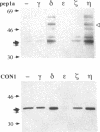
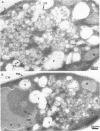
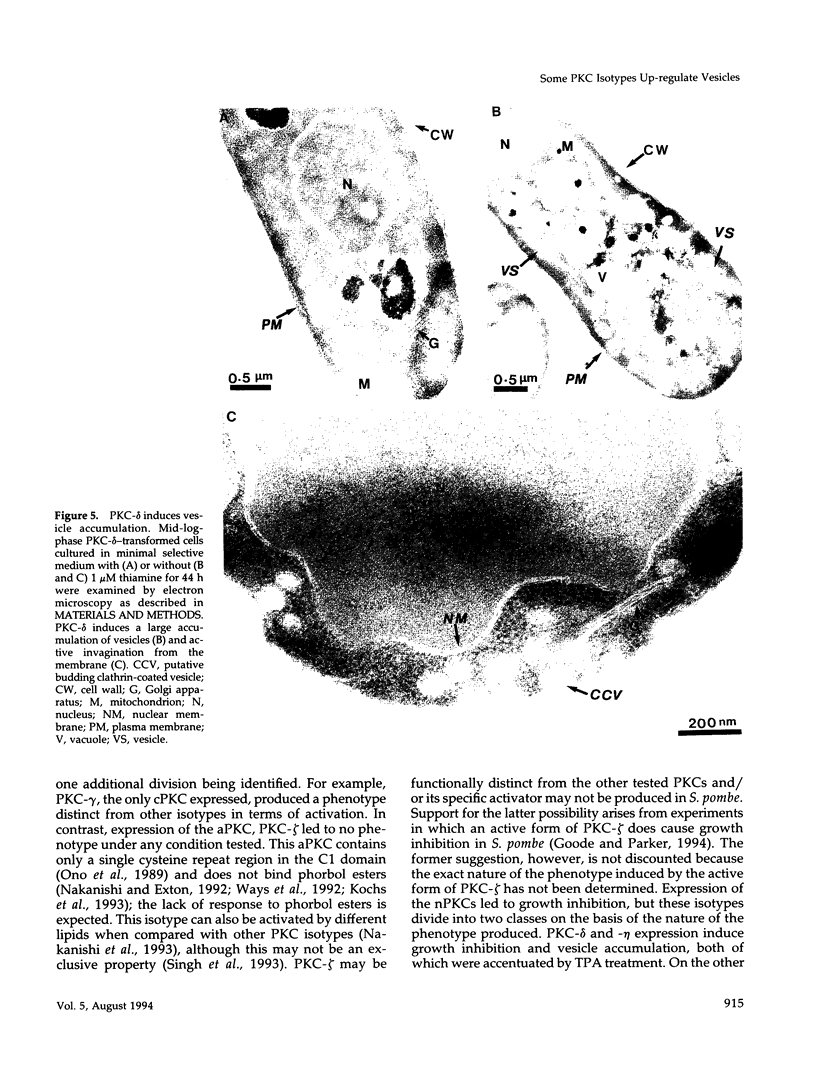
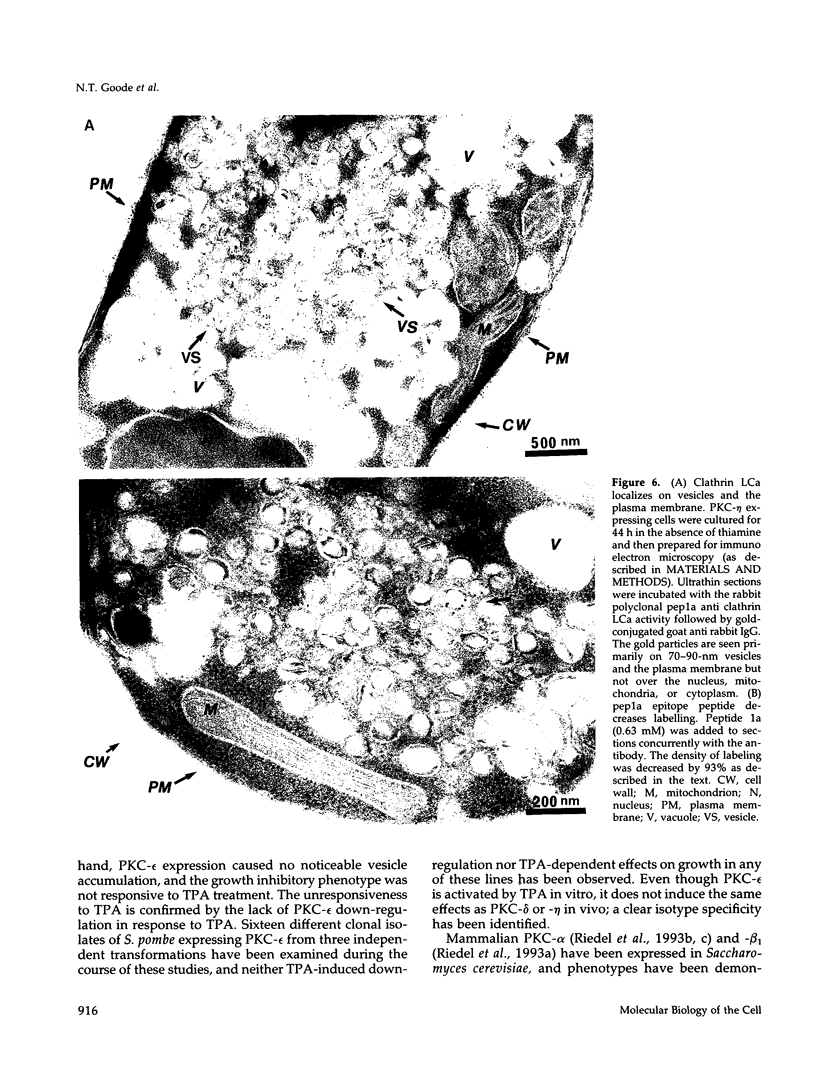
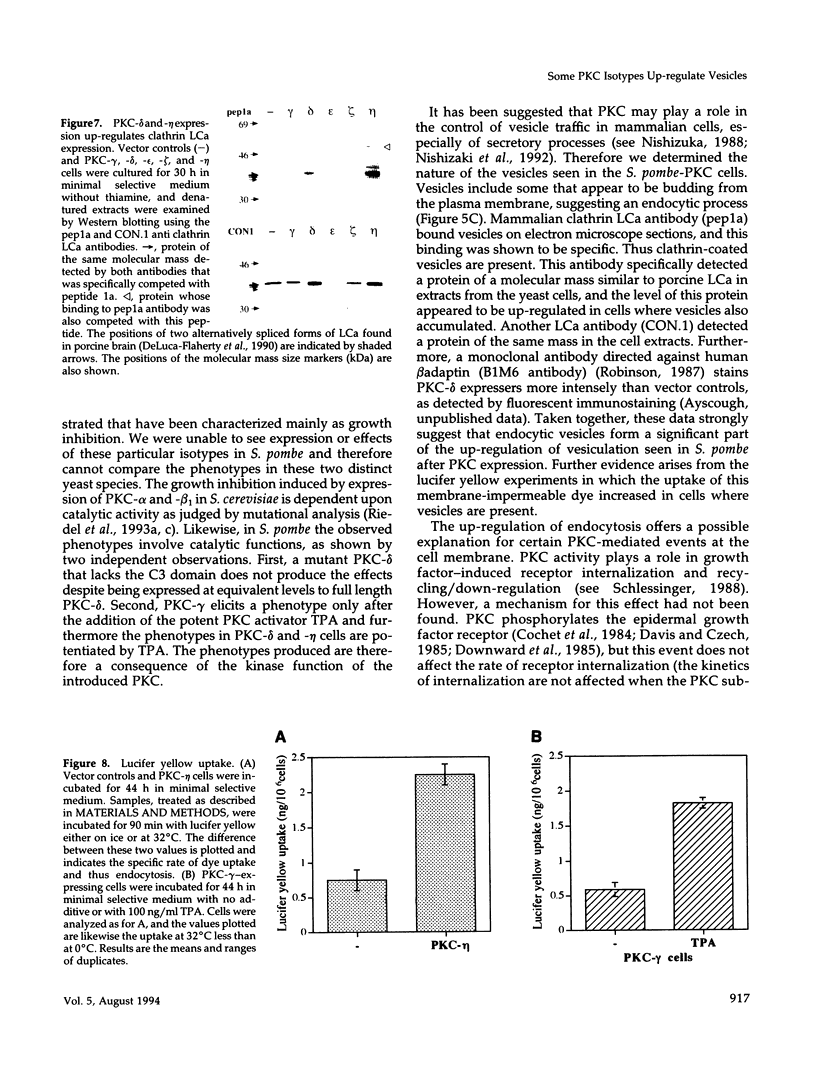
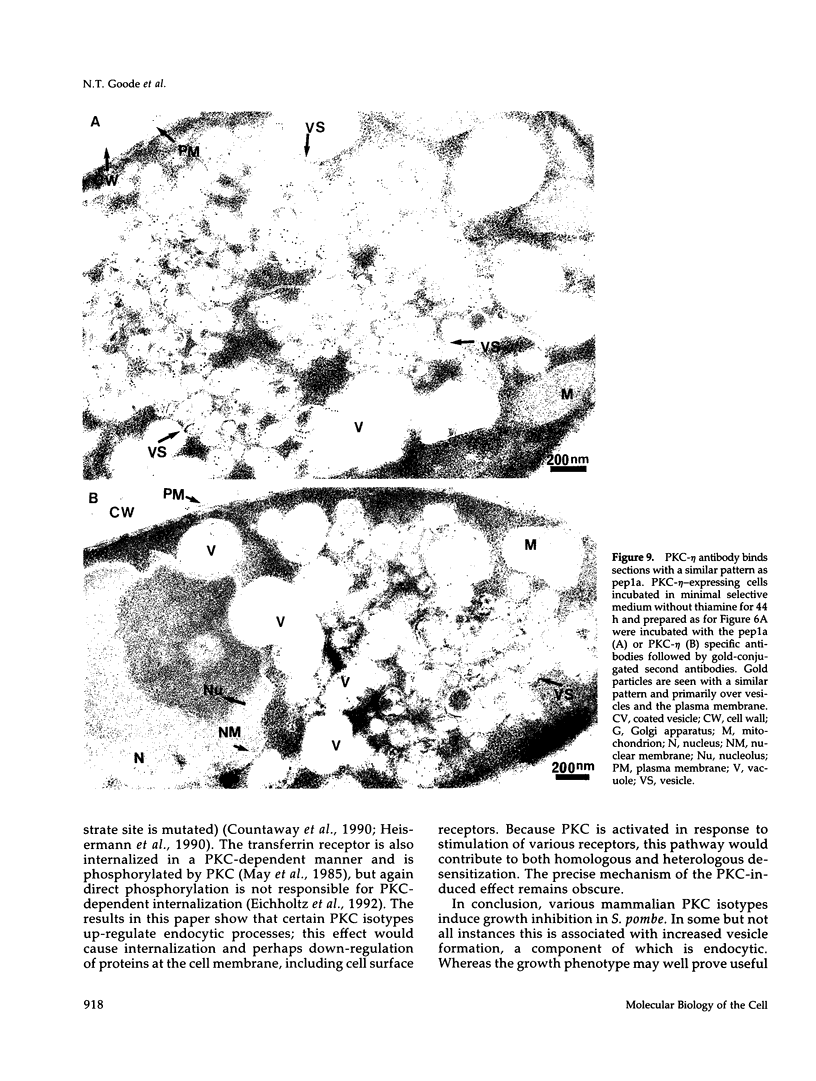
Abstract
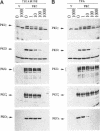
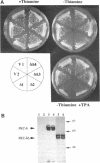
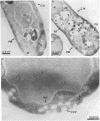
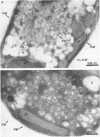
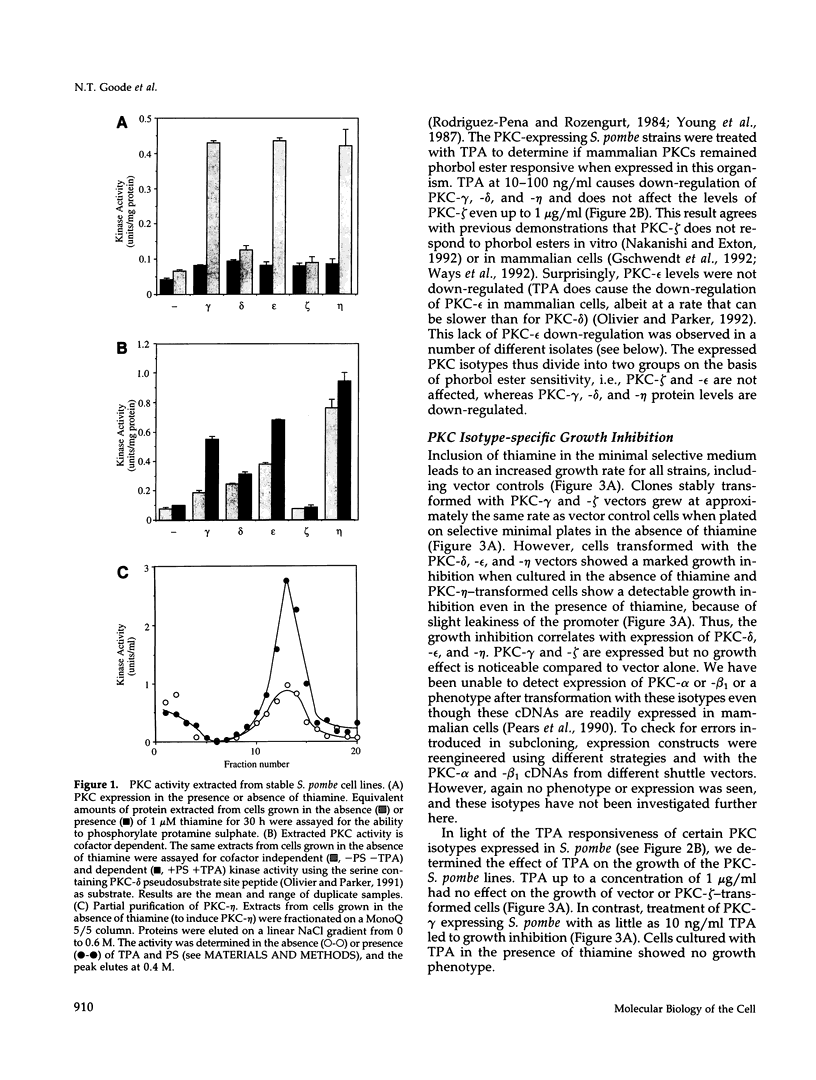
Mammalian protein kinase C (PKC) isotypes elicit a number of effects on expression in Schizosaccharomyces pombe. A small decrease in growth rate results from PKC-gamma expression, and treatment of these cells with phorbol esters leads to marked growth inhibition and vesicle formation. PKC-delta and -eta expression causes growth inhibition and vesiculation, and the magnitude of both of these effects is increased by phorbol esters. In contrast, PKC-epsilon expression produces growth inhibition but no vesicle accumulation, and this effect is not responsive to phorbol ester. Finally, PKC-zeta has no observable effect. Thus, isotype-specific biological effects are observed. The accumulation of vesicles correlates with phorbol ester-dependent growth inhibition and occurs only with expression of those isotypes that down-regulate in response to phorbol esters in these cells. Antibodies against mammalian clathrin light chain 1a identified clathrin-coated vesicles and up-regulation of clathrin expression in those cells where vesicles accumulate; the increased vesicular traffic includes an element of endocytosis. Thus expression of specific mammalian PKC isotypes up-regulates endocytosis in S. pombe, providing a likely explanation for PKC-mediated receptor internalization in higher eukaryotes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Nelsestuen G. L. Substrate-specific stimulation of protein kinase C by polyvalent anion. Biochem Biophys Res Commun. 1987 Aug 31;147(1):248–253. doi: 10.1016/s0006-291x(87)80113-4. [DOI] [PubMed] [Google Scholar]
