Abstract
A splicing mutation in the IKBKAP gene encoding the IKAP/hELP1 (IKAP) protein was found to be the major cause of Familial Dysautonomia (FD). This mutation affects both the normal development and survival of sensory and sympathetic neurons of the peripheral nervous system (PNS). To understand the FD phenotype it is important to study the specific role played by IKAP in developing and mature PNS neurons. We used the neuroblastoma SHSY5Y cell line, originated from neural crest adrenal tumor and simulated the FD phenotype by reducing IKAP expression with retroviral constructs. We observed that IKAP-downregulated cells formed cell clusters compared to control cells under regular culture conditions. We examined the ability of these cells to differentiate into mature neurons in the presence of laminin, an essential extracellular matrix for developing PNS neurons. We found that the cells showed reduced attachment to laminin, morphological changes and increased cell-to-cell adhesion resulting in cell aggregates. We identified Contactin as the adhesion molecule responsible for this phenotype. We show that Contactin expression is related to IKAP expression, suggesting that IKAP regulates Contactin levels for appropriate cell-cell adhesion that could modulate neuronal growth of PNS neurons during development.
Key words: Familial Dysautonomia, IKAP/hELP1, neuronal differentiation, laminin, contactin, peripheral nervous system
Introduction
Familial Dysautonomia (FD) is an autosomal recessive neurodegenerative disease characterized by abnormal development and function of the sensory and autonomic nervous systems.1,2 Among the neuronal pathology findings are decreased numbers of sympathetic neurons as well as the absence of autonomic nerve terminals on peripheral blood vessels. Also, the development and maintenance of sensory neurons in the dorsal root ganglia and spinal cord are affected, exhibiting further depletion with age, especially of sensory myelinated axons.2
In 99.5% of the diagnosed patients a mutation in the donor splice site of intron 20 of the IKBKAP gene was found. This mutation causes skipping of exon 20 and premature open reading frame termination of the IKBKAP gene. However, the expression pattern of IKAP in FD patients (homozygous for the splicing mutation) is unique: In non-neuronal cells both the wild-type mRNA and the expected mutant mRNA lacking exon 20 can be found, the latter being more abundant. In contrast, in neuronal tissues, the wt mRNA cannot be detected and the mutant mRNA levels are very low demonstrating that in neuronal tissues the splicing of IKAP is severely hampered, leading to the absence (below detectable levels) of the 150 kDa mature IKAP protein in a tissue-specific manner.1,3,4 The other minor mutation found in FD patients is a G → C change at base pair 2,397 in exon 19, which causes an Arginine to Proline missense mutation. This mutation was shown, in vitro, to disrupt a potential Threonine phosphorylation site at residue 699.3
The function of IKAP in human cells in general and in neural cells in particular has not yet been elucidated. The protein contains WD40 motifs and TPR domains (Cohen-Kupiec R, unpublished), implicated in protein-protein interactions5,6 suggesting that IKAP functions as a scaffold for protein interactions. IKAP/Elp1 was indeed shown to be a subunit of Elongator complex, in both yeast and mammalian cells.7,8 The complex binds RNA polymerase II and possesses a histone acetyl transferase (HAT) activity, through its catalytic subunit Elp3.8 A few functions have been attributed to the Elongator complex in yeast, among which are transcription elongation through histones acetylation by Elp3,9 polarized exocytosis,10 and tRNA modification.11 As a complex involved in transcription, IKAP in HeLa cells was shown to be involved in the transcription of genes of diverse molecular functions.12 Recently, a role for Elongator complex in zygotic paternal demethylation through the SAM radical domain, but not the HAT domain of ELP3 was demonstrated in the mouse.13 Also, involvement of IKAP in cytoskeleton-dependent functions such as cellular spreading, adhesion and migration was demonstrated in murine fibroblasts and primary cerebral granule neurons, where depletion of IKAP affected Filamin A distribution and actin organization.14 It has also been shown that defective Elongator caused reduced acetylated alpha tubulin levels, which affected the cytoskeleton of cortical neurons, leading to reduced migration of projection neurons to the cerebral cortex in mice.15
The crucial role of IKAP in early development was demonstrated in experiments where IKAP-knocked out mouse embryos died at day 12 post coitum because of poor development.16 It is clear that the differential splicing and hence, the expression of mutant IKAP in neuronal tissues compared to other tissues, defines the FD phenotype. The peripheral nervous system (PNS) which includes the sensory and autonomic nervous systems, defective in FD, develops from the embryonic neural crest cells. To date there is no good model in which the importance of IKAP in early developmental stages (and particularly those of the peripheral nervous system) can be examined. The reduced numbers of autonomic and sensory neurons in FD patients suggest that IKAP-deficient cells either have difficulties to develop into mature peripheral neurons or have a low survival rate as mature neurons. In order to examine the importance of IKAP in the ability of cells from neural crest origin to differentiate into mature neurons, we used SHSY5Y cells in which we downregulated IKAP expression. Our results show that Contactin 1, a GPI-anchored cell surface protein with a role in cell adhesion, was upregulated in IKAP-depleted cells. This caused the cells to adhere strongly to each other and affected their attachment to laminin, and therefore the outgrowth of neurites.
Results
Downregulation of IKAP expression in SH-SY5Y neuroblastoma cells causes cells to aggregate.
