa (187.1) and anti-Ki67 (SP6). Ethidium monoazide (EMA) was used for livedead discrimination. Cells were analyzed on a LSRII instrument (BD).
Immunofluorescence
Kidneys were fixed in 0.7% paraformaldehyde-lysine-periodate (PLP) solution and frozen in OCT (TissueTek) after dehydration in 30% sucrose solution. Cryostat sections (7 μm) were stained with the following antibodies: Alexa Fluor (AF) 647 labeled anti-CD11c (N418), AF488 labeled anti-CD4 (GK1.5), rabbit anti-Ki67 (SP6) followed by AF568 labeled goat anti-rabbit IgG (Invitrogen). To detect glomerular immune complexes sections were stained with AF555 labeled goat anti-mouse IgG (highly cross-adsorbed, Southern Biotech), rat anti-mouse C3 (RmC11H9) followed by AF647 labeled goat anti-rat IgG (cross-adsorbed against mouse IgG, Southern Biotech). BrdU staining was performed as described previously (Hauser et al., 2007). Spleens were frozen in OCT and cryostat sections fixed in acetone. Spleen sections were stained with biotinylated anti-CD4 (GK1.5) and AF647 labeled anti-CD169 (MOMA-1), or AF647 labeled anti-CD4 and biotinylated anti-CD209b (ER-TR9). Streptavidin-AF555 (Invitrogen) was used as a secondary reagent. Epidermal sheets were prepared as described previously (Kaplan et al., 2005). Epidermal sheets were stained with biotinylated rat anti-mouse I-A/I-E (M5/114) followed by AF555 conjugated streptavidin (Invitrogen). Tissues were mounted in Prolong Gold anti-fade mounting medium (Invitrogen, with or without DAPI). HEp-2 immunofluorescence assays (Antibodies Inc.) were performed as previously described (Christensen et al., 2005) with serum diluted at 1:100. Images were captured on an Olympus BX-40, a Nikon Eclipse Ti-U or for confocal images on a Zeiss LSM 510 microscope and processed in Adobe Photoshop.
ELISA and Luminex
Anti-nucleosome ELISA was performed essentially as previously described (Nickerson et al., 2010). Specific antibodies were detected with alkaline phosphatase-conjugated goat anti-mouse IgG or IgM (Southern Biotech). The monoclonal antibody PL2-3 (Losman et al., 1993) was used as a standard for IgG anti-nucleosome measurement and 3H9/Vκ1 (Shlomchik et al., 1987) for IgM anti-nucleosome. Anti-Sm and anti-IgG2a rheumatoid factor concentrations were determined by ELISA as previously described (Christensen et al., 2005; Nickerson et al., 2010). Serum concentrations of individual Ig isotypes were measured by Luminex assay (Millipore) according to the manufacturer’s protocol.
Evaluation of clinical disease
For kidney disease, formalin-fixed and paraffin-embedded sections, stained with either H&E or PAS, were scored for glomerular and interstitial nephritis by a pathologist (M.K.) who was blinded to the genotype of the mice. H&E-stained slides were photographed to determine the total area of renal perivascular infiltrates relative to the total area of the kidney section using ImageJ software (http://rsb.info.nih.gov/ij/). Proteinuria was measured using Bayer Albustix reagent strips. Mice were classified as positive for dermatitis upon development of typical lesions on the dorsum of the neck and back.
Highlights.
The net effect of DC function in lupus is to promote disease and tissue infiltrates
Surprisingly, DCs are not required for initial T cell activation
DCs promote expansion and differentiation of preactivated T cells
The extrafollicular B cell response—the main source of autoantibodies—is DC-dependent
Supplementary Material
Acknowledgements
We thank J. Martinez-Barbera for the generous gift of the Rosa26-eGFP-DTA mice. We thank Yale Animal Resources Center for outstanding animal husbandry. Supported by National Institutes of Health grant R01-AR044077 (M.J.S.), Deutsche Forschungsge-meinschaft (fellowship to L.L.T.); and the Lupus Research Institute and the Yale Skin Diseases Research Center (D.H.K).