- Choi K. W., Smith R. F., Buratowski R. M., Quinn W. G. Deficient protein kinase C activity in turnip, a Drosophila learning mutant. J Biol Chem. 1991 Aug 25;266(24):15999–15606. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Countaway J. L., McQuilkin P., Gironès N., Davis R. J. Multisite phosphorylation of the epidermal growth factor receptor. Use of site-directed mutagenesis to examine the role of serine/threonine phosphorylation. J Biol Chem. 1990 Feb 25;265(6):3407–3416. [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B., Parham P., Hill B. L. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell. 1990 Sep 7;62(5):875–887. doi: 10.1016/0092-8674(90)90263-e. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J., McIntyre P. Biochemical properties of rat protein kinase C-eta expressed in COS cells. FEBS Lett. 1992 Nov 9;312(2-3):195–199. doi: 10.1016/0014-5793(92)80934-9. [DOI] [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Eichholtz T., Vossebeld P., van Overveld M., Ploegh H. Activation of protein kinase C accelerates internalization of transferrin receptor but not of major histocompatibility complex class I, independent of their phosphorylation status. J Biol Chem. 1992 Nov 5;267(31):22490–22495. [PubMed] [Google Scholar]
- Goode N. T., Parker P. J. A phorbol ester-responsive PKC-zeta generated by fusion with the regulatory domain of PKC-delta. FEBS Lett. 1994 Feb 28;340(1-2):145–150. doi: 10.1016/0014-5793(94)80190-8. [DOI] [PubMed] [Google Scholar]
- Goode N., Hughes K., Woodgett J. R., Parker P. J. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992 Aug 25;267(24):16878–16882. [PubMed] [Google Scholar]