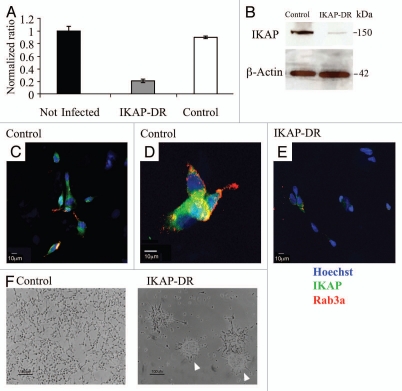
We have used lentiviruses carrying IKAP shRNA sequences to downregulate the expression of IKAP in the neuroblastoma cell line SHSY5Y. This cell line is of neural crest origin17 and was selected by three rounds of subcloning from an original clone derived from human metastatic neuroblastoma tissue.18,19 We used five different individual IKAP shRNA sequences and examined the expression of IKAP in all SHSY5Y stable cell lines generated. Out of them, we chose clone SHSY5Y-V71 in which IKAP expression was significantly downregulated (designated IKAP-DR). As a control we used cells infected with an empty viral vector (designated control). Figure 1A shows that IKAP mRNA expression was reduced by more than 5-fold in IKAP-DR cells Followed by significantly reduced protein levels in this cell line, as determined by western blot analysis (Fig. 1B). The expression of IKAP was also measured by immunocytochemistry of the IKAP-DR and control cell lines. Figure 1C–E shows a significant reduction of IKAP protein expression in IKAP-DR cells, compared to the control. In addition, these IKAP-DR cells were not stained with rab3A, a neuronal vesicular marker that stained the neurites of the control cells (Fig. 1D and E). In IKAP-DR, clear morphological changes could be detected that can be attributed to the reduction in IKAP expression. Unlike the monolayers formed by the control cells, IKAP-DR cells tended to aggregate with time in what is considered to be neuroblastic immature spheres17 (Fig. 1F). These sphere-like aggregates detached at a certain point from the plate surface and floated in the medium as indicated by arrowheads in Figure 1F.
Figure 1.
IKAP downregulation. (A) Graphic summary of qRT-PCR showing the relative expression of IKBAP mRNA in control and IKAP shRNA infected cells (IKAP-DR) compared to SHSY5Y non-infected cells. (B) Western blot analysis showing the reduced IKAP protein expression in IKAP-DR cells compared to control, beta actin expression was used for normalization. (C–E) Immunofluorescence staining of control [C and D (higher magnification)] and of IKAP-DR cells (E), IKAP is stained in green, nuclei are stained in blue (Hoechst) and the neuronal marker Rab3a is stained in red. (F) control and IKAP-DR cells morphology when grown on regular plates with selective growth medium. Arrow heads show the spheres of IKAP-DR cells.
IKAP downregulation affects the adhesion and spreading of SHSY5Y cells.
The morphological changes we observed in the IKAP-DR cells suggested that these cells went back to neuroblastic spheres and the question of whether they can be differentiated into neurons was raised. We hence carried out a protocol for enhanced neuronal differentiation that has been established before for SHSY5Y cells.20,21 This protocol includes the incubation of the cells with retinoic acid (RA) for five days followed by incubation in a serum-free medium supplemented with brain-derived neurotrophic factor (BDNF). At this stage, SHSY5Y cells should develop typical bipolar neuronal morphology.
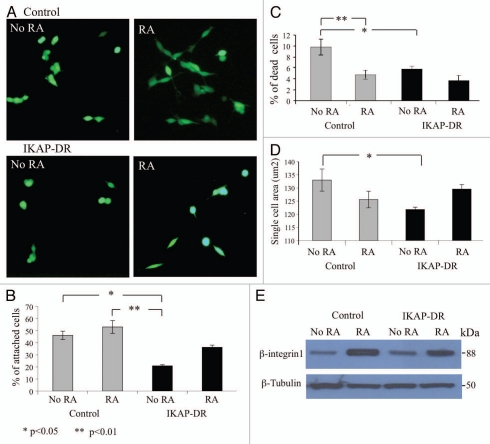
In order to investigate the IKAP downregulation effect on IKAP-DR cells throughout the differentiation protocol we examined first in the presence or absence of RA the ability of IKAP-DR and control cells to attach to plates coated with laminin, which is known to support differentiation of PNS neurons,22–24 followed by viability assay as described in the Materials and Methods section. Figure 2 shows that IKAP-DR cells exhibited a delayed and reduced attachment to laminin, compared to the control: only about 20% of the IKAP-DR cells adhered to the plates (Fig. 2B). The addition of RA to the medium improved the ability of both control and IKAP-DR cells to adhere to laminin. Cell death of IKAP-DR cells (as determined by the number of PI-positive cells) at this stage was surprisingly low compared to the control cells and considering the fact that they did not adhere well to the plate surface (Fig. 2C). Here too, RA helped reduce cell death, although not significantly for the IKAP-DR cells. Because many IKAP-DR cells that were not stained with PI remained floating, we assume that cells eventually adhered to each other on the plate surface.
Figure 2.
Attachment and spreading assays. (A) Calcein stained control or IKAP-DR cells plated on laminin-coated plates without retinoic acid (NO RA) or with 10 µM retinoic acid (RA). (B) Graphic summary describing the averaged percentage of cells of three different experiments, that attached to the laminin coated plates in the indicated growth conditions. (C) Graphic summary of cell death indicated as percent of plated control or IKAP-DR cells. (D) Cell spreading described as the average cell area of a single cell of each strain in the indicated growth conditions. Statistical significance in (B–D) was determined using one-way ANOVA program, values are depicted at the bottom left corner of the figure. (E) Western blot analysis showing the expression of β-integrin1 in control and IKAP-DR cells grown on laminin without or with RA addition. Beta tubulin expression was used for normalization.
IKAP reduction in SHSY5Y cells affected also the spreading of the cells on laminin under the same culture conditions: IKAP-DR cells tended to have a rounded morphology (Fig. 2A) that resulted in a reduced surface size compared to control cells. Here too, RA improved the spreading of these cells as determined by the measured area of the cells (Fig. 2D).
Because IKAP-DR cells attachment to laminin was low we looked, by western blot analysis, at the expression of beta integrin1, a major subunit of the laminin receptors25,26 in IKAP-DR and control cells. Figure 2E shows that beta integrin1 expression was similar for the control and IKAP-DR cells in regular medium and was elevated in both cell lines upon incubation with RA (Fig. 2E), regardless of IKAP reduction. This result is in accordance with the enhanced cell attachment we observed on laminin upon addition of RA but could not explain the reduced attachment to laminin of IKAP-DR cells (Fig. 2B).
Effect of IKAP downregulation on differentiation.
