Abstract
The establishment of sex-specific neural morphology, which underlies sex-specific behaviors, occurs during a perinatal sensitive window in which brief exposure to gonadal steroid hormones produces permanent masculinization of the brain. In the rodent, estradiol derived from testicular androgens is a principle organizational hormone. The mechanism by which transient estradiol exposure induces permanent differences in neuronal anatomy has been widely investigated, but remains elusive. Epigenetic changes, such as DNA methylation, allow environmental influences to alter long-term gene expression patterns and therefore may be a potential mediator of estradiol-induced organization of the neonatal brain. Here we review data that demonstrate sex and estradiol-induced differences in DNA methylation on the estrogen receptor α (ERα), estrogen receptor β (ERβ), and progesterone receptor (PR) promoters in sexually dimorphic brain regions across development. Contrary to the overarching view of DNA methylation as a permanent modification directly tied to gene expression, these data demonstrate that methylation patterns on steroid hormone receptors change across the life span and do not necessarily predict expression. Although further exploration into the mechanism and significance of estradiol-induced alterations in DNA methylation patterns in the neonatal brain is necessary, these results provide preliminary evidence that epigenetic alterations can occur in response to early hormone exposure and may mediate estradiol-induced organization of sex differences in the neonatal brain.
Keywords: Sex differences, DNA Methylation, Estradiol, Estrogen Receptor α, Estrogen Receptor β, Progesterone Receptor
I. Organization of Sex Differences in the Brain
Many sex differences in the rodent brain are the result of permanent, gonadal steroid-induced alterations in neural morphology arising during a perinatal sensitive period that begins embryonically with the onset of gonadal steroidogenesis and ends within the first two postnatal weeks. Testosterone, synthesized by the male testis, is locally aromatized to estradiol within developing neurons; and estradiol, via activation of its nuclear receptor, mediates several developmental sex differences in cell anatomy and physiology. These sex differences underlie sex-specific regulation of gonadotropin secretion from the anterior pituitary and sexual behavior, as well as other behaviors exhibited by both males and females that differ in their frequency or intensity (see for review McCarthy, 2008).
Estradiol derived from testicular androgens during the perinatal sensitive period is necessary for both defeminization and masculinization of the developing male brain (Gorski, 1985). Masculinization is the process by which estradiol organizes male-specific neuronal characteristics and enables the expression of male sexual behavior in the adult. Defeminization is the process by which female-specific characteristics are removed from or suppressed in the bi-potential brain, eliminating the capacity for surge release of gonadotropins (LH and FSH) from the anterior pituitary and female sexual receptivity in adulthood (Todd et al., 2005).
Two brain regions that show robust estradiol-induced organizational changes are the preoptic area (POA) and medial basal hypothalamus (MBH), both of which are critical for sexual behavior. Estradiol-induced organizational changes are perhaps best exemplified within the POA, an area containing the medial preoptic nucleus (MPN), the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the anteroventral periventricular nucleus (AVPV). The MPN is critical to the control of male sexual behavior and the SDN-POA is a subregion within this nucleus that has been implicated in partner preference (Houtsmuller et al., 1994). The AVPV is important for gonadotropin secretion and believed to be the source of control of the LH surge essential for ovulation in adult females (reviewed by Dungan et al., 2006). In the perinatal male brain, estradiol derived from testosterone stimulates opposing events in the SDN and AVPV, protecting neurons within the SDN-POA from apoptosis by enhancing NMDA receptor expression (Hsu et al., 2001), while provoking expression of pro-apoptotic proteins such as TRIP, Bad, and Bax to induce apoptosis in the AVPV (Krishnan et al., 2009; Forger et al., 2004). In addition to modulating cell death, estradiol also mediates sex differences in synaptic patterning in the MPN by inducing synthesis of the cyclooxygenase enzymes, COX-1 and COX-2, thereby increasing the production of prostaglandin-E2 (PGE2; Amateau and McCarthy, 2004). Acting via the EP2 and EP4 receptors, PGE2 activates protein kinase A and allows for glutamate-induced activation of AMPA receptors and formation of dendritic spines (Wright and McCarthy, 2009), the postsynaptic contact points for excitatory synapses. Ultimately, the increased production of PGE2 in the male brain results in a two to three times higher density of dendritic spine synapses compared to females and, interestingly, this higher spine density positively correlates with the degree of masculinization of sexual behavior (Wright et al., 2008). Thus three key cellular responses, cell survival, cell death and synaptogenesis, are all mediated by estradiol within one brain region, the preoptic area. The divergent effects of estradiol are mediated via the estrogen receptor (ER), in particular the ERα isoform (see for review, McCarthy et al., 2009a).
