Abstract
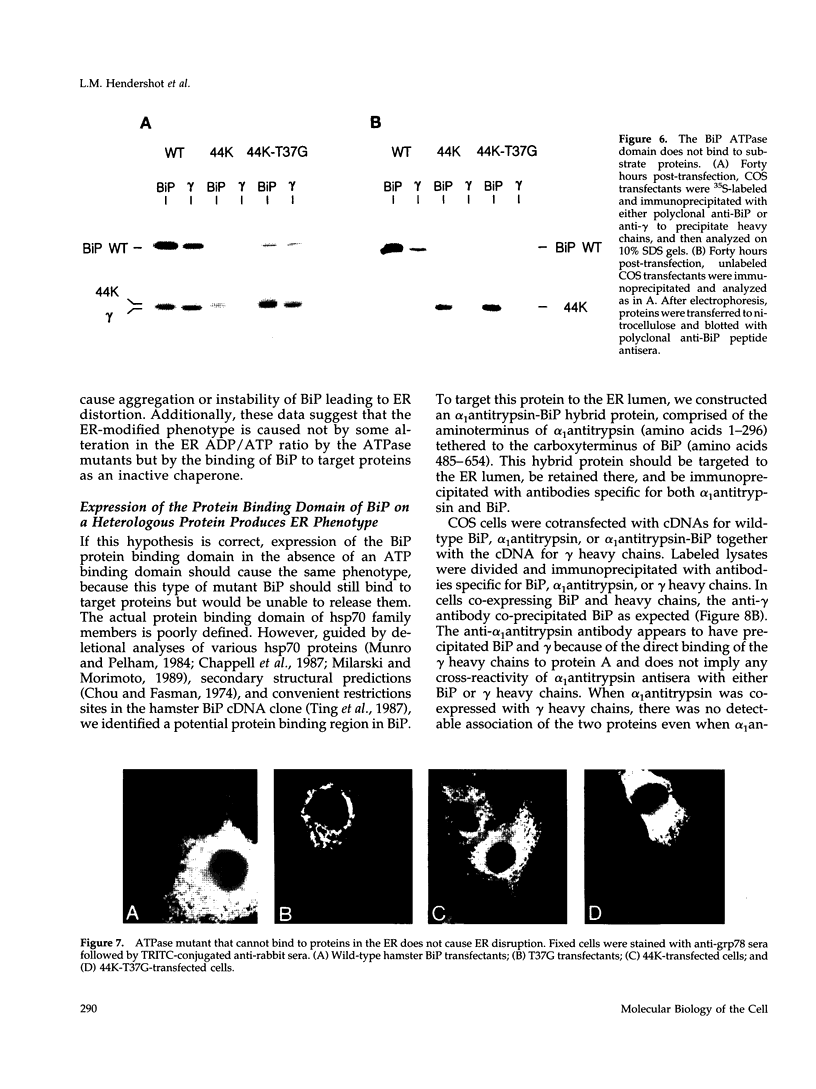
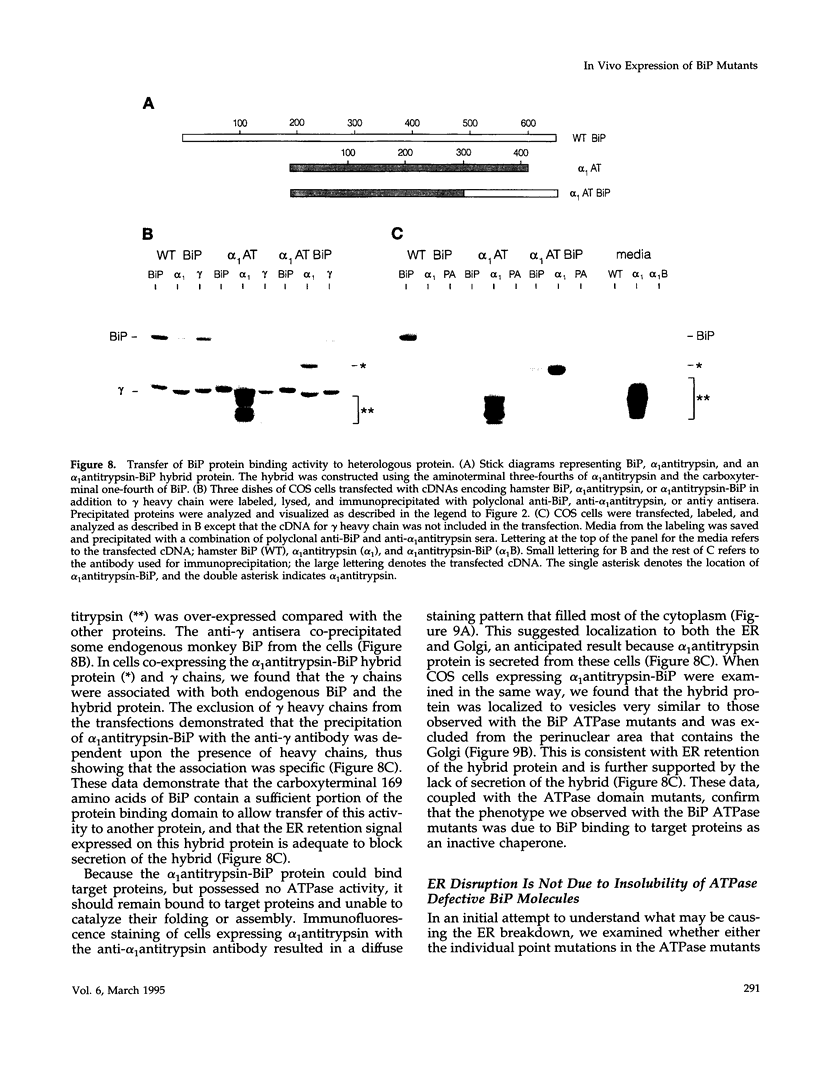
BiP possesses ATP binding/hydrolysis activities that are thought to be essential for its ability to chaperone protein folding and assembly in the endoplasmic reticulum (ER). We have produced a series of point mutations in a hamster BiP clone that inhibit ATPase activity and have generated a species-specific anti-BiP antibody to monitor the effects of mutant hamster BiP expression in COS monkey cells. The enzymatic inactivation of BiP did not interfere with its ability to bind to Ig heavy chains in vivo but did inhibit ATP-mediated release of heavy chains in vitro. Immunofluorescence staining and electron microscopy revealed vesiculation of the ER membranes in COS cells expressing BiP ATPase mutants. ER disruption was not observed when a "44K" fragment of BiP that did not include the protein binding domain was similarly mutated but was observed when the protein binding region of BiP was expressed without an ATP binding domain. This suggests that BiP binding to target proteins as an inactive chaperone is responsible for the ER disruption. This is the first report on the in vivo expression of mammalian BiP mutants and is demonstration that in vitro-identified ATPase mutants behave as dominant negative mutants when expressed in vivo.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahluwalia N., Bergeron J. J., Wada I., Degen E., Williams D. B. The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J Biol Chem. 1992 May 25;267(15):10914–10918. [PubMed] [Google Scholar]
- Alanen A., Pira U., Colman A., Franklin R. M. Mott cells: a model to study immunoglobulin secretion. Eur J Immunol. 1987 Nov;17(11):1573–1577. doi: 10.1002/eji.1830171108. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberger A., Valencia A., McMacken R., Sander C., Bukau B. The chaperone function of DnaK requires the coupling of ATPase activity with substrate binding through residue E171. EMBO J. 1994 Apr 1;13(7):1687–1695. doi: 10.1002/j.1460-2075.1994.tb06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Wooden S. K., Nakaki T., Kim Y. K., Lin A. Y., Kung L., Attenello J. W., Lee A. S. Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression. Proc Natl Acad Sci U S A. 1987 Feb;84(3):680–684. doi: 10.1073/pnas.84.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell T. G., Konforti B. B., Schmid S. L., Rothman J. E. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987 Jan 15;262(2):746–751. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Clairmont C. A., De Maio A., Hirschberg C. B. Translocation of ATP into the lumen of rough endoplasmic reticulum-derived vesicles and its binding to luminal proteins including BiP (GRP 78) and GRP 94. J Biol Chem. 1992 Feb 25;267(6):3983–3990. [PubMed] [Google Scholar]