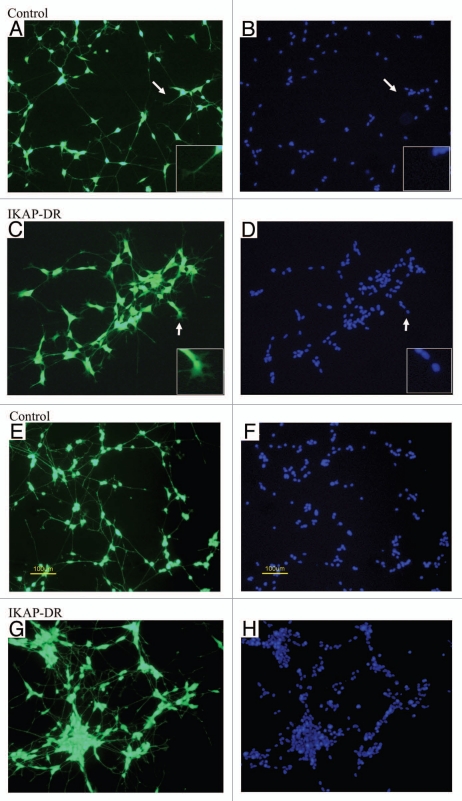
We checked the capacity of IKAP-DR cells to further differentiate after the addition of BDNF, according to the protocol described above. To do that, we sheared the cells in calcium and magnesium-free PBS and plated them on laminin in medium supplemented with 10 µM RA for 5 days, followed by serum-free medium with 2 nM BDNF for 3 days. Cells were then stained with vitality examination dyes and were photographed. At first sight we saw that both the control cells and IKAP-DR cells grew neurites upon differentiation (Fig. 3A and B). Closer examination of the cells revealed major morphological differences between the control and the IKAP-DR cells. While the control cells had a typical neuronal morphology (a small elliptical cell body with one neurite extending from each pole), the IKAP-DR cells adhered to each other, forming small colonies with no clear septa between the clumped cells. The adherence of the cells was also evident when looking at the distribution of the nuclei in IKAP-DR cells compared to control cells: in the IKAP-DR cells they tended to form clusters (Hoechst staining, Fig. 3C and D). This tendency to aggregate caused the cells to look triangular in shape, while the neurites extensions were thicker than and not as regular as in the control cells. Some IKAP-DR cells clusters had short and branched neurites that at places looked like filopodia compared to the long and extended neurites of control cells (see inserts and arrows in Fig. 3B and A, respectively). It is clear that downregulation of IKAP led to enhanced cell-to-cell adhesion and to differences in the pattern of differentiation. In order to check whether the IKAP downregulated cells indeed adhered stronger to each other than control cells, we disrupted the cells after BDNF treatment by pipetting them thoroughly and then plated them in fresh serum-free medium with 2 nM BDNF for 3 additional days. As can be seen in Figure 3E–H, the differences between the control and the IKAP-DR cells are remarkable. While most of the control cells remained isolated and maintained their morphology, renewing the network of neurites that connected them (Fig. 3E and G), the IKAP-DR cells formed large conglomerates with fewer connecting neurites (considering the large number of cells in each conglomerate) (Fig. 3F and H). The same phenomenon was observed at several cell densities tested (data not shown). Cell death of both cell lines was higher at this point, probably because of the relatively long incubation in serum-free medium and the physical stress that the cells went through in the disruption process (data not shown).
Figure 3.
Differentiation on laminin. (A and C) control and IKAP-DR cells stained with calcein, respectively. (B and D) control and IKAP-DR cells nuclei staining. Arrows show the area in the inserts (X4). (E and G) Calcein-stained differentiated control and IKAP-DR cells respectively, disrupted by thorough pipetting after differentiation as described in Materials and Methods and grown for three more days in serum-free medium with BDNF. (F and H) nuclei staining of control and IKAP-DR cells shown in (E and G). Size bars in (E and F) apply to all pictures in this figure.
Contactin causes the enhanced adhesion of IKAP-DR cells on laminin.
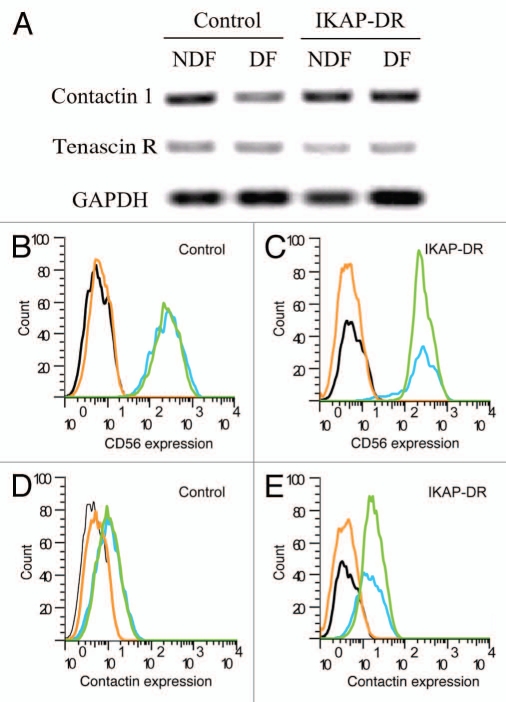
In order to pinpoint the adherence molecules that may cause the phenotype we observed, we performed microarray analysis of control and IKAP-DR cells that were grown on laminin either in regular medium (NDF) or under differentiating conditions (DF). We screened the data for adhesion molecules that were upregulated in the IKAP-DR cells in differentiation compared to control cells. One gene that was overexpressed 2.1-fold compared to control cells already in non-differentiating cells and 3.8-fold under differentiating conditions was CNTC1, encoding Contactin (Fig. 4A). These results were also confirmed by real-time PCR of the same cell lines (S1A) and two more IKAP-DR cell lines (S1B). Contactin is a GPI-anchored cell surface protein that belongs to the family of immunoglobulin domain-containing cell adhesion molecules (CAMs) that also includes NCAM among others. Contactin has been shown to bind several proteins, one of which is TenascinR,27,28 TenascinR is an extracellular matrix glycoprotein expressed in neural tissues (reviewed in ref. 29). The expression of TenascinR was reduced in IKAP-DR cells compared to control in our microarrays and RT-PCR experiments as a result of IKAP downregulation (Fig. 4A). To confirm the importance of Contactin in our experimental system, we checked its expression in both control and IKAP-DR cells by FACS analysis, in non-differentiating conditions or after differentiation on laminin. As a reference, we also analyzed by FACS the expression of CD56, a neural cell adhesion molecule (NCAM) known to be expressed in neurons, glia, skeletal muscle and NK cells.30 CD56 did not show differential expression between IKAP-DR and control cells in our DNA microarray analysis. We found that both control (Fig. 4B and blue and green lines) and IKAP-DR cells (Fig. 4C and blue and green lines) expressed CD56 at the same intensity in both NDF and DF conditions, as can be judged by the position of the fluorescence peak for both cell lines. In contrast, the results of the FACS analysis with antibodies against Contactin showed that IKAP-DR cells expressed higher levels of Contactin compared to control cells (Fig. 4D and E) in both NDF and DF conditions. We thus conclude that IKAP-DR cells express higher levels of Contactin. The Contactin expression was also verified by western blot analysis (described below).