In addition to exerting organizational effects on the physiology of the POA, estradiol also induces permanent changes in synaptic connectivity in the MBH. The ventromedial nucleus of the hypothalamus (VMN) is a key region for regulating female sexual behavior. Within the VMN, male neurons have twice the number of dendritic spines and more dendritic branches than females as a result of neonatal hormone exposure (Matsumoto and Arai, 1983; Matsumoto and Arai, 1986; Todd et al, 2007). Estradiol produces sex differences in synaptic organization in this region by rapid activation of PI3 kinase which enhances glutamate release from presynaptic cells, thus provoking dendritic spine outgrowth from postsynaptic neurons (Schwarz et al., 2008). Here too, the ERα isoform is the critical mediator of estradiol action though the initiating steps in the signal transduction cascade appear to begin at the membrane via rapid activation of PI3 kinase and, more interestingly, the requirement for ER is restricted to the presynaptic membrane despite the induction of changes in neuronal morphology within the postsynaptic neuron.
The enduring organizational changes produced by estradiol within the neonatal brain enable circulating gonadal hormones in the adult to activate sexually differentiated brain regions, such as the POA and the MBH, in a sex-specific manner. Thus, in adulthood estrogens and progesterone act on a female brain to regulate pulsatile LH release, induce estrous cyclicity, and female sexual receptivity; whereas testosterone reaches a masculinized adult brain to activate male sexual behavior. Although the Organizational / Activational Hypothesis (Phoenix et al., 1959) did not specifically articulate the notion of permanent changes to the neural circuits controlling physiology or behavior, this concept became embedded in the collective view as details emerged on how steroids altered the developing brain (i.e. cell death and synaptic patterning). Both of these endpoints, neuron number and circuit wiring, were considered immutable once established during a sensitive period, and hence the perfect substrate for “organizational” effects. There was no need for any additional maintenance once organizational effects occurred, as they were considered permanent. However, in the intervening years our understanding of the brain has changed such that we now appreciate that neurogenesis goes on throughout life and that dendritic morphology and synaptic profiles continuously change in response to extrinsic and intrinsic stimuli. This altered view requires that we also re-examine the notion of organizational effects of steroids and reconcile how effects can be permanent in light of such dynamism. Put another way, if we accept that new neurons are continuously being born in the mature brain and that synapses are continuously being broken and formed anew, in order for the pattern of responses established early in life to be maintained there must be some sort of cellular memory. A potential source of this memory is epigenetic changes that provide maintenance of long-term hormonal effects on the brain (McCarthy et al., 2009b). The study of epigenetics has provided insight not only into the ability of extrinsic factors to influence gene expression, but also on how environmental influences during development can shape neuronal physiology and behavior later in life. In this review, we aim to summarize what is known about the impact of neonatal estradiol on the epigenetic process of DNA methylation. We seek only to demonstrate that such a process occurs and leave evidence of the functional significance of these epigenetic modifications for future studies.
II. DNA Methylation and its Role in Development
Methylation at cytosine-guanine (CpG) dinucleotides is one of the most prevalent epigenetic modifications on the vertebrate genome and plays a critical role in chromatin remodeling and subsequent gene expression. DNA methylation is an enzymatically-driven process during which a methyl group (CH3) is added to a cytosine residue at the 5-position of the pyrimadine ring. Methylation of CpGs changes chromatin conformation and can alter the transcriptional capacity of a particular gene. For instance, CpG methylation is known to lead to the recruitment of DNA methyl-binding proteins, such as MeCP2, leading to tightening of chromatin structure and the repression of gene transcription (Hansen and Gartler, 1990; Fan and Hutnick, 2005). CpG islands are regions of the genome that contain a high level of these CpG dinucleotides (> 60%), and are generally localized to the 5′ promoter region of genes, establishing their importance for gene expression (Caiafa and Zampieri, 2005). While nearly 90% of the CpG dinucleotides outside of a CpG island are methylated, CpGs within a CpG island are predominantly unmethylated (6-8%), allowing for differential methylation and subsequently differential gene expression (Jaenisch and Bird, 2003; Illingworth et al., 2008) as well as other possible regulatory effects.
DNA methyltransferases (DNMTs) are the enzymes that establish and maintain the methylation of DNA at individual CpGs. The activity of DNMTs, and hence the timing and pattern of methylation, is critically important to numerous developmental processes and therefore tightly regulated (Okano et al., 1999, Robertson and Wolffe, 2000). There appear to be two distinct phases of DNA methylation during development (see Geiman and Muegge, 2009 for review). The first occurs during early embryogenesis with the silencing of genes from either maternal or paternal origins prior to implantation (Mayer et al., 2000; Oswald et al., 2000; Rougier et al., 1998). This process predominantly requires DNMT1, as its activity can maintain or prevent the replication of DNA methylation patterns during rapid cell division (Cardoso and Leonhardt, 1999). A second phase of DNA methylation occurs after implantation and into postnatal development wherein cell-specific methylation mediates tissue-specific gene expression. This process predominantly requires the activity of the de novo DNMT enzymes, DNMT3a and DNMT3b (Okano et al., 1999). In general the endpoint of both DNA methylation processes is long-term silencing or expression of genes from development into adulthood. Hormones, including estrogens, can up-regulate (Cui et al., 2009; Shafiei et al., 2007; Zama and Uzumcu, 2009) or down-regulate (Yamagata et al., 2009) DNMT enzymes in peripheral tissues and cancer cells, and this may also be true in the brain.