- Dorner A. J., Wasley L. C., Kaufman R. J. Protein dissociation from GRP78 and secretion are blocked by depletion of cellular ATP levels. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7429–7432. doi: 10.1073/pnas.87.19.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dycaico M. J., Grant S. G., Felts K., Nichols W. S., Geller S. A., Hager J. H., Pollard A. J., Kohler S. W., Short H. P., Jirik F. R. Neonatal hepatitis induced by alpha 1-antitrypsin: a transgenic mouse model. Science. 1988 Dec 9;242(4884):1409–1412. doi: 10.1126/science.3264419. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., Wilbanks S. M., DeLuca-Flaherty C., McKay D. B. Structural basis of the 70-kilodalton heat shock cognate protein ATP hydrolytic activity. II. Structure of the active site with ADP or ATP bound to wild type and mutant ATPase fragment. J Biol Chem. 1994 Apr 29;269(17):12899–12907. [PubMed] [Google Scholar]
- Gaut J. R., Hendershot L. M. Mutations within the nucleotide binding site of immunoglobulin-binding protein inhibit ATPase activity and interfere with release of immunoglobulin heavy chain. J Biol Chem. 1993 Apr 5;268(10):7248–7255. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham K. S., Le A., Sifers R. N. Accumulation of the insoluble PiZ variant of human alpha 1-antitrypsin within the hepatic endoplasmic reticulum does not elevate the steady-state level of grp78/BiP. J Biol Chem. 1990 Nov 25;265(33):20463–20468. [PubMed] [Google Scholar]
- Haas I. G. BiP--a heat shock protein involved in immunoglobulin chain assembly. Curr Top Microbiol Immunol. 1991;167:71–82. doi: 10.1007/978-3-642-75875-1_4. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Meo T. cDNA cloning of the immunoglobulin heavy chain binding protein. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2250–2254. doi: 10.1073/pnas.85.7.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kassenbrock C. K., Kelly R. B. Interaction of heavy chain binding protein (BiP/GRP78) with adenine nucleotides. EMBO J. 1989 May;8(5):1461–1467. doi: 10.1002/j.1460-2075.1989.tb03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Chandra T., Degen S. J., White T. T., Marchioro T. L., Woo S. L., Davie E. W. Cloning and sequence of cDNA coding for alpha 1-antitrypsin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6826–6830. doi: 10.1073/pnas.78.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latterich M., Schekman R. The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell. 1994 Jul 15;78(1):87–98. doi: 10.1016/0092-8674(94)90575-4. [DOI] [PubMed] [Google Scholar]
- Liberek K., Marszalek J., Ang D., Georgopoulos C., Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Mack P. W., Champion C. I., Robinson R. R. Expression of mouse::human immunoglobulin heavy-chain cDNA in lymphoid cells. Gene. 1987;54(1):33–40. doi: 10.1016/0378-1119(87)90344-1. [DOI] [PubMed] [Google Scholar]
- Mazzarella R. A., Srinivasan M., Haugejorden S. M., Green M. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J Biol Chem. 1990 Jan 15;265(2):1094–1101. [PubMed] [Google Scholar]