Figure 4.
(A) Expression of Contactin-1 and Tenascin R as measured by RT PCR of mRNA extracted from control and IKAP-DR cells grown in non differentiation (NDF) or differentiation (DF) conditions. GAPDH expression was used for normalization. (B) FACS analysis of control cells grown in NDF (blue) and DF (green) conditions, stained with anti-CD56 (NCAM) antibody or IgG2a isotype as a reference for control cells in NDF (black) and DF (orange) conditions. (C) Same as in (B), but for IKAP-DR cells: isotype in black (NDF) and orange (DF) and anti-CD56 in blue (NDF) and green (DF). (D) FACS analysis of control cells stained with anti-Contactin1 in NDF (blue) and DF (green) conditions or control cells stained with only secondary antibody as a reference in NDF (black) and DF (orange) conditions. (E) same as in (D) but for IKAP-DR cells: secondary antibody staining in black (NDF) and orange (DF) and anti-Contactin1 in blue (NDF) and green (DF).
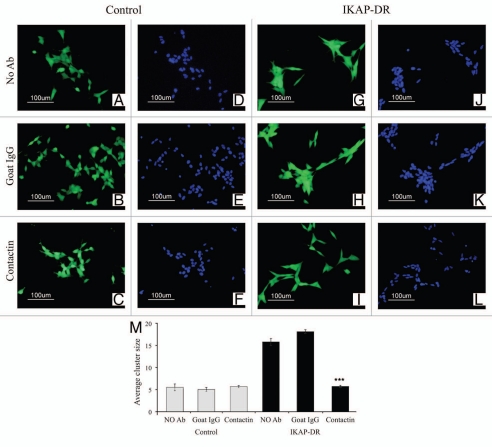
To test whether the increased adhesion of the IKAP-DR cells to each other, compared to control cells (Fig. 3) was due to the high level of Contactin, we tested whether antibodies against Contactin can reduce the adhesion phenotype. We therefore plated the IKAP-DR and control cells on laminin with added goat anti-Contactin antibody. We also used two experimental controls, one without any antibody and one with goat IgG. Cells were incubated for 24 h in the respective media, then the relevant antibodies were re-added to the medium and cells were incubated for 24 additional hours. Viability assay dyes were added and the cells were photographed. As shown in Figure 5, the control cells that were incubated with no added antibodies (Fig. 5A), with goat IgG (Fig. 5B) or with anti Contactin antibodies (Fig. 5C) were not affected by the different treatments and showed relatively good cell separation, as observed also by the Hoechst nuclear staining (Fig. 5D–F). On the other hand, while IKAP-DR cells incubated with no antibodies (Fig. 5G and J) or with goat IgG antibodies (Fig. 5H and K) formed relatively large clusters that appeared also very compact, the cells that were incubated with the anti-Contactin antibody grew in much smaller clusters and spread better on the plate surface (Fig. 5I and L). Figure 5M summarizes the results quantitatively: the average number of cells per cluster in the control cells was around 5, and this number was not affected by either treatment. In contrast, the IKAP-DR cells incubated with anti-goat antibodies or with no antibodies showed a much larger number of cells (16–18) per cluster. This enhanced clustering, however, was completely abolished by the anti-Contactin antibody: the number of cells per cluster under these conditions was similar to those observed in the control cells (Fig. 5I and L).
Figure 5.
Control cells stained with calcein or Hoechst stain and grown on laminin with no added antibody (A and D), with goat IgG (B and E) or with anti-Contactin1 antibody (C and F), compared to IKAP-DR cells grown with no added antibody (G and J), with goat IgG (H and K) or with anti-Contactin1 antibody (I and L). In (M), a graphic summary of the average number of cells in a cluster for each of the cell lines and growth conditions is shown. ***p value < 0.001.
IKAP expression is naturally reduced in differentiating SHSY5Y cells.