- Gschwendt M., Leibersperger H., Kittstein W., Marks F. Protein kinase C zeta and eta in murine epidermis. TPA induces down-regulation of PKC eta but not PKC zeta. FEBS Lett. 1992 Jul 28;307(2):151–155. doi: 10.1016/0014-5793(92)80756-7. [DOI] [PubMed] [Google Scholar]
- Ha K. S., Exton J. H. Differential translocation of protein kinase C isozymes by thrombin and platelet-derived growth factor. A possible function for phosphatidylcholine-derived diacylglycerol. J Biol Chem. 1993 May 15;268(14):10534–10539. [PubMed] [Google Scholar]
- Heisermann G. J., Wiley H. S., Walsh B. J., Ingraham H. A., Fiol C. J., Gill G. N. Mutational removal of the Thr669 and Ser671 phosphorylation sites alters substrate specificity and ligand-induced internalization of the epidermal growth factor receptor. J Biol Chem. 1990 Aug 5;265(22):12820–12827. [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O'Brian C. A., Murphy J. P., Kirschmeier P., Weinstein I. B. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988 Feb 12;52(3):343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- Ido M., Sekiguchi K., Kikkawa U., Nishizuka Y. Phosphorylation of the EGF receptor from A431 epidermoid carcinoma cells by three distinct types of protein kinase C. FEBS Lett. 1987 Jul 13;219(1):215–218. doi: 10.1016/0014-5793(87)81219-x. [DOI] [PubMed] [Google Scholar]
- Kochs G., Hummel R., Meyer D., Hug H., Marmé D., Sarre T. F. Activation and substrate specificity of the human protein kinase C alpha and zeta isoenzymes. Eur J Biochem. 1993 Sep 1;216(2):597–606. doi: 10.1111/j.1432-1033.1993.tb18179.x. [DOI] [PubMed] [Google Scholar]
- Marais R. M., Nguyen O., Woodgett J. R., Parker P. J. Studies on the primary sequence requirements for PKC-alpha, -beta 1 and -gamma peptide substrates. FEBS Lett. 1990 Dec 17;277(1-2):151–155. doi: 10.1016/0014-5793(90)80831-3. [DOI] [PubMed] [Google Scholar]