The importance of DNA methylation in development is exemplified by its role in the etiology of several human disorders. Rett syndrome, a neurodevelopmental disorder involving mental retardation, results from a mutation in the methylation binding protein, MeCP2. ICF (immunodeficiency, centromeric instability, and facial anomalies) syndrome results from the loss of DNMT3b function. Fragile X syndrome is characterized by severe mental retardation as a result of increased DNA methylation along the FMR1 gene, resulting in decreased expression of FMR1 protein. Notably, both Rett and Fragile X syndromes exhibit sex differences in their incidences and symptomologies, as the genes carrying these mutations are X-linked and therefore more severe in males (see Geiman and Muegge, 2009 for review).
III. Sex and Estradiol-induced Regulation of ER Methylation Patterns
As mentioned above, estradiol exerts its organizational impact primarily by binding to and activating estrogen receptors (ERs). These ligand-activated transcription factors belong to the same nuclear receptor super-family as receptors for other steroid hormones, thyroid hormone, retinoids, and vitamin D (Beato et al., 1995; Mangelsdorf et al., 1995). Although they share an endogenous ligand, the two classic ERs, ERα and ERβ, are the products of two independent genes (Greene et al., 1986; Tremblay et al., 1997) and have distinctive distribution patterns in the brain (Shughrue et al., 1997) and periphery (Hiroi et al., 1999; Kuiper et al., 1996). The temporal expression patterns for ERα and ERβ are also distinct, suggesting they mediate distinct developmental events. ERβ is first detected as early as embryonic day 10.5, whereas ERα does not appear until E16.5, (Lemmen et al., 1999).
Despite their essential role in brain development, sex differences in ER expression in the neonatal brain are generally minor and localized to small subregions. Gene expression of ERα and ERβ is tightly auto-regulated by estradiol activation of the receptors themselves (Orikasa and Sakuma, 2004; Stoica et al., 2003). Neonatal exposure to estrogenic compounds has been shown to induce hypermethylation of the ERβ promoter and in turn decrease the expression of ERβ in the ovary (Zama and Uzumcu, 2009), which suggests that the ER genes may be targets for sex and/or estradiol-induced differences in promoter methylation in the brain. In the neonatal female, there are higher levels of ERα in the POA and VMN, which may be due to estradiol-induced down regulation in males (Yokosuka et al., 1997). However, functional ERα is critical for masculinization of the neonatal rodent brain (Kudwa et al., 2006) and administration of the ERα-specific agonist, PPT, to female rat pups during the critical period induces cell death in the AVPV and promotes cell survival in the SDN-POA, resulting in male-like morphology of these regions and a loss of female sexual receptivity (Patchev et al., 2004). Knockdown of ERα with antisense oligonucleotides impairs hormonally-induced sexual differentiation of the brain (McCarthy et al., 1993) and ERα knockout mice show impaired female (Ogawa et al., 1996) and male sex behavior (Ogawa et al., 1997).
Methylation patterns on the ERα promoter within the POA have been extensively classified in response to alterations in maternal care during early development. Sex differences in maternal licking and grooming have been shown to alter methylation of the ERα promoter, ultimately influencing ER expression (Champagne et al., 2006; Kurian et al., 2010). Further exploration is needed to determine how these alterations in ERα promoter methylation, as well epigenetic changes induced by other environmental influences, contribute to the process of sexual differentiation of the brain.
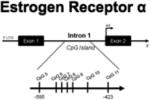
In order to gain insight into the stability of methylation across development and in response to changes in hormonal milieu, our lab conducted a region-specific analysis of 7 sites within a CpG island in intron 1 of the ERα 5′ non-coding region during the critical period for sexual differentiation, prior to the onset of puberty, and in adulthood (Table 1). In the POA, the overall level of methylation in this region of the ERα gene was significantly higher in newborn females compared to males and estradiol-treated females, most notably at two CpG sites. The sex and hormone-mediated difference in methylation diminished by around three weeks of age, a time point outside the sensitive period but before the onset of hormone secretion at puberty. In adulthood, a new significant difference in methylation at a different CpG site emerged, but the magnitude and direction of the difference was consistent with the pattern observed in newborns (Table 1). The mechanistic basis for how early sex differences in CpG methylation can recede and reemerge later in life is not yet understood. Considering that the differences in methylation disappear when gonadal steroids are low, around three weeks of age, there may be a requirement for circulating hormones to maintain methylation patterns on this gene.
Table 1. Summary of region-specific, sex and estradiol-induced differences in hormone receptor methylation across development.