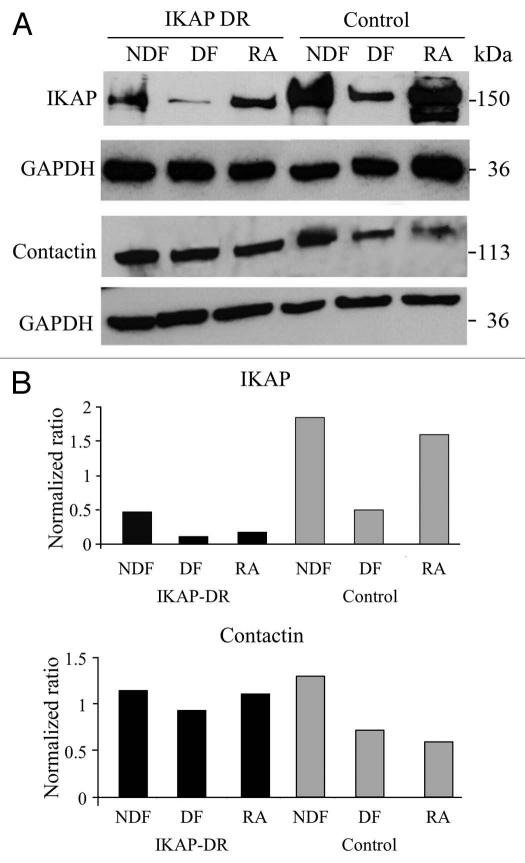
In order to verify the correlation between the expression of IKAP and Contactin, we checked the expression of both proteins in control and IKAP-DR cells by western blot analysis in non-differentiation (NDF) conditions, after full differentiation (DF) and at the intermediate stage with RA only. Figure 6A (top right) and B (upper graph), show that IKAP expression in control cells was not changed by RA treatment, but surprisingly was significantly reduced after differentiation (DF). In the IKAP-DR cells, as expected, the level of IKAP protein was initially low; however, upon addition of RA and further incubation with BDNF, IKAP expression was further reduced in these cells. As opposed to this, expression of Contactin in control cells was reduced already upon RA incubation and remained low in differentiated cells. Importantly, in contrast to control cells, Contactin levels, although reduced in differentiation, were still higher in IKAP-DR cells compared to control cells (Fig. 6A and B). The expression of Contactin under differentiation was verified in two more IKAP-DR cell lines (S2). These results suggest that IKAP is needed at the RA conditioning step for the Contactin levels to be reduced during differentiation. Low levels of IKAP seem to disrupt the regulation of Contactin expression and lead to higher Conatctin levels, which cause enhanced cell to cell adherence and disrupt neurites outgrowth.
Figure 6.
(A) Western blot analyses showing the expression of IKAP and of Contactin1 proteins in IKAP-DR or control cells grown in non-differentiation (NDF), in differentiation (DF) or with retinoic acid only (RA) conditions. For both analyses, GAPDH expression was used for normalization. In (B) the ratio between the expression levels of IKAP and GAPDH (upper graph) or Contactin1 (lower graph) and GAPDH is shown.
Discussion
The balance between cell-to-cell contact and cell-to-matrix contact needs to be carefully monitored and regulated in developing networks like the neuronal system. The extracellular matrix composition promotes signal transduction pathways that lead to specific developmental steps. Cell-to-cell contact helps neurons to migrate and also determines their location and hence more specific differentiation. We demonstrate in this manuscript that SHSY5Y neuroblastoma cells from neural crest origin in which IKAP is downregulated exhibit low attachment to laminin and increased cell-to-cell interactions. Laminin is the extracellular matrix that promotes PNS development.22 It is also a crucial matrix for regeneration of peripheral neurons and for axon myelination and Schwann cells differentiation.24 The reduced attachment of the IKAP-DR cells to laminin suggests that IKAP plays a role in the signaling pathways that enable PNS neurons to differentiate on laminin. Our results, however, point to the fact that this differentiation does not proceed through receptors containing beta integrin 1, a major subunit of laminin receptors, since there was no difference in its expression in the IKAP-DR cells compared to control cells (Fig. 2E). Moreover, RA improved the attachment of both control and IKAP-DR cells to laminin and beta integrin 1 expression was enhanced under these conditions, indicating that the improved attachment was a result of enhanced expression of integrins. IKAP downregulation did not inhibit the positive effect of RA on cell-to-matrix contact, suggesting that the inhibition in attachment does not involve the same integrins that mediate laminin attachment. We hence propose that the inhibited attachment of IKAP-DR cells to laminin is an indirect effect of the enhanced adhesion of these cells to each other. Indeed, as shown in Figure 3B, IKAP-DR cells adhered strongly to each other and this adherence was intensified upon differentiation.
We have identified Contactin as the molecule responsible for the enhanced adhesion of the IKAP-DR cell line. Contactin is expressed in axonal paranodes31 and is involved in Schwann cell migration and myelination.32 Our results demonstrate that in SHSY5Y control cells, Contactin expression is reduced upon differentiation. We do not know the reason for this, but we can postulate that this reduction happens in order to help the neurons separate in order to extend their axons and make them accessible to myelinating cells like Schwann cells in the peripheral nervous system.33,34
Looking at the expression of IKAP and Contactin proteins, we show that in the control cells, the expression of both was reduced upon differentiation. In IKAP-DR cells, in which IKAP expression was artificially reduced, IKAP expression was further diminished during differentiation, demonstrating that indeed the expression of this protein is naturally reduced in differentiating conditions. In the control cells, Contactin downregulation occurred already upon exposure to RA while IKAP reduction could be observed only upon BDNF induction. In contrast to this, in the IKAP-DR cells, Contactin expression, although reduced during differentiation, remained higher than in control cells. These results demonstrate that downregulation of Contactin happened at the conditioning stage (RA) and when IKAP levels were low at this stage (as in the IKAP-DR cells only), Contactin was not downregulated enough, thus remained higher through differentiation. Our results therefore show that IKAP influences Contactin expression and suggests that this downregulation during differentiation plays a critical role in cell-cell adhesion. The mechanism by which downregulation of these genes is achieved remains to be elucidated: (i) IKAP is a subunit of the Elongator complex that was found to be involved in transcriptional regulation of genes through chromatin modification.12 In our case IKAP depletion could cause Elongator deficiencies, affecting the expression of genes important for neuronal differentiation, such as Contactin and Tenascin. (ii) Elongator has also been implicated in cytoskeleton modifications through reduced acetylation of α-tubulin.15,35 This in turn can affect the mobilization of adhesion molecules to their final destination and can cause excessive adhesion.
The overexpression of Contactin caused the IKAP-DR cells to grow as if they were glued together, with almost undetectable septa. Although the IKAP-DR cells could grow neurites, the neurites were thicker and their growth looked distorted, as judged by their endings (Fig. 3B and insert). By neutralizing Contactin using anti-Contactin antibodies, we showed that the enhanced clustering of the cells could be suppressed, restoring cell-to-cell contacts to the level of control cells. The effect of the anti-Contactin antibody was evident only in IKAP-DR cells, where Contactin was overexpressed. This result suggests that only Contactin molecules not bound to their ligands because of overexpression were neutralized by the antibodies.