- McCarty J. S., Walker G. C. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J., Aviel S., Argon Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. J Biol Chem. 1992 Oct 25;267(30):21303–21306. [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J Cell Biol. 1989 Nov;109(5):1947–1962. doi: 10.1083/jcb.109.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. Use of peptide tagging to detect proteins expressed from cloned genes: deletion mapping functional domains of Drosophila hsp 70. EMBO J. 1984 Dec 20;3(13):3087–3093. doi: 10.1002/j.1460-2075.1984.tb02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normington K., Kohno K., Kozutsumi Y., Gething M. J., Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989 Jun 30;57(7):1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- O'Brien M. C., McKay D. B. Threonine 204 of the chaperone protein Hsc70 influences the structure of the active site, but is not essential for ATP hydrolysis. J Biol Chem. 1993 Nov 15;268(32):24323–24329. [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., ROBINSON A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. 1958 Dec 1;108(6):945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleros D. R., Reid K. L., Shi L., Welch W. J., Fink A. L. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993 Oct 14;365(6447):664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- Pathak R. K., Merkle R. K., Cummings R. D., Goldstein J. L., Brown M. S., Anderson R. G. Immunocytochemical localization of mutant low density lipoprotein receptors that fail to reach the Golgi complex. J Cell Biol. 1988 Jun;106(6):1831–1841. doi: 10.1083/jcb.106.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Bruns D., Jarett L. A sensitive and precise isotopic assay of ATPase activity. Anal Biochem. 1978 Oct 15;90(2):785–795. doi: 10.1016/0003-2697(78)90169-0. [DOI] [PubMed] [Google Scholar]
- Suzuki C. K., Bonifacino J. S., Lin A. Y., Davis M. M., Klausner R. D. Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca(2+)-dependent association with BiP. J Cell Biol. 1991 Jul;114(2):189–205. doi: 10.1083/jcb.114.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J., Lee A. S. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988 May;7(4):275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Ting J., Wooden S. K., Kriz R., Kelleher K., Kaufman R. J., Lee A. S. The nucleotide sequence encoding the hamster 78-kDa glucose-regulated protein (GRP78) and its conservation between hamster and rat. Gene. 1987;55(1):147–152. doi: 10.1016/0378-1119(87)90258-7. [DOI] [PubMed] [Google Scholar]
- Vogel J. P., Misra L. M., Rose M. D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990 Jun;110(6):1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbanks S. M., DeLuca-Flaherty C., McKay D. B. Structural basis of the 70-kilodalton heat shock cognate protein ATP hydrolytic activity. I. Kinetic analyses of active site mutants. J Biol Chem. 1994 Apr 29;269(17):12893–12898. [PubMed] [Google Scholar]
- Wild J., Kamath-Loeb A., Ziegelhoffer E., Lonetto M., Kawasaki Y., Gross C. A. Partial loss of function mutations in DnaK, the Escherichia coli homologue of the 70-kDa heat shock proteins, affect highly conserved amino acids implicated in ATP binding and hydrolysis. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7139–7143. doi: 10.1073/pnas.89.15.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]