- Marais R. M., Parker P. J. Purification and characterisation of bovine brain protein kinase C isotypes alpha, beta and gamma. Eur J Biochem. 1989 Jun 1;182(1):129–137. doi: 10.1111/j.1432-1033.1989.tb14809.x. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- May W. S., Sahyoun N., Jacobs S., Wolf M., Cuatrecasas P. Mechanism of phorbol diester-induced regulation of surface transferrin receptor involves the action of activated protein kinase C and an intact cytoskeleton. J Biol Chem. 1985 Aug 5;260(16):9419–9426. [PubMed] [Google Scholar]
- Mazzei G. J., Schmid E. M., Knowles J. K., Payton M. A., Maundrell K. G. A Ca(2+)-independent protein kinase C from fission yeast. J Biol Chem. 1993 Apr 5;268(10):7401–7406. [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Brewer K. A., Exton J. H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993 Jan 5;268(1):13–16. [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- Nishizaki T., Walent J. H., Kowalchyk J. A., Martin T. F. A key role for a 145-kDa cytosolic protein in the stimulation of Ca(2+)-dependent secretion by protein kinase C. J Biol Chem. 1992 Nov 25;267(33):23972–23981. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Näthke I. S., Heuser J., Lupas A., Stock J., Turck C. W., Brodsky F. M. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992 Mar 6;68(5):899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J Biol Chem. 1994 Jan 28;269(4):2758–2763. [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Expression and characterization of protein kinase C-delta. Eur J Biochem. 1991 Sep 15;200(3):805–810. doi: 10.1111/j.1432-1033.1991.tb16248.x. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Identification of multiple PKC isoforms in Swiss 3T3 cells: differential down-regulation by phorbol ester. J Cell Physiol. 