In Familial Dysautonomia, the sensory and autonomic nervous systems develop abnormally and this abnormality is manifested partially by demyelination at various loci in the nervous system.36 Indeed, genes involved in myelin formation were shown to be downregulated in FD but not control brains.37 We showed here that Contactin, an adhesion molecule involved in Schwann cells myelination, is responsible for the enhanced adhesion of IKAP-DR SHSY5Y cells. It is tempting to hypothesize that IKAP reduction in neurons, which affects Contactin expression and neurites growth, may affect the myelinating process of PNS neurons by Schwann cells. Since this process requires adequate contact and recognition between cells, the upregulation of Contactin that leads to clumping of neurons together on one hand and downregulation of myelinating genes in Schwann cells with reduced IKAP on the other, may be implicated in the developmental malformation of the PNS in FD patients.
Materials and Methods
Cell lines and cultures.
SHSY5Y cells were grown in medium containing DMEM with glutamine (Gibco), 10% heat-inactivated FCS (Hyclone), 2 mM sodium pyruvate (Invitrogen) and antibiotics (50 U/ml of penicillin, streptomycin and nystatin) (Biological Industries, Israel). Cells were split by trypsinization and were not grown for longer than 5–6 passages. HEK293-T cells were grown in the same medium as above but with untreated FCS.
Virus production and shRNA downregulation.
Plasmid DNA carrying shRNA sequences to IKBKAP gene (Mission shRNA Plasmid DNA, Sigma NM_003640, a kit containing 5 plasmids: TRCN0000037869-73) was prepared using standard methods. HEK293-T cells were transfected using MISSION Lentiviral Packaging plasmids (Sigma-Aldrich Corp., USA) with individual shRNA plasmids according to manufacturer instructions, using Fugene 6 reagent (Roche). Viruses were collected 24 and 48 h post transfection, filtered and used to infect SHSY5Y cells that were 60–80% confluent. Selection with 1 µg/ml puromycin (Sigma-Aldrich Corp., Israel) was started 24 h post infection. Stable cell lines were analyzed for IKBKAP RNA expression and IKAP protein expression. The cell line generated with shRNA TRCN0000037871 was used for all experiments. Control viruses were produced with Mission pLKO.1-Puro control vector (Sigma-Aldrich Corp., USA), using the same procedures.
Attachment, neuronal differentiation experiments and viability assay.
Cells were washed in Ca++ and Mg++ free PBS (Biological Industries, Israel) and harvested using Trypsin-EDTA (Biological Industries, Israel) and centrifuged at 750 g for 5 min. They were then suspended in Ca++ and Mg++ free PBS and disrupted into single cells by gentle shearing using 21 gauge needle and 1 ml syringe, counted and plated on laminin coated plates or 12 mm cover slips (De-Groot Laboratory Equipment LTD, Israel). Cover slips were first coated with 10 µg/ml Poly-D-Lysine (30–70 kDa, Sigma-Aldrich Corp., Israel) for 1 h at room temperature. At the end of incubation the cover slips were rinsed once with PBS and incubated with 4 µg/ml Laminin (Sigma-Aldrich Corp., Israel) overnight at 4°C. The cover slips were rinsed twice with PBS before cells were plated. Plates were coated overnight in 4 µg/ml laminin and rinsed as above. For cell attachment experiments, 25,000 cells were incubated either with or without 10 µM retinoic acid (PeproTech Asia, Israel) for 20 h. For differentiation experiments, cells were incubated with 10 µM retinoic acid for 5 days and then in serum-free medium with 2 nM BDNF (PeproTech Asia, Israel) for 3 days or as indicated. Following the incubations for either experiment, cell viability was assayed as previously reported.38 Briefly, 25 µl of a solution containing: 20 µg/mL calcein acetoxymethyl ester (Calcein AM; Invitrogen, OR, USA) (a cell permeable nonfluorescent dye that undergoes conversion to a green fluorescent calcein by intracellular esterases hydrolysis), 2 µg/mL Bisbenzimide (Hoechst 33342) (Sigma-Aldrich Corp., Israel) (which stains all nuclei) and 4 µg/mL propidium iodide (PI) (Sigma-Aldrich Corp., Israel) (a non-cell-permeable dye that stains only apoptotic cells) in PBS, was added to the cells for incubation for 20 min at 37°C. Then, green (calcein), blue (Hoechst) and red (PI) cells were counted.
All quantifications and Image acquisitions were made using a Nikon DXM1200f digital camera (Nikon, Japan) mounted on an inverted fluorescence Nikon Eclipse TE2000-S microscope (Nikon, Japan).
Cell data and statistical analyses.
Tif images were analyzed using SlideBook 4.2 software (Intelligent Imaging Innovations, Inc., USA). Data were expressed as the mean ± SE. Statistical significance was analyzed using one-way ANOVA program and Post hoc analysis using Tuki follow up test (STATISTICA 8).
RNA preps, real time PCR and RT PCR.
Cells were harvested as described above and the PBS washed pellets were used for total RNA isolation using Trisol reagent (Invitrogen, NY, USA), according to reagent-supplemented protocol. Total RNA (0.3–0.5 µg) was reverse-transcribed into complementary DNA (cDNA) with Thermo Scientific Verso cDNA Kit (ABgene, Surrey, UK) using random hexamers and according to the manufacturer's instructions. Real-Time PCR was carried out using Hot Start Taq Cyber green ready mix (ABgene, Surrey, UK) and specific primers. The Expression level of IKBKAP (IKAP gene) in the different clones is expressed as the ratio between the IKBKAP (gene of interest) and RS9 (normalizer) PCR products copy number using the ratio of the uninfected cells as a calibrator. RT-PCR reactions were carried out using Ready mix (ABgene, Surrey, UK). Primers used to check gene expression were as follows: IKBAP, Fwd: ATC ATC GAG CCC TGG TTT TAG, Rev: ATT GAT TCT CAG CTT TCT CAT GC; RS9, Fwd: CGG AGA CCC TTC GAG AAA TCT, Rev: GCC CAT ACT CGC CGA TCA, Contactin1, Fwd: ACC TGA ACG AAC AAC AAA ACC, Rev: ACA GGA TTT CCA AGT GCA AAA C; TenascinR, Fwd: CCA TCT CTC CAC TCC TCA AG, Rev: CCA TCG AAG GAA AAT GAG AAG; GAPDH, Fwd: GCT CTC TGC TCC TCC TGT T, Rev: CCA TGG TGT CTG AGC GAT GT.
