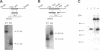Abstract
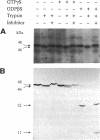
GTP-binding protein (G protein)-mediated signal transduction pathways play essential roles during the aggregation and differentiation process of Dictyostelium. In addition to the five known G protein alpha-subunit genes, we recently identified three novel alpha-subunit genes, G alpha 6, G alpha 7, and G alpha 8, using the polymerase chain reaction technique. We present here a more complete analysis of G alpha 7 and G alpha 8. The cDNAs of these two genes were cloned, and their complete nucleotide sequences were determined. Sequence analyses indicate that G alpha 8 possesses some unusual features. It lacks the "TCATDT" motif, a sequence of amino acids highly conserved among G alpha subunits, and has an additional 50 amino acids at its C-terminus consisting of long stretches of asparagine. Moreover, G alpha 8 is unusually resistant to protease digestion, which may indicate a slow GTP hydrolysis rate. The possible functions of these alpha-subunits were assessed by generating mutants lacking G alpha 7 or G alpha 8 by gene targeting through homologous recombination and by overexpressing G alpha 7 or G alpha 8 protein. Overexpression of G alpha 7 resulted in abnormal morphogenesis starting at the slug stage, whereas analysis of the other strains failed to reveal any obvious growth or developmental defects under either normal or stressful conditions. The implications of these results are discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlot C. H., Bourne H. R. Identification of effector-activating residues of Gs alpha. Cell. 1992 Mar 6;68(5):911–922. doi: 10.1016/0092-8674(92)90034-a. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bürki E., Anjard C., Scholder J. C., Reymond C. D. Isolation of two genes encoding putative protein kinases regulated during Dictyostelium discoideum development. Gene. 1991 Jun 15;102(1):57–65. doi: 10.1016/0378-1119(91)90538-m. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Fong H. K., Simon M. I., Gilman A. G. Gz, a guanine nucleotide-binding protein with unique biochemical properties. J Biol Chem. 1990 Feb 5;265(4):2383–2390. [PubMed] [Google Scholar]
- Caterina M. J., Milne J. L., Devreotes P. N. Mutation of the third intracellular loop of the cAMP receptor, cAR1, of Dictyostelium yields mutants impaired in multiple signaling pathways. J Biol Chem. 1994 Jan 14;269(2):1523–1532. [PubMed] [Google Scholar]
- Conklin B. R., Bourne H. R. Structural elements of G alpha subunits that interact with G beta gamma, receptors, and effectors. Cell. 1993 May 21;73(4):631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Farfel Z., Lustig K. D., Julius D., Bourne H. R. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993 May 20;363(6426):274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- Cubitt A. B., Carrel F., Dharmawardhane S., Gaskins C., Hadwiger J., Howard P., Mann S. K., Okaichi K., Zhou K., Firtel R. A. Molecular genetic analysis of signal transduction pathways controlling multicellular development in Dictyostelium. Cold Spring Harb Symp Quant Biol. 1992;57:177–192. doi: 10.1101/sqb.1992.057.01.023. [DOI] [PubMed] [Google Scholar]
- Deretic D., Hamm H. E. Topographic analysis of antigenic determinants recognized by monoclonal antibodies to the photoreceptor guanyl nucleotide-binding protein, transducin. J Biol Chem. 1987 Aug 5;262(22):10839–10847. [PubMed] [Google Scholar]
- Devreotes P. Dictyostelium discoideum: a model system for cell-cell interactions in development. Science. 1989 Sep 8;245(4922):1054–1058. doi: 10.1126/science.2672337. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Signal transduction pathways controlling multicellular development in Dictyostelium. Trends Genet. 1991 Nov-Dec;7(11-12):381–388. doi: 10.1016/0168-9525(91)90260-w. [DOI] [PubMed] [Google Scholar]