Methylation status of individual CpG sites within CpG islands in or around the estrogen receptor α, estrogen receptor β, and progesterone receptor promoter regions was analyzed in males (M), females (F), and females neonatally treated with estradiol (F+E2). Methylation at different CpG sites varied between groups based on age and brain region.
| Gene of Interest |
Brain Region |
 |
Postnatal Day 20 |  |
|---|---|---|---|---|
|
POA | Site 7: F > M, F+E2 Site 9: F > M, F+E2 |
No differences | Site 5: F > M, F+E2 |
| MBH | Site 6: F > M Site 9: F > M |
No differences | No differences | |
| HIPP | -------- | No differences | No differences | |
|
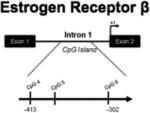
POA | Site 5: M > F (p=0.07) |
Site 6: F+E2 > M | Site 5: M > F, F + E2 |
| MBH | No differences | Site 6: F < M, F+E2 | Site 4: F > M, F+E2 Site 5: F > F+E2 > M |
|
| HIPP | No differences | No differences | Site 4: F+E2 > M, F | |
|
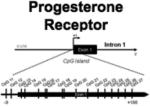
POA | Site 11: F+E2 > M, F Site 12: F+E2 > F |
No differences | No differences |
| MBH | Site 13: F+E2 > M, F | Site 11: F< M, F+E2 Site 13: F< M, F+E2 Site 17: F< M, F+E2 Site 27: F+E2 > M, F |
Site 27: F+E2 > M, F |
Methylation of the same 7 CpG sites was examined in the hypothalamus of the same animals. Similar to the POA, newborn females had significantly higher levels of methylation at 2 CpG sites on the ERα gene (Table 1). However, estradiol treatment of females did not significantly impact the methylation status of ERα in this region. Although testosterone-derived estradiol is a critical hormone for sexual differentiation of the male brain, effects of genetic sex or other testosterone metabolites, such as DHT, may also influence sex differences in CpG methylation.
Interestingly, we found that methylation levels on the ERα promoter were nearly doubled at PN20 and PN60 compared to PN1 in both the POA and hypothalamus (Schwarz et al., 2010). Developmental increases in methylation of the ERα gene have also been observed in the cortex (Westberry et al., 2010). Increased methylation of the ERα promoter during maturation of these brain regions may reflect an overall decrease in ERα receptor sensitivity following the critical period for sexual differentiation of the brain.
While ERα activation is necessary for masculinization of the neonatal brain, ERβ has been implicated in defeminization of the brain (Kudwa et al., 2005) and its overlapping distribution and co-localization with important neuroendocrine peptide hormones, such as oxytocin, vasopressin, prolactin, corticotropin-releasing hormone, and gonadatropin-releasing hormone, suggest it is an important player in neuroendocrine functions (for review see Bodo and Rissman, 2006). ERβ's involvement in defeminization has mainly been understood through the use of ERβ knockout mice and the ERβ-selective agonist, DPN. Administration of DPN during the perinatal sensitive period impairs lordosis behavior (Kudwa et al., 2006) and causes defeminization of the AVPV gonadotropin surge generator (Bodo et al., 2006), resulting in persistent estrous (Patchev et al., 2004). Consistent with a role for ERβ in defeminization of sexual behavior, male ERβ knockout mice show higher levels of lordosis behavior than their wild-type littermates (Kudwa et al., 2005). Its localization to regions associated with sexual behavior, ERβ is highly expressed in the hippocampus (Shughrue et al., 1997) and has been implicated in controlling stress and learned-helplessness and related behaviors associated with depression (Kreżel et al., 2001; Imwalle et al., 2005; Rocha et al., 2005).
One day after birth, the methylation patterns on 3 CpG sites within exon 1 of the ERβ gene in the POA, hypothalamus and hippocampus are not significantly impacted by sex or hormonal manipulation (Table 1). This is not necessarily surprising given that ERβ-mediated defeminization occurs closer to the end of the developmental sensitive period (Kudwa et al., 2005). In adulthood, we found region-specific differences in methylation levels in all three brain areas. Males have significantly higher methylation at one CpG site in the POA compared to both vehicle and estradiol-treated females, however the opposite pattern is observed in the hypothalamus where females have significantly higher CpG methylation at two sites compared to both males and estradiol-treated females. In the hippocampus, estradiol-treated females had significantly higher methylation at one CpG site compared to both males and females. Just as activation of ERα and ERβ contribute to independent aspects of developmental organization of the brain, sex and estradiol appear to exert differential patterns of control over the DNA methylation of these genes. Whereas differences in ERα promoter methylation are most prevalent during the critical period, hormonally-induced differences in methylation of the ERβ promoter do not appear until after sexual differentiation of the brain. Puberty is now considered a second sensitive period during which further organizational effects can occur (reviewed in Schulz et al., 2009) and perhaps the changes we observed in adolescents are related to this phenomenon.
V. Regulation of Progesterone Receptor Methylation
One of the most dynamically regulated hormone receptors within the brain is the progesterone receptor (PR). Neuronal expression of PR is necessary for neonatal masculinization of the brain (Lonstein et al., 2001), critical for the ovulatory surge in luteinizing hormone (LH), and essential for the expression of female sexual behavior in adulthood (Parsons et al., 1982; Brown et al., 1990; Shughrue et al., 1997). Progesterone receptor expression in specific brain regions is dependent on prior exposure to estradiol (MacLusky and McEwen, 1980); but even this regional specificity is dependent on sex, age, and previous hormonal exposure during development (Lauber, Romano, and Pfaff, 1991). Within the developing POA, males have significantly greater progesterone receptor expression than females as early as embryonic day 17 (Wagner et al., 1998), and this sex difference in PR expression persists throughout the critical period for sexual differentiation (Wagner et al., 2001). In spite of this, we found no sex differences in the pattern or levels of methylation at any CpG site analyzed across a large CpG island within exon 1 of the PR gene at three developmental time points (Table 1), suggesting that methylation of the PR gene is not the mechanism by which sex differences in PR expression within the POA are regulated.
