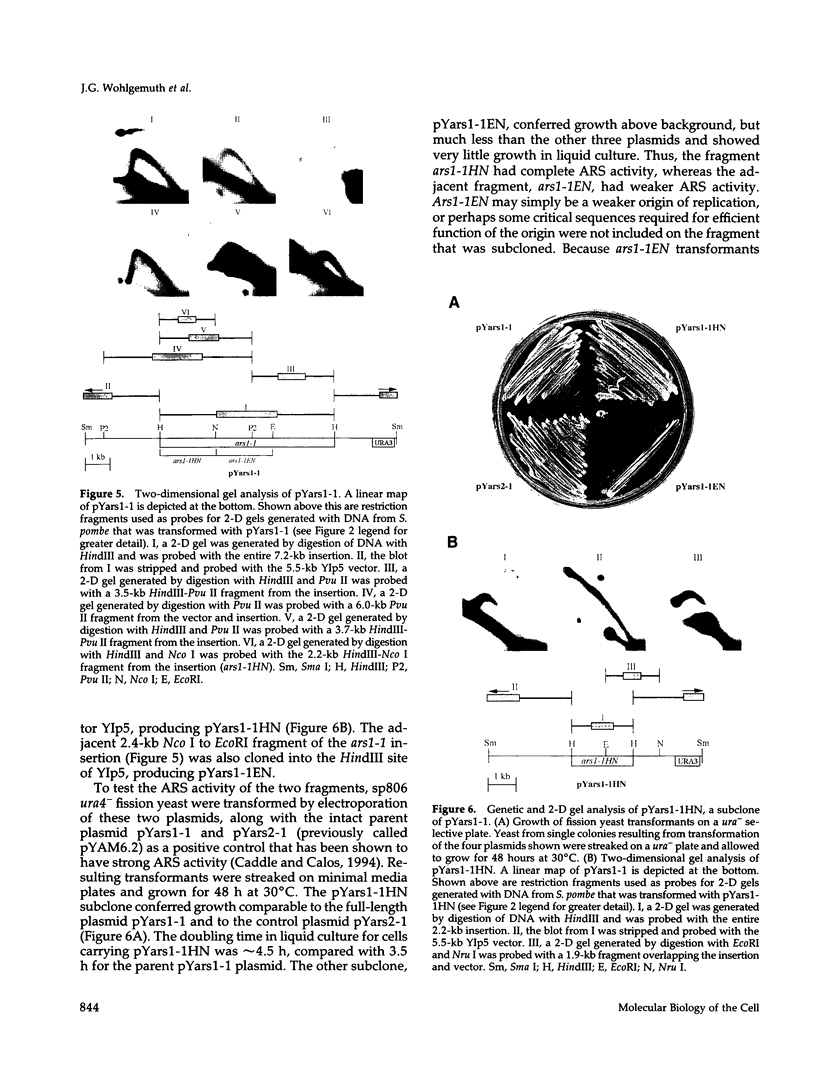
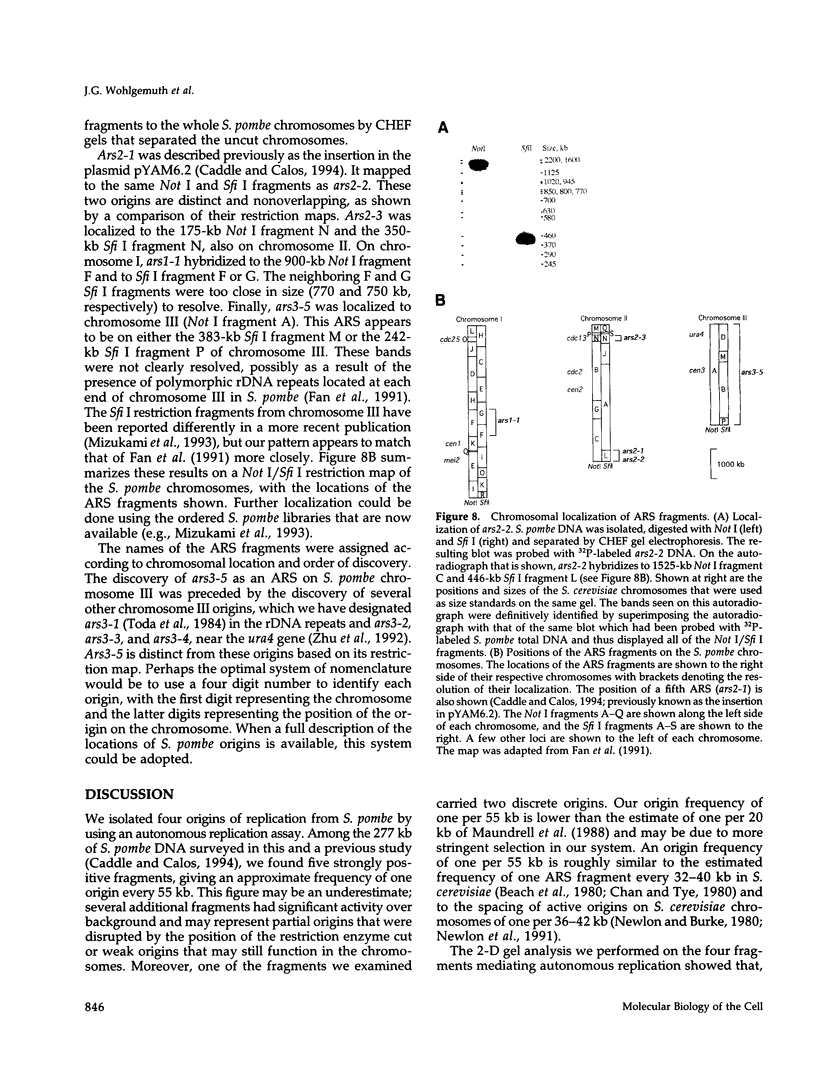
Abstract
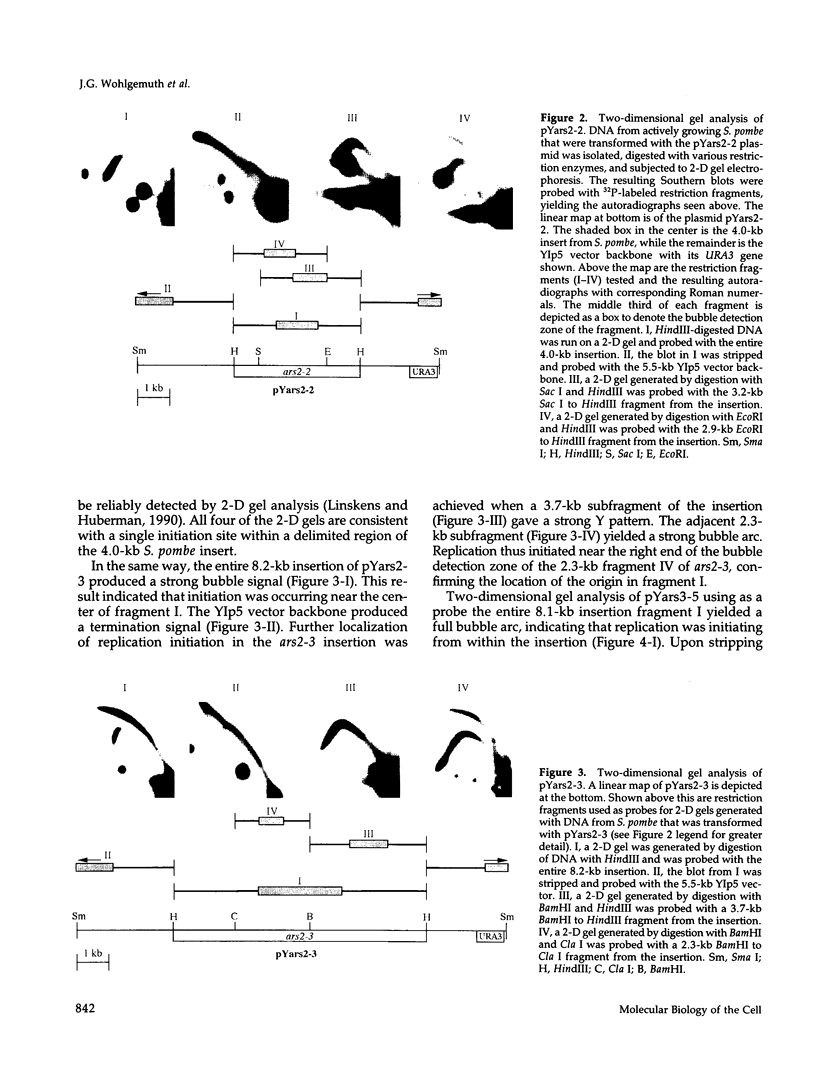
We isolated four fragments from the Schizosaccharomyces pombe genome that mediate autonomous replication. A two-dimensional gel analysis revealed that in each case initiation could be mapped to within the S. pombe sequences. In three of the fragments, initiation could be mapped to one discrete location. In the fourth fragment, subcloning and two-dimensional gel analysis suggested that two discrete origins of replication were located within 3 kb of each other. When in proximity, usually only one of these origins fired, suggesting origin interference. Two-dimensional gel analysis of the four origin fragments at their genomic locations demonstrated that each is used in the chromosomes, but in only a subset of cells or cell divisions. The S. pombe genome appears to contain many discrete origins, not all of which fire in any given cell and some of which are closely spaced. Not I/Sfi I mapping of the five origins from this and a previous study indicates that they are randomly distributed throughout the genome and appear to be representative of chromosomal origins of replication in this organism. We compare the features of S. pombe replication origins with those of S. cerevisiae and animal cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D., Piper M., Shall S. Isolation of chromosomal origins of replication in yeast. Nature. 1980 Mar 13;284(5752):185–187. doi: 10.1038/284185a0. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. Initiation at closely spaced replication origins in a yeast chromosome. Science. 1993 Dec 10;262(5140):1728–1731. doi: 10.1126/science.8259517. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. Initiation preference at a yeast origin of replication. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3418–3422. doi: 10.1073/pnas.91.8.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Caddle M. S., Calos M. P. Specific initiation at an origin of replication from Schizosaccharomyces pombe. Mol Cell Biol. 1994 Mar;14(3):1796–1805. doi: 10.1128/mcb.14.3.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Autonomously replicating sequences in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6329–6333. doi: 10.1073/pnas.77.11.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L. Centromeres of budding and fission yeasts. Trends Genet. 1990 May;6(5):150–154. doi: 10.1016/0168-9525(90)90149-z. [DOI] [PubMed] [Google Scholar]