Protein preps and western blot analysis.
Cells were washed and scrapped from the plates in PBS followed by centrifugation at 750 g for 5 min. Proteins were extracted from cell pellets using Ripa buffer (Sigma-Aldrich Corp., Israel). Protein concentrations were checked using BCA kit (Pierce Biotechnology, IL, USA). For western analysis, 30 µg proteins were loaded on 10% acrylamide gels or 4–15% gradient gels (Bio Rad) in Tris-glycin buffer. Proteins were transferred to nitrocellulose membrane and blocked in 3% low fat milk in TBST for 1 h. Primary antibodies (at 1:1,000) were applied for 1 h at room temperature or overnight at 4°C. Antibodies used were as follows: mouse anti hIKAP (BD Biosciences, Franklin Lakes, NJ, USA), rabbit anti IKAP (Anaspec, Fremont, CA, USA), goat anti Rab3a (Santa Cruz Biotechnology, Inc., CA, USA), mouse anti beta actin and mouse anti beta tubulin (Sigma-Aldrich Corp., USA), goat anti Contactin1 (Biolegend, San Diego, CA, USA), mouse anti GAPDH-HRP (Sigma-Aldrich Corp., USA). Secondary antibodies: Donkey anti goat, Donkey anti mouse and Donkey anti rabbit, all HRP conjugated (at 1:6,000) (Jackson ImmunoResearch laboratories, West Grove, PA, USA). For ECL, Super signal (Pierce Biotechnology, IL, USA) was used.
FACS analysis.
IKAP-DR and control cells were grown on laminin-coated plates in a regular medium (NDF) or differentiation medium (DF: incubation for 5 days in medium supplemented with 10 µM retinoic acid, followed by serum-free medium with 2 nM BDNF for 3 days). Cells were incubated briefly in trypsin-EDTA and washed twice with PBS containing 1% FCS (FACS buffer). To determine CD56 (NCAM) expression, cells were incubated with Alexa-488-labeled mouse anti-human CD56 or Alexa-488-labeled mouse IgG2a isotype control (both from Biolegend, San Diego, CA, USA) for 30 min on ice and washed twice with FACS buffer before subjected to flow cytometry analysis. To determine Contactin expression, cells were incubated with Goat anti-human contactin-1 (1:200) (R & D systems, Minneapolis, MN, USA) for 30 min on ice, then washed twice with FACS buffer and incubated for 30 min on ice with a donkey anti-goat-Cy2 antibody (1:1,000) (Jackson ImmunoResearch laboratories, West Grove, PA, USA), then washed twice with FACS buffer and subjected to flow cytometry analysis.
At least 10,000 events were determined for each test sample using a Becton Dickinson FACScan™ (BD Biosciences, Franklin Lakes, New Jersey, USA) and analyzed using a flowjo™ software (Ashland, OR, USA). Excitation was by a single 15 mW argon- ion laser beam (488 nm). Emission was collected through a 530 nm band pass filter.
Immunocytochemistry analysis.
Cells grown on laminin-coated cover slips were gently washed in PBS and fixed with 4% paraformaldehyde/PBS for 10 minutes. The cover-slips were incubated for 20 minutes in blocking solution containing 2% bovine serum albumin and 0.05% Triton in PBS. Antibodies were used as follows: rabbit anti hIKAP (Anaspec, Fremont, CA, USA. Used at 1:300), Goat anti rab3A (Santa Cruz Biotechnology, Inc., CA, USA. Used at 1:200). Secondary antibodies were mouse anti rabbit-Cy2 (Jackson ImmunoResearch laboratories, West Grove, PA, USA) and Donkey anti goat-Alexa 488 (R & D systems, Minneapolis, MN, USA, both used at 1:1,000). For nuclei staining, we used Hoechst 33258 (0.1 µg/ml). Cover slips were incubated with the primary antibodies for 1 h at room temperature or overnight at 4°C and rinsed four times with PBS. The secondary antibodies were applied for 1 h with added Hoechst reagent. Cover slips were then rinsed in PBS, mounted on microscope slides in gel mount (BD Biosciences, Franklin Lakes, NJ, USA) and sealed with nail polish. Cells were photographed using a fluorescent microscope (Carl Zeiss, Oberkochen, Germany). Images were analyzed using Slidebook (Intelligent Imaging Innovations, Inc.).
Neutralizing experiments with anti-contactin antibodies.
IKAP-DR or control cells were washed and sheared as described above and plated at 50,000 cells/well in six-well plates coated with laminin. Anti Contactin antibody (R & D Systems, Minneapolis, MN, USA) or goat IgG (Sigma-Aldrich Corp., Israel) were filter sterilized and added at 5 µg/ml to the medium. Cells were incubated for 24 h at 37°C and fresh aliquotes of the antibody or IgG at 2.5 µg/ml were added for additional 24 h. Cells were also incubated with no added antibodies for control. Cells were than incubated with a mix of calcein, Hoechst and PI and analyzed as described above in the attachment and neuronal differentiation experiments paragraph.