Footnotes
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal H, Jacob N, Carreras E, Bajana S, Putterman C, Turner S, Neas B, Mathian A, Koss MN, et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol. 2009;183:6021–6029. doi: 10.4049/jimmunol.0803872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. The Journal of Immunology. 2007;179:3351. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev. 2010;234:76–89. doi: 10.1111/j.0105-2896.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragán L, Makia D, Krauthgamer R, Brenner O, Ludewig B, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. The Journal of Immunology. 2006;177:3260. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999a;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OT, Madaio MP, Shlomchik MJ. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999b;163:3592–3596. [PubMed] [Google Scholar]
- Cheung YH, Loh C, Pau E, Kim J, Wither J. Insights into the genetic basis and immunopathogenesis of systemic lupus erythematosus from the study of mouse models. Semin Immunol. 2009;21:372–382. doi: 10.1016/j.smim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse-Jèze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Trez C, Magez S, Akira S, Ryffel B, Carlier Y, Muraille E. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS Pathog. 2009;5:e1000494. doi: 10.1371/journal.ppat.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5:433–441. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- García De Vinuesa C, Gulbranson-Judge A, Khan M, O’Leary P, Cascalho M, Wabl M, Klaus GG, Owen MJ, MacLennan IC. Dendritic cells associated with plasmablast survival. Eur J Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- Hauser AE, Junt T, Mempel TR, Sneddon MW, Kleinstein SH, Henrickson SE, von Andrian UH, Shlomchik MJ, Haberman AM. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. Journal of Experimental Medicine. 2001;194:769. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- Hebel K, Griewank K, Inamine A, Chang HD, Müller-Hilke B, Fillatreau S, Manz RA, Radbruch A, Jung S. Plasma cell differentiation in T-independent type 2 immune responses is independent of CD11c(high) dendritic cells. Eur J Immunol. 2006;36:2912–2919. doi: 10.1002/eji.200636356. [DOI] [PubMed] [Google Scholar]
- Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyarto BZ, Jenison MC, Dudda JC, Roers A, Müller W, Koni PA, Campbell DJ, Shlomchik MJ, Kaplan DH. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183:5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43:129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs DA, Burek CL, Hu Q, Kuppers RC, Lee B, Prendergast RA. Anti-CD4 monoclonal antibody therapy suppresses autoimmune disease in MRL/Mp-lpr/lpr mice. Cell Immunol. 1992;141:496–507. doi: 10.1016/0008-8749(92)90166-m. [DOI] [PubMed] [Google Scholar]
- Jevnikar AM, Grusby MJ, Glimcher LH. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. Journal of Experimental Medicine. 1994;179:1137. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsdóttir T, Gunnarsson I, Risselada A, Henriksson EW, Klareskog L, van Vollenhoven RF. Treatment of refractory SLE with rituximab plus cyclophos-phamide: clinical effects, serological changes, and predictors of response. Ann Rheum Dis. 2008;67:330–334. doi: 10.1136/ard.2007.079095. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Looney RJ. B cell-targeted therapies for systemic lupus erythematosus: an update on clinical trial data. Drugs. 2010;70:529–540. doi: 10.2165/11535420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Losman MJ, Fasy TM, Novick KE, Monestier M. Relationships among antinuclear antibodies from autoimmune MRL mice reacting with histone H2A-H2B dimers and DNA. Int Immunol. 1993;5:513–523. doi: 10.1093/intimm/5.5.513. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Mehal WZ, Crispe IN. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol. 1998;161:1686–1693. [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 Regulates TLR7- and MyD88-Dependent Autoantibody Production and Disease in a Murine Model of Lupus. J Immunol. 2010 doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud’homme GJ, Kono DH, Theofilopoulos AN. Quantitative polymerase chain reaction analysis reveals marked overexpression of interleukin-1 beta, interleukin-1 and interferon-gamma mRNA in the lymph nodes of lupus-prone mice. Mol Immunol. 1995;32:495–503. doi: 10.1016/0161-5890(95)00024-9. [DOI] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys. 2009;53:1–16. doi: 10.1007/s12013-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- Walsh M, Jayne D. Rituximab in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis and systemic lupus erythematosus: past, present and future. Kidney Int. 2007;72:676–682. doi: 10.1038/sj.ki.5002395. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, Wakeland EK, Mohan C. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest. 2005;115:1869–1878. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.