- Franke J., Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 May;74(5):2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Nash C. R. Characterization of transducin from bovine retinal rod outer segments. II. Evidence for distinct binding sites and conformational changes revealed by limited proteolysis with trypsin. J Biol Chem. 1983 Sep 10;258(17):10503–10510. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Firtel R. A. Analysis of G alpha 4, a G-protein subunit required for multicellular development in Dictyostelium. Genes Dev. 1992 Jan;6(1):38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Wilkie T. M., Strathmann M., Firtel R. A. Identification of Dictyostelium G alpha genes expressed during multicellular development. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8213–8217. doi: 10.1073/pnas.88.18.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard P. K., Ahern K. G., Firtel R. A. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 1988 Mar 25;16(6):2613–2623. doi: 10.1093/nar/16.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Saxe C. L., 3rd, Gollop R., Kimmel A. R., Devreotes P. N. Identification and targeted gene disruption of cAR3, a cAMP receptor subtype expressed during multicellular stages of Dictyostelium development. Genes Dev. 1993 Feb;7(2):273–282. doi: 10.1101/gad.7.2.273. [DOI] [PubMed] [Google Scholar]
- Kesbeke F., Snaar-Jagalska B. E., Van Haastert P. J. Signal transduction in Dictyostelium fgd A mutants with a defective interaction between surface cAMP receptors and a GTP-binding regulatory protein. J Cell Biol. 1988 Aug;107(2):521–528. doi: 10.1083/jcb.107.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Hadwiger J. A., Pupillo M., Firtel R. A. Molecular genetic analysis of two G alpha protein subunits in Dictyostelium. J Biol Chem. 1991 Jan 15;266(2):1220–1228. [PubMed] [Google Scholar]
- Kumagai A., Pupillo M., Gundersen R., Miake-Lye R., Devreotes P. N., Firtel R. A. Regulation and function of G alpha protein subunits in Dictyostelium. Cell. 1989 Apr 21;57(2):265–275. doi: 10.1016/0092-8674(89)90964-1. [DOI] [PubMed] [Google Scholar]
- Lilly P., Wu L., Welker D. L., Devreotes P. N. A G-protein beta-subunit is essential for Dictyostelium development. Genes Dev. 1993 Jun;7(6):986–995. doi: 10.1101/gad.7.6.986. [DOI] [PubMed] [Google Scholar]
- Mann S. K., Firtel R. A. A developmentally regulated, putative serine/threonine protein kinase is essential for development in Dictyostelium. Mech Dev. 1991 Sep;35(2):89–101. doi: 10.1016/0925-4773(91)90060-j. [DOI] [PubMed] [Google Scholar]
- Mann S. K., Firtel R. A. cAMP-dependent protein kinase differentially regulates prestalk and prespore differentiation during Dictyostelium development. Development. 1993 Sep;119(1):135–146. doi: 10.1242/dev.119.1.135. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Sullivan K. A., Miller R. T., Beiderman B., Lopez N. G., Ramachandran J., Bourne H. R. Carboxyl terminal domain of Gs alpha specifies coupling of receptors to stimulation of adenylyl cyclase. Science. 1988 Jul 22;241(4864):448–451. doi: 10.1126/science.2899356. [DOI] [PubMed] [Google Scholar]
- Miller R. T., Masters S. B., Sullivan K. A., Beiderman B., Bourne H. R. A mutation that prevents GTP-dependent activation of the alpha chain of Gs. Nature. 1988 Aug 25;334(6184):712–715. doi: 10.1038/334712a0. [DOI] [PubMed] [Google Scholar]