1992 Aug;152(2):240–244. doi: 10.1002/jcp.1041520204. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Patel G., Stabel S. Expression of a functional protein kinase C-gamma using a baculovirus vector: purification and characterisation of a single protein kinase C iso-enzyme. Cell Signal. 1989;1(3):227–240. doi: 10.1016/0898-6568(89)90040-5. [DOI] [PubMed] [Google Scholar]
- Pears C. J., Kour G., House C., Kemp B. E., Parker P. J. Mutagenesis of the pseudosubstrate site of protein kinase C leads to activation. Eur J Biochem. 1990 Nov 26;194(1):89–94. doi: 10.1111/j.1432-1033.1990.tb19431.x. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Crowther R. A. Structure and assembly of coated vesicles. Annu Rev Biophys Biophys Chem. 1987;16:49–68. doi: 10.1146/annurev.bb.16.060187.000405. [DOI] [PubMed] [Google Scholar]
- Riedel H., Hansen H., Parissenti A. M., Su L., Shieh H. L., Zhu J. Phorbol ester activation of functional rat protein kinase C beta-1 causes phenotype in yeast. J Cell Biochem. 1993 Jul;52(3):320–329. doi: 10.1002/jcb.240520308. [DOI] [PubMed] [Google Scholar]
- Riedel H., Parissenti A. M., Hansen H., Su L., Shieh H. L. Stimulation of calcium uptake in Saccharomyces cerevisiae by bovine protein kinase C alpha. J Biol Chem. 1993 Feb 15;268(5):3456–3462. [PubMed] [Google Scholar]
- Riedel H., Su L., Hansen H. Yeast phenotype classifies mammalian protein kinase C cDNA mutants. Mol Cell Biol. 1993 Aug;13(8):4728–4735. doi: 10.1128/mcb.13.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985 Apr;40(4):1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987 Apr;104(4):887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J., Bristol A., Kriz R., Knopf J. Unique substrate specificity and regulatory properties of PKC-epsilon: a rationale for diversity. FEBS Lett. 1989 Jan 30;243(2):351–357. doi: 