- Dershowitz A., Newlon C. S. The effect on chromosome stability of deleting replication origins. Mol Cell Biol. 1993 Jan;13(1):391–398. doi: 10.1128/mcb.13.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A. M., Newlon C. S. The ARS consensus sequence is required for chromosomal origin function in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Oct;12(10):4305–4313. doi: 10.1128/mcb.12.10.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Hamlin J. L. Initiation of DNA replication in the dihydrofolate reductase locus is confined to the early S period in CHO cells synchronized with the plant amino acid mimosine. Mol Cell Biol. 1992 Sep;12(9):3715–3722. doi: 10.1128/mcb.12.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey D. D., Davis L. R., Greenfeder S. A., Ong L. Y., Zhu J. G., Broach J. R., Newlon C. S., Huberman J. A. Evidence suggesting that the ARS elements associated with silencers of the yeast mating-type locus HML do not function as chromosomal DNA replication origins. Mol Cell Biol. 1991 Oct;11(10):5346–5355. doi: 10.1128/mcb.11.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. B., Grothues D., Smith C. L. Alignment of Sfi I sites with the Not I restriction map of Schizosaccharomyces pombe genome. Nucleic Acids Res. 1991 Nov 25;19(22):6289–6294. doi: 10.1093/nar/19.22.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L., Brewer B. J. Activation of replication origins within yeast chromosomes. Annu Rev Cell Biol. 1991;7:375–402. doi: 10.1146/annurev.cb.07.110191.002111. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Greenfeder S. A., Newlon C. S. A replication map of a 61-kb circular derivative of Saccharomyces cerevisiae chromosome III. Mol Biol Cell. 1992 Sep;3(9):999–1013. doi: 10.1091/mbc.3.9.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S. S., Krysan P. J., Tran C. T., Calos M. P. Autonomous DNA replication in human cells is affected by the size and the source of the DNA. Mol Cell Biol. 1991 Apr;11(4):2263–2272. doi: 10.1128/mcb.11.4.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. L., Carbon J. High-frequency transformation of yeast by plasmids containing the cloned yeast ARG4 gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3829–3833. doi: 10.1073/pnas.76.8.