- Milne J. L., Devreotes P. N. The surface cyclic AMP receptors, cAR1, cAR2, and cAR3, promote Ca2+ influx in Dictyostelium discoideum by a G alpha 2-independent mechanism. Mol Biol Cell. 1993 Mar;4(3):283–292. doi: 10.1091/mbc.4.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen W., Datta S., Reymond C., Sivertsen A., Mann S., Crowley T., Firtel R. A. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 1987;28:67–100. doi: 10.1016/s0091-679x(08)61637-4. [DOI] [PubMed] [Google Scholar]
- Okaichi K., Cubitt A. B., Pitt G. S., Firtel R. A. Amino acid substitutions in the Dictyostelium G alpha subunit G alpha 2 produce dominant negative phenotypes and inhibit the activation of adenylyl cyclase, guanylyl cyclase, and phospholipase C. Mol Biol Cell. 1992 Jul;3(7):735–747. doi: 10.1091/mbc.3.7.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt G. S., Milona N., Borleis J., Lin K. C., Reed R. R., Devreotes P. N. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell. 1992 Apr 17;69(2):305–315. doi: 10.1016/0092-8674(92)90411-5. [DOI] [PubMed] [Google Scholar]
- Pupillo M., Kumagai A., Pitt G. S., Firtel R. A., Devreotes P. N. Multiple alpha subunits of guanine nucleotide-binding proteins in Dictyostelium. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4892–4896. doi: 10.1073/pnas.86.13.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., McInnis M. G., Margolis R. L., Li S. H. Genes with triplet repeats: candidate mediators of neuropsychiatric disorders. Trends Neurosci. 1993 Jul;16(7):254–260. doi: 10.1016/0166-2236(93)90175-l. [DOI] [PubMed] [Google Scholar]
- Saxe C. L., 3rd, Ginsburg G. T., Louis J. M., Johnson R., Devreotes P. N., Kimmel A. R. CAR2, a prestalk cAMP receptor required for normal tip formation and late development of Dictyostelium discoideum. Genes Dev. 1993 Feb;7(2):262–272. doi: 10.1101/gad.7.2.262. [DOI] [PubMed] [Google Scholar]
- Saxe C. L., 3rd, Johnson R. L., Devreotes P. N., Kimmel A. R. Expression of a cAMP receptor gene of Dictyostelium and evidence for a multigene family. Genes Dev. 1991 Jan;5(1):1–8. doi: 10.1101/gad.5.1.1. [DOI] [PubMed] [Google Scholar]
- Saxe C. L., 3rd, Johnson R., Devreotes P. N., Kimmel A. R. Multiple genes for cell surface cAMP receptors in Dictyostelium discoideum. Dev Genet. 1991;12(1-2):6–13. doi: 10.1002/dvg.1020120104. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Snaar-Jagalska B. E., Kesbeke F., Van Haastert P. J. G-proteins in the signal-transduction pathways of Dictyostelium discoideum. Dev Genet. 1988;9(4-5):215–226. doi: 10.1002/dvg.1020090404. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Miller R. T., Masters S. B., Beiderman B., Heideman W., Bourne H. R. Identification of receptor contact site involved in receptor-G protein coupling. Nature. 1987 Dec 24;330(6150):758–760. doi: 10.1038/330758a0. [DOI] [PubMed] [Google Scholar]
- Sun T. J., Devreotes P. N. Gene targeting of the aggregation stage cAMP receptor cAR1 in Dictyostelium. Genes Dev. 1991 Apr;5(4):572–582. doi: 10.1101/gad.5.4.572. [DOI] [PubMed] [Google Scholar]
- Sun T. J., Van Haastert P. J., Devreotes P. N. Surface cAMP receptors mediate multiple responses during development in Dictyostelium: evidenced by antisense mutagenesis. J Cell Biol. 1990 May;110(5):1549–1554. doi: 10.1083/jcb.110.5.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theibert A., Klein P., Devreotes P. N. Specific photoaffinity labeling of the cAMP surface receptor in Dictyostelium discoideum. J Biol Chem. 1984 Oct 25;259(20):12318–12321. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. J., Devreotes P. N. Dictyostelium transiently expresses eight distinct G-protein alpha-subunits during its developmental program. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1141–1147. doi: 10.1016/0006-291x(91)91690-e. [DOI] [PubMed] [Google Scholar]