Acknowledgements
We thank David Cheichashvili for technical support, Anastasia Abashidze and David Shitrit for assistance in image cell analysis. We also thank Aharon Razin and Martin Kupiec for useful comments on this manuscript. This study was supported by a grant from the FD Hope foundation and by a grant from the FD research consortium (FD Hope, FD Foundation Inc., FD Israel Foundation).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/12923
Supplementary Material
References
- 1.Slaugenhaupt SA, Gusella JF. Familial dysautonomia. Curr Opin Genet Dev. 2002;12:307–311. doi: 10.1016/s0959-437x(02)00303-9. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod FB. Familial dysautonomia. Muscle Nerve. 2004;29:352–363. doi: 10.1002/mus.10499. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SL, Coli R, Daly IW, Kichula EA, Rork MJ, Volpi SA, et al. Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet. 2001;68:753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- 6.Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Krogan NJ, Kim M, Ahn SH, Zhong G, Kobor MS, Cagney G, et al. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol. 2002;22:6979–6992. doi: 10.1128/MCB.22.20.6979-6992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkes NA, Otero G, Winkler GS, Marshall N, Dahmus ME, Krappmann D, et al. Purification and characterization of the human elongator complex. J Biol Chem. 2002;277:3047–3052. doi: 10.1074/jbc.M110445200. [DOI] [PubMed] [Google Scholar]
- 9.Winkler GS, Kristjuhan A, Erdjument-Bromage H, Tempst P, Svejstrup JQ. Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc Natl Acad Sci USA. 2002;99:3517–3522. doi: 10.1073/pnas.022042899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahl PB, Chen CZ, Collins RN. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol Cell. 2005;17:841–853. doi: 10.1016/j.molcel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Huang B, Johansson MJ, Bystrom AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11:424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Close P, Hawkes N, Cornez I, Creppe C, Lambert CA, Rogister B, et al. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol Cell. 2006;22:521–531. doi: 10.1016/j.molcel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Okada Y, Yamagata K, Hong K, Wakayama T, Zhang Y. A role for the elongator complex in zygotic paternal genome demethylation. Nature. 463:554–558. doi: 10.1038/nature08732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen LD, Naumanen T, Knudsen A, Westerlund N, Gromova I, Junttila M, et al. IKAP localizes to membrane ruffles with filamin A and regulates actin cytoskeleton organization and cell migration. J Cell Sci. 2008;121:854–864. doi: 10.1242/jcs.013722. [DOI] [PubMed] [Google Scholar]
- 15.Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 16.Chen YT, Hims MM, Shetty RS, Mull J, Liu L, Leyne M, et al. Loss of mouse Ikbkap, a subunit of elongator, leads to transcriptional deficits and embryonic lethality that can be rescued by human IKBKAP. Mol Cell Biol. 2009;29:736–744. doi: 10.1128/MCB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiele CJ. Masters J, editor. Neuroblastoma. Human Cell Culture. 1998;1:21–53. [Google Scholar]
- 18.Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. [PubMed] [Google Scholar]
- 19.Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. [PubMed] [Google Scholar]
- 20.Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, et al. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 21.Jamsa A, Hasslund K, Cowburn RF, Backstrom A, Vasange M. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer's disease-like tau phosphorylation. Biochem Biophys Res Commun. 2004;319:993–1000. doi: 10.1016/j.bbrc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 22.Carbonetto S, Cochard P. In vitro studies on the control of nerve fiber growth by the extracellular matrix of the nervous system. J Physiol (Paris) 1987;82:258–270. [PubMed] [Google Scholar]
- 23.Carbonetto S, Evans D, Cochard P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J Neurosci. 1987;7:610–620. doi: 10.1523/JNEUROSCI.07-02-00610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietri T, Eder O, Breau MA, Topilko P, Blanche M, Brakebusch C, et al. Conditional beta1-integrin gene deletion in neural crest cells causes severe developmental alterations of the peripheral nervous system. Development. 2004;131:3871–3883. doi: 10.1242/dev.01264. [DOI] [PubMed] [Google Scholar]
- 26.Denda S, Reichardt LF. Studies on integrins in the nervous system. Methods Enzymol. 2007;426:203–221. doi: 10.1016/S0076-6879(07)26010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaughan L, Weber P, D'Alessandri L, Zisch AH, Winterhalter KH. Tenascin-contactin/F11 interactions: a clue for a developmental role? Perspect Dev Neurobiol. 1994;2:43–52. [PubMed] [Google Scholar]
- 28.Zacharias U, Rauch U. Competition and cooperation between tenascin-R, lecticans and contactin 1 regulate neurite growth and morphology. J Cell Sci. 2006;119:3456–3466. doi: 10.1242/jcs.03094. [DOI] [PubMed] [Google Scholar]
- 29.Brellier F, Tucker RP, Chiquet-Ehrismann R. Tenascins and their implications in diseases and tissue mechanics. Scand J Med Sci Sports. 2009;19:511–519. doi: 10.1111/j.1600-0838.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- 30.Mechtersheimer G, Staudter M, Moller P. Expression of the natural killer (NK) cell-associated antigen CD56(Leu-19), which is identical to the 140 kDa isoform of N-CAM, in neural and skeletal muscle cells and tumors derived therefrom. Ann NY Acad Sci. 1992;650:311–316. doi: 10.1111/j.1749-6632.1992.tb49143.x. [DOI] [PubMed] [Google Scholar]
- 31.Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- 32.Falk J, Bonnon C, Girault JA, Faivre-Sarrailh C. F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol Cell. 2002;94:327–334. doi: 10.1016/s0248-4900(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 33.Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol. 1999;9:293–311. doi: 10.1111/j.1750-3639.1999.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster HD. Myelin injury and repair. Adv Neurol. 1993;59:67–73. [PubMed] [Google Scholar]
- 35.Solinger JA, Paolinelli R, Kloss H, Scorza FB, Marchesi S, Sauder U, et al. The Caenorhabditis elegans Elongator complex regulates neuronal alpha-tubulin acetylation. PLoS Genet. 6:1000820. doi: 10.1371/journal.pgen.1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fogelson MH, Rorke LB, Kaye R. Spinal cord changes in familial dysautonomia. Arch Neurol. 1967;17:103–108. doi: 10.1001/archneur.1967.00470250107012. [DOI] [PubMed] [Google Scholar]
- 37.Cheishvili D, Maayan C, Smith Y, Ast G, Razin A. IKAP/hELP1 deficiency in the cerebrum of familial dysautonomia patients results in downregulation of genes involved in oligodendrocyte differentiation and in myelination. Hum Mol Genet. 2007;16:2097–2104. doi: 10.1093/hmg/ddm157. [DOI] [PubMed] [Google Scholar]
- 38.Solmesky LJ, Abekasis M, Bulvik S, Weil M. Bone morphogenetic protein signaling is involved in human mesenchymal stem cell survival in serum-free medium. Stem Cells Dev. 2009;18:1283–1292. doi: 10.1089/scd.2009.0020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.