10.1016/0014-5793(89)80160-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer E., Smith D., Mardon G., Quinn W., Zuker C. Isolation and characterization of two new drosophila protein kinase C genes, including one specifically expressed in photoreceptor cells. Cell. 1989 May 5;57(3):403–412. doi: 10.1016/0092-8674(89)90915-x. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988 May 3;27(9):3119–3123. doi: 10.1021/bi00409a002. [DOI] [PubMed] [Google Scholar]
- Sheu F. S., Marais R. M., Parker P. J., Bazan N. G., Routtenberg A. Neuron-specific protein F1/GAP-43 shows substrate specificity for the beta subtype of protein kinase C. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1236–1243. doi: 10.1016/0006-291x(90)90818-8. [DOI] [PubMed] [Google Scholar]
- Singh S. S., Chauhan A., Brockerhoff H., Chauhan V. P. Activation of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. Biochem Biophys Res Commun. 1993 Aug 31;195(1):104–112. doi: 10.1006/bbrc.1993.2016. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Ranganathan R., Hardy R. W., Marx J., Tsuchida T., Zuker C. S. Photoreceptor deactivation and retinal degeneration mediated by a photoreceptor-specific protein kinase C. Science. 1991 Dec 6;254(5037):1478–1484. doi: 10.1126/science.1962207. [DOI] [PubMed] [Google Scholar]
- Stabel S., Parker P. J. Protein kinase C. Pharmacol Ther. 1991;51(1):71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Yanagida M. Two novel protein kinase C-related genes of fission yeast are essential for cell viability and implicated in cell shape control. EMBO J. 1993 May;12(5):1987–1995. doi: 10.1002/j.1460-2075.1993.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways D. K., Cook P. P., Webster C., Parker P. J. Effect of phorbol esters on protein kinase C-zeta. J Biol Chem. 1992 Mar 5;267(7):4799–4805. [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]