In contrast to the POA, the female hypothalamus contains significantly higher levels of the progesterone receptor than the male hypothalamus throughout the critical period of sexual differentiation. This neonatal sex difference in PR expression, however, is neither established by, nor responsive to estradiol. After the close of the critical period, at around 2 weeks of age, treatment of females with estradiol results in a reliable increase in PR expression within the hypothalamus similar to that of adult cycling females. In males, however, estradiol treatment after the critical period of sexual differentiation does not induce PR expression, suggesting males have become refractory to the PR-inducing effects of estradiol (Quadros and Wagner, 2008). Whether epigenetic changes play a role in this process remains to be determined but based on these observations we predicted that a late emerging male bias in PR promoter methylation would appear. This was precisely the pattern we observed, with no sex difference in PR promoter methylation in newborns but significantly greater levels in males than females when assessed in adolescence. Treatment of neonatal females with estradiol during the critical period for sexual differentiation induced a pattern similar to that seen in males. By adulthood, there were no sex differences in methylation levels detected at any of the CpG sites along the PR promoter, indicating that the organizational sex difference detected at adolescence is eliminated in the mature adult, possibly via the influence of circulating hormones in the adult. These findings support the hypothesis that PR expression in the adult hypothalamus is epigenetically silenced by estradiol exposure during the critical period of sexual differentiation in the male, a process that is essential for the defeminization of behavior into adulthood. However, other aspects of this data suggest that PR expression is not directly tied to methylation levels of the promoter, and vice versa, methylation changes in the promoter do not necessarily predict sex differences in PR expression.
VI. Additional Thoughts and Unanswered Questions
Many questions remain regarding the mechanisms of hormonally-induced neural organization and how neonatal changes are manifested across the lifespan. The term “organization” carries the inherent expectation that structural changes established early in life are permanent, a notion pushed further by use of terms such as “neuroarchitechture” and “circuit wiring”, as so often done by ourselves and others. Nevertheless, it is time for a reexamination of this old idea in light of new knowledge depicting the myriad of ways in which the brain continues to change into adulthood, a concept captured by the ubiquitous use of the word “plastic”. We now know that many, if not all, synapses are temporary, axons and dendrites sprout and retract, astrocytes reshape themselves and new neurons continue to be born, differentiate, and integrate into the neuropil. All of these endpoints are profoundly and importantly altered by gonadal steroids in adulthood, and estradiol in particular has been shown to affect each one (Olmos et al., 1989; Woolley et al., 1990; Burke et al., 1998; Goldstein and Sengelaub, 2004; Torres-Aleman et al., 1992; Pawluski et al., 2009; Brock et al., 2010). This leaves us with a conundrum: how can the effects of estradiol on the developing brain be permanent while many of the same effects in the adult are temporary? Moreover, in light of this, how is it that early hormonal exposure dictates hormonal and behavioral responses elicited in the adult? Epigenetic changes to the genome are often referred to as a form of memory, a way in which experience or environment can remind the body and, in particular, the brain of past events. In the context of sexual differentiation, epigenetic changes offer a plausible mechanism by which developmental hormonal effects could be coded into the genome to restrict and direct adult hormonal and behavioral responses. The challenge is to identify which genes might be the source of this “memory”. The data reviewed here demonstrate the potential for estradiol to influence gene methylation on the promoters of its own and related receptors in discrete brain regions of the developing brain and attest to the potential for many other yet to be discovered epigenetic changes.
The notion that steroid receptors themselves are a source of genetic memory is worthy of consideration. Steroid hormone receptors are dynamically regulated during the organization of the brain (Walker et al., 2009). A change in the expression or the activity of ERs or PR can disrupt the differentiation of sexually dimorphic brain regions and impair sexual behavior, supporting the concept that proper regulation of these receptors is critical. The determinants of ER and PR expression are subtle, as evidenced by the presence of multiple complex promoters and receptor isoforms (Kos et al., 2001; Flouriot et al., 1998; Price et al., 2000; Giangrande and McDonnell, 1999). Estradiol tightly regulates the expression of both varieties of ER as well as PR. Traditionally this regulation is achieved via activation of ERs, which exert direct transcriptional control over gene promoters containing estrogen response elements. Additional control may be achieved by influencing proteasome-mediated receptor turnover to ensure continual estrogen responsiveness (Reid et al., 2003). Estrogen action impacts the methylation status of both ER and PR promoters (Zama and Uzumcu, 2009; Schwarz et al., 2010), providing an additional mechanism by which estradiol maintains control over the expression of its own receptors. Thus, differing estradiol levels in distinct cell types and brain regions can also influence receptor responsiveness (Schreihofer et al., 2000).