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Spotila L. D., Nawotka K. A., el-Assouli S. M., Davis L. R. The in vivo replication origin of the yeast 2 microns plasmid. Cell. 1987 Nov 6;51(3):473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Zhu J. G., Davis L. R., Newlon C. S. Close association of a DNA replication origin and an ARS element on chromosome III of the yeast, Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Jul 25;16(14A):6373–6384. doi: 10.1093/nar/16.14.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O., Méchali M. Plasmid replication in Xenopus eggs and egg extracts: a 2D gel electrophoretic analysis. Nucleic Acids Res. 1992 Apr 11;20(7):1463–1469. doi: 10.1093/nar/20.7.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan P. J., Calos M. P. Replication initiates at multiple locations on an autonomously replicating plasmid in human cells. Mol Cell Biol. 1991 Mar;11(3):1464–1472. doi: 10.1128/mcb.11.3.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan P. J., Smith J. G., Calos M. P. Autonomous replication in human cells of multimers of specific human and bacterial DNA sequences. Mol Cell Biol. 1993 May;13(5):2688–2696. doi: 10.1128/mcb.13.5.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens M. H., Huberman J. A. Ambiguities in results obtained with 2D gel replicon mapping techniques. Nucleic Acids Res. 1990 Feb 11;18(3):647–652. doi: 10.1093/nar/18.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbubani H. M., Paull T., Elder J. K., Blow J. J. DNA replication initiates at multiple sites on plasmid DNA in Xenopus egg extracts. Nucleic Acids Res. 1992 Apr 11;20(7):1457–1462. doi: 10.1093/nar/20.7.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahrens Y., Stillman B. A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science. 1992 Feb 14;255(5046):817–823. doi: 10.1126/science.1536007. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Hutchison A., Shall S. Sequence analysis of ARS elements in fission yeast. EMBO J. 1988 Jul;7(7):2203–2209. doi: 10.1002/j.1460-2075.1988.tb03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami T., Chang W. I., Garkavtsev I., Kaplan N., Lombardi D., Matsumoto T., Niwa O., Kounosu A., Yanagida M., Marr T. G. A 13 kb resolution cosmid map of the 14 Mb fission yeast genome by nonrandom sequence-tagged site mapping. Cell. 1993 Apr 9;73(1):121–132. doi: 10.1016/0092-8674(93)90165-m. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Newlon C. S., Lipchitz L. R., Collins I., Deshpande A., Devenish R. J., Green R. P., Klein H. L., Palzkill T. G., Ren R. B., Synn S. Analysis of a circular derivative of Saccharomyces cerevisiae chromosome III: a physical map and identification and location of ARS elements. Genetics. 1991 Oct;129(2):343–357. doi: 10.1093/genetics/129.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon C. S., Theis J. F. The structure and function of yeast ARS elements. Curr Opin Genet Dev. 1993 Oct;3(5):752–758. doi: 10.1016/s0959-437x(05)80094-2. [DOI] [PubMed] [Google Scholar]
- Newlon C. S. Yeast chromosome replication and segregation. Microbiol Rev. 1988 Dec;52(4):568–601. doi: 10.1128/mr.52.4.568-601.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice H. L. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1992 Feb 11;20(3):621–621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. Schizosaccharomyces pombe and Saccharomyces cerevisiae: a look at yeasts divided. Cell. 1986 Jun 20;45(6):781–782. doi: 10.1016/0092-8674(86)90550-7. [DOI] [PubMed] [Google Scholar]
- Shinomiya T., Ina S. Analysis of chromosomal replicons in early embryos of Drosophila melanogaster by two-dimensional gel electrophoresis. Nucleic Acids Res. 1991 Jul 25;19(14):3935–3941. doi: 10.1093/nar/19.14.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Ina S. DNA replication of histone gene repeats in Drosophila melanogaster tissue culture cells: multiple initiation sites and replication pause sites. Mol Cell Biol. 1993 Jul;13(7):4098–4106. doi: 10.1128/mcb.13.7.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahige K., Iwasaki T., Rashid M. B., Ogasawara N., Yoshikawa H. Location and characterization of autonomously replicating sequences from chromosome VI of Saccharomyces cerevisiae. Mol Cell Biol. 1993 Aug;13(8):5043–5056. doi: 10.1128/mcb.13.8.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. P., Dijkwel P. A., Hamlin J. L. Replication initiates in a broad zone in the amplified CHO dihydrofolate reductase domain. Cell. 1990 Jun 15;61(6):1075–1087. doi: 10.1016/0092-8674(90)90071-l. [DOI] [PubMed] [Google Scholar]
- Zhu J., Brun C., Kurooka H., Yanagida M., Huberman J. A. Identification and characterization of a complex chromosomal replication origin in Schizosaccharomyces pombe. Chromosoma. 1992;102(1 Suppl):S7–16. doi: 10.1007/BF02451780. [DOI] [PubMed] [Google Scholar]