However, a straightforward regulation of gene expression may not be the entire story of epigenetic influence on sexual differentiation of the brain. There is clear evidence that early life experience exerts enduring effects on the methylation status and subsequent expression levels of several genes associated with changes in adult behavior (see Roth and Sweatt, this issue). But so far this does not appear to be the case for the enduring consequences of early hormone exposure during the process of sexual differentiation, at least not for the three genes explored to-date. Results from the recent analyses of 16,000 promoters in the human epigenome reveal that while DNA methylation is sufficient to inactivate CpG rich gene promoters, it is not necessary as many hypomethylated CpG rich promoters are inactive (Weber et al., 2007). This and other studies are beginning to reveal the potential for other regulatory aspects of epigenetic changes, or even that epigenetics reflect past history of gene expression as much as future regulation. The complexity of ER expression is expanded by the finding that methylation at individual ERα promoters causes differential expression of ERα isoforms (Sasaki et al., 2001a) and promoter utilization and hormone receptor isoform expression can be cell line and tissue-specific (Grandien et al., 1993; Grandien et al., 1995). For example, in endometrial cells selective methylation at the ERα-C and PR-B promoters can silence the expression of the ERα-C and PR-B isoforms, whereas ERα-A, ERα-B, ERβ, and PR-A remain freely expressed (Sasaki et al., 2001a; Sasaki et al., 2001b). The opposite is true in prostate cancer cell lines, which have high levels of methylation on the ERα-A and B promoters and low expression levels of their corresponding transcripts, and no differences in PR promoter methylation or expression (Sasaki et al., 2002). Estrogens either increase or decrease the use of individual ERα promoters depending on the cell line (Donaghue et al., 1999), and this effect may be the result of estrogen-induced differences in promoter methylation. Furthermore, estrogenic compounds can influence differential expression of ERα isoforms in the developing POA (Monje et al., 2007). Although the function of individual ER and PR isoforms in sexual differentiation of the brain is unknown, estradiol-induced differences in methylation at specific promoters may influence the expression of receptor subtypes and potentially result in differential estrogen-mediated responses.
The data presented here highlight the undiscovered potential for sex differences in promoter methylation that may be involved in masculinization and defeminization of the neonatal brain. However, a functional role for these changes has yet to be discovered. A fair assessment of the current understanding of dynamic changes in gene methylation in the brain across the lifespan is, well, dynamic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat. Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Bodo C, Kudwa AE, Rissman EF. Both Estrogen Receptor-{alpha} and-{beta} Are Required for Sexual Differentiation of the Anteroventral Periventricular Area in Mice. Endocrinology. 2006;147:415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. New roles for estrogen receptor β in behavior and neuroendocrinology. Front. Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Brock O, Keller M, Veyrac A, Douhard Q, Bakker J. Short term treatment with estradiol decreases the rate of newly generated cells in the subventricular zone and main olfactory bulb of adult female mice. Neuroscience. 2010;166:368–376. doi: 10.1016/j.neuroscience.2009.12.050. [DOI] [PubMed] [Google Scholar]
- Brown TJ, MacLusky NJ, Shanabrough M, Naftolin F. Comparison of age-and sex-related changes in cell nuclear estrogen-binding capacity and progestin receptor induction in the rat brain. Endocrinology. 1990;126:2965–2972. doi: 10.1210/endo-126-6-2965. [DOI] [PubMed] [Google Scholar]
- Burke KA, Widows MR, Sengelaub DR. Synergistic effects of testosterone metabolites on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. Dev. Neurobiol. 1998;33:1–10. doi: 10.1002/(sici)1097-4695(199707)33:1<1::aid-neu1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Caiafa P, Zampieri M. DNA methylation and chromatin structure: the puzzling CpG islands. J. Cell. Biochem. 2005;94:257–265. doi: 10.1002/jcb.20325. [DOI] [PubMed] [Google Scholar]
- Cardoso MC, Leonhardt H. DNA methyltransferase is actively retained in the cytoplasm during early development. J. Cell Biol. 1999;147:25–32. doi: 10.1083/jcb.147.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-{alpha} 1b promoter and estrogen receptor-{alpha} expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Cui M, Wen Z, Yang Z, Chen J, Wang F. Estrogen regulates DNA methyltransferase 3B expression in Ishikawa endometrial adenocarcinoma cells. Mol. Biol. Rep. 2009:1–7. doi: 10.1007/s11033-008-9435-9. [DOI] [PubMed] [Google Scholar]
- Donaghue C, Westley BR, May FEB. Selective promoter usage of the human estrogen receptor-{alpha} gene and its regulation by estrogen. Molecular Endocrinol. 1999;13:1934–1950. doi: 10.1210/mend.13.11.0366. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Fan G, Hutnick L. Methyl-CpG binding proteins in the nervous system. Cell Res. 2005;15:255–261. doi: 10.1038/sj.cr.7290294. [DOI] [PubMed] [Google Scholar]
- Flouriot G, Griffin C, Kenealy M, Sonntag-Buck V, Gannon F. Differentially expressed messenger RNA isoforms of the human estrogen receptor-{alpha} gene are generated by alternative splicing and promoter usage. Mol. Endocrinol. 1998;12:1939–1954. doi: 10.1210/mend.12.12.0209. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, De Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiman TM, Muegge K. DNA methylation in early development. Mol. Reprod. Dev. 2009;77:105–113. doi: 10.1002/mrd.21118. [DOI] [PubMed] [Google Scholar]
- Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog. Horm. Res. 1999;54:291–313. [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Differential effects of dihydrotestosterone and estrogen on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. Dev. Neurobiol. 2004;25:878–892. doi: 10.1002/neu.480250711. [DOI] [PubMed] [Google Scholar]
- Gorski RA. Sexual dimorphisms of the brain. J. Anim. Sci. 1985;61:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- Grandien K, Backdahl M, Ljunggren O, Gustafsson JA, Berkenstam A. Estrogen target tissue determines alternative promoter utilization of the human estrogen receptor gene in osteoblasts and tumor cell lines. Endocrinology. 1995;136:2223–2229. doi: 10.1210/endo.136.5.7720671. [DOI] [PubMed] [Google Scholar]
- Grandien KFH, Berkenstam A, Nilsson S, Gustafsson JA. Localization of DNase I hypersensitive sites in the human oestrogen receptor gene correlates with the transcriptional activity of two differentially used promoters. J. Mol. Endocrinol. 1993;10:269–277. doi: 10.1677/jme.0.0100269. [DOI] [PubMed] [Google Scholar]
- Greene G, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Gartler SM. 5-Azacytidine-induced reactivation of the human X chromosome-linked PGK1 gene is associated with a large region of cytosine demethylation in the 5′CpG island. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4174–4178. doi: 10.1073/pnas.87.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi H, Inoue S, Watanabe T, Goto W, Orimo A, Momoeda M, Tsutsumi O, Taketani Y, Muramatsu M. Differential immunolocalization of estrogen receptor alpha and beta in rat ovary and uterus. J. Mol. Endocrinol. 1999;22:37. doi: 10.1677/jme.0.0220037. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol. Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Hsu HK, Yang RC, Shih HC, Hsieh YL, Chen U. Prenatal exposure of testosterone prevents SDN-POA neurons of postnatal male rats from apoptosis through NMDA receptor. J. Neurophysiol. 2001;86:2374. doi: 10.1152/jn.2001.86.5.2374. [DOI] [PubMed] [Google Scholar]
- Illingworth R, Kerr A, DeSousa D, Jorgensen H, Ellis P, Stalker J, Jackson D, Clee C, Plumb R, Rogers J. A novel CpG island set identifies tissue-specific methylation at developmental gene loci. PLoS Biol. 2008;6:e22. doi: 10.1371/journal.pbio.0060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JÅ, Rissman EF. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol. Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Kos M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ER {alpha} gene promoter region. Mol. Endocrinol. 2001;15:2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreżel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JÅ, Rissman EF. A previously uncharacterized role for estrogen receptor β: Defeminization of male brain and behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4608. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa A, Michopoulos V, Gatewood J, Rissman E. Roles of estrogen receptors α and β in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5925. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. Sex Differences in Epigenetic Regulation of the Estrogen Receptor-{alpha} Promoter within the Developing Preoptic Area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Pfaff DW. Gene expression for estrogen and progesterone receptor mRNAs in rat brain and possible relations to sexually dimorphic functions. J. Steroid Biochem. Mol. Biol. 1991;40:53–62. doi: 10.1016/0960-0760(91)90167-4. [DOI] [PubMed] [Google Scholar]
- Lemmen JG, Broekhof JLM, Kuiper GGJM, Gustafsson JÅ, van der Saag PT, van der Burg B. Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mech. Dev. 1999;81:163–167. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Quadros PS, Wagner CK. Effects of neonatal RU486 on adult sexual, parental, and fearful behaviors in rats. Behav. Neurosci. 2001;115:58–70. doi: 10.1037/0735-7044.115.1.58. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS. Progestin receptors in the developing rat brain and pituitary. Brain Res. 1980;189:262–268. doi: 10.1016/0006-8993(80)90026-8. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schuetz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendo. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol. Jpn. 1983;30:277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- Mayer W, Smith A, Fundele R, Haaf T. Spatial separation of parental genomes in preimplantation mouse embryos. J. Cell Biol. 2000;148:629–634. doi: 10.1083/jcb.148.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: Mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm. Behav. 2009a;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The Epigenetics of Sex Differences in the Brain. J. Neurosci. 2009b;29:12815. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M, Schlenker E, Pfaff D. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor {alpha} transcripts with alternative 5′-untranslated regions in the female rat preoptic area. J. Endocrinol. 2007;194:201–212. doi: 10.1677/JOE-07-0014. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:1476. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Taylor J, Lubahn D, Korach K, Pfaff D. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendo. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–258. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Olmos G, Naftolin F, Perez J, Tranque PA, Garcia-Segura LM. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32:663–667. doi: 10.1016/0306-4522(89)90288-1. [DOI] [PubMed] [Google Scholar]
- Orikasa C, Sakuma Y. Estrogen down-regulates estrogen receptor beta expression in the rat ventromedial hypothalamus. Jpn. J. Physiol. 2004;54:S223. [Google Scholar]
- Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J. Active demethylation of the paternal genome in the mouse zygote. Cur. Biol. 2000;10:475–478. doi: 10.1016/s0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J. Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev AV, Gotz F, Rohde W. Differential role of estrogen receptor isoforms in sex-specific brain organization. FASEB. 2004;18:1568–1570. doi: 10.1096/fj.04-1959fje. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LAM. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front. Neuroendocrinol. 2009;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizational action of prenatally administered testosterone propionate on the tissues mediating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Price RH, Lorenzon N, Handa RJ. Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Mol. Brain Res. 2000;80:260–268. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Wagner CK. Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology. 2008;149:3054–1133. doi: 10.1210/en.2007-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol. Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat. Rev. Gen. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17β-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (BERKO) mice. Psychopharmacology. 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Epigenetic marking of the BDNF gene by early-life adverse experiences. Horm. Behav. 2010 doi: 10.1016/j.yhbeh.2010.05.005. current issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier N, Bourc'his D, Gomes DM, Niveleau A, Plachot M, Pàldi A, Viegas-Péquignot E. Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 1998;12:2108–2113. doi: 10.1101/gad.12.14.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Kotcherguina L, Dharia A, Fujimoto S, Dahiya R. Cytosinephosphoguanine methylation of estrogen receptors in endometrial cancer. Cancer Res. 2001a;61:3262–3266. [PubMed] [Google Scholar]
- Sasaki M, Dharia A, Oh BR, Tanaka Y, Fujimoto S, Dahiya R. Progesterone receptor B gene inactivation and CpG hypermethylation in human uterine endometrial cancer. Cancer Res. 2001b;61:97–102. [PubMed] [Google Scholar]
- Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. JNCI. 2002;94:384–390. doi: 10.1093/jnci/94.5.384. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Stoler MH, Shupnik MA. Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ER {alpha} protein and estrogen responsiveness. Endocrinology. 2000;141:2174–2184. doi: 10.1210/endo.141.6.7505. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Hormonally-induced epigenetic changes to estrogen and progesterone receptor genes in brain regions critical to sex differences in behavior. Endo. 2010 doi: 10.1210/en.2010-0142. published onilne August 11, 2010 as doi:10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei F, Rahnama F, Pawella L, Mitchell M, Gluckman P, Lobie P. DNMT3A and DNMT3B mediate autocrine hGH repression of plakoglobin gene transcription and consequent phenotypic conversion of mammary carcinoma cells. Oncogene. 2007;27:2602–2612. doi: 10.1038/sj.onc.1210917. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative Distribution of Estrogen Receptor-α and-β mRNA in the Rat Central Nervous System. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Stoica GE, Franke TF, Moroni M, Mueller S, Morgan E, Iann MC, Winder AD, Reiter R, Wellstein A, Martin MB. Effect of estradiol on estrogen receptor-α gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003;22:7998–8011. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm. Behav. 2005;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA/kainate receptors, not GABAA receptors, mediate estradiol-induced sex differences in the hypothalamus. J. Neurobiol. 2007;67:304–315. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- Torres-Aleman I, Rejas MT, Pons S, Garcia-Segura LM. Estradiol promotes cell shape changes and glial fibrillary acidic protein redistribution in hypothalamic astrocytes in vitro: a neuronal-mediated effect. Glia. 1992;6:180–187. doi: 10.1002/glia.440060305. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor {beta} Mol. Endocrinol. 1997;11:353. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology. 1998;139:3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor expression. J. Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]
- Walker DM, Juenger TE, Gore AC. Developmental Profiles of Neuroendocrine Gene Expression in the Preoptic Area of Male Rats. Endocrinology. 2009;150:2308–2316. doi: 10.1210/en.2008-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schübeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat. Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic Regulation of Estrogen Receptor {alpha} Gene Expression in the Mouse Cortex during Early Postnatal Development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev. Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM. Prostaglandin E2-Induced Masculinization of Brain and Behavior Requires Protein Kinase A, AMPA/Kainate, and Metabotropic Glutamate Receptor Signaling. J. Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y, Asada H, Tamura I, Lee L, Maekawa R, Taniguchi K, Taketani T, Matsuoka A, Tamura H, Sugino N. DNA methyltransferase expression in the human endometrium: down-regulation by progesterone and estrogen. Hum. Reprod. 2009;24:1126–1132. doi: 10.1093/humrep/dep015. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal Development and Sex Difference in Neurons Containing Estrogen Receptor-α Immunoreactivity in the Preoptic Brain, the Diencephalon, and the Amygdala in the Rat. J. Comp. Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150:4681–4691. doi: 10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]


