Abstract
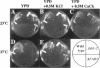
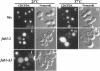
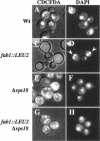
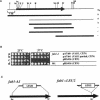
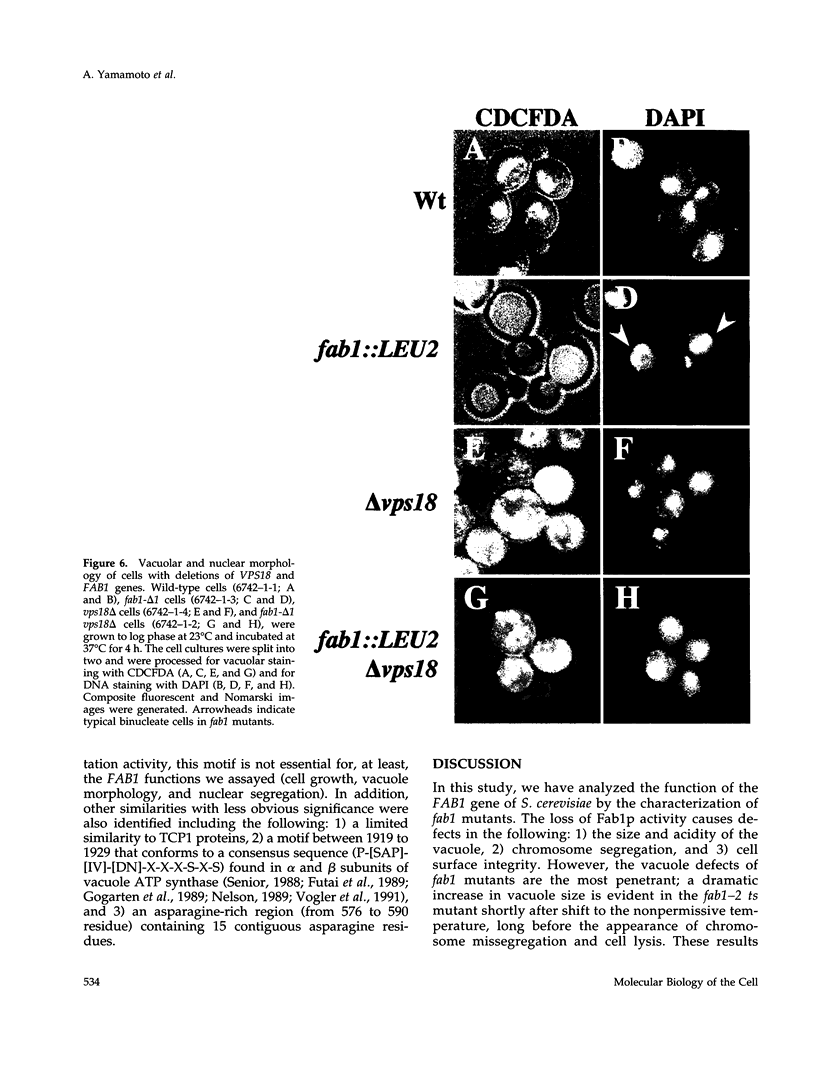
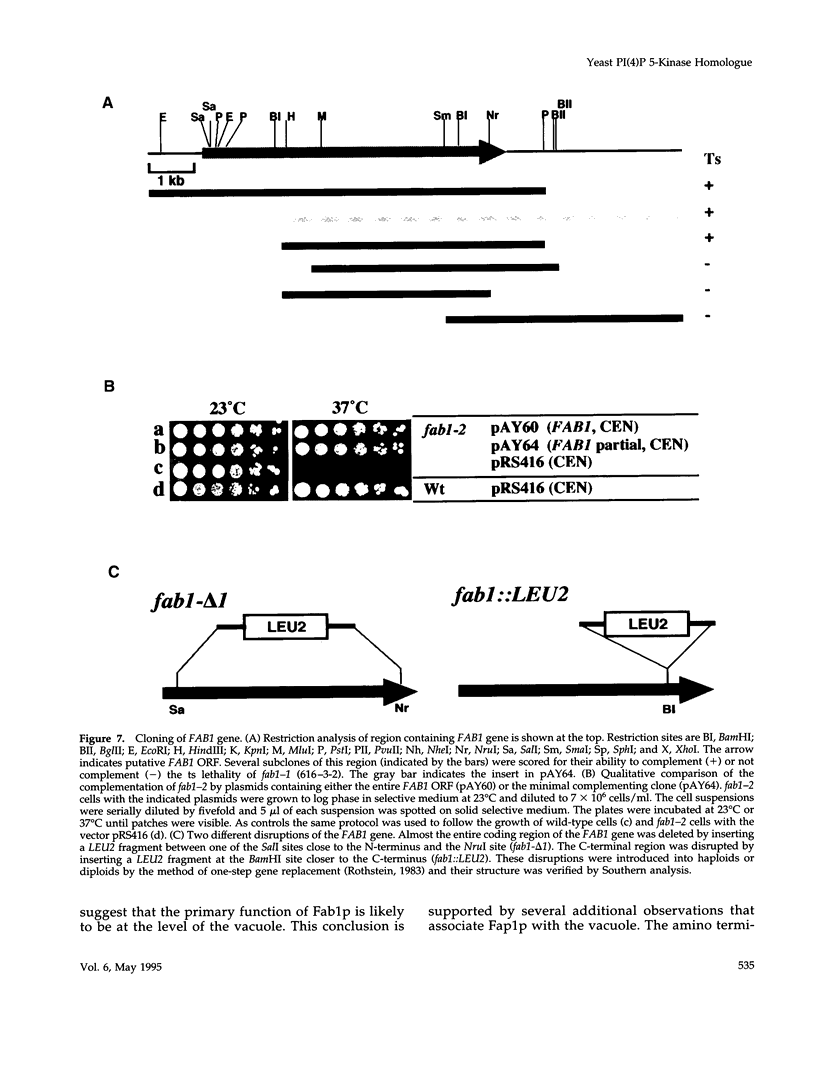
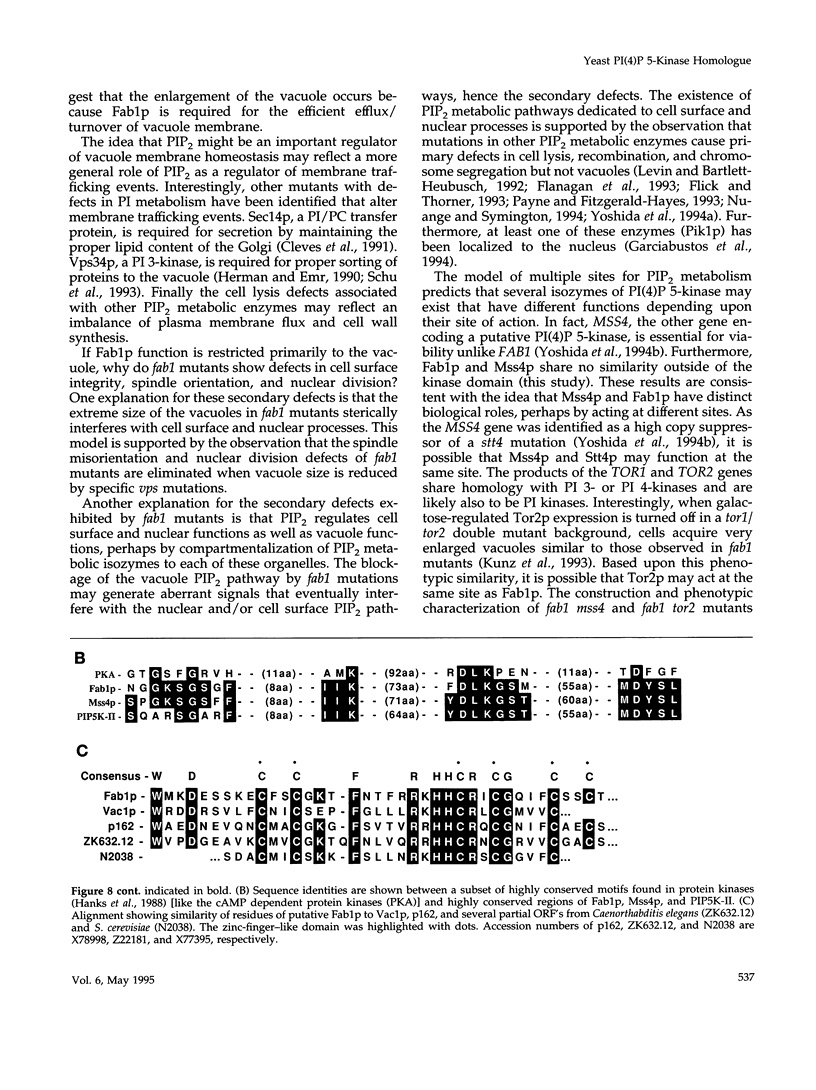
The FAB1 gene of budding yeast is predicted to encode a protein of 257 kDa that exhibits significant sequence homology to a human type II PI(4)P 5-kinase (PIP5K-II). The recently cloned human PIP5K-II specifically converts PI(4)P to PI(4,5)P2 (Boronenkov and Anderson, 1995). The region of highest similarity between Fab1p and PIP5K-II includes a predicted nucleotide binding motif, which is likely to correspond to the catalytic domain of the protein. Interestingly, neither PIP5K-II nor Fab1p exhibit significant homology with cloned PI 3-kinases or PI 4-kinases. fab1 mutations result in the formation of aploid and binucleate cells (hence the name FAB). In addition, loss of Fab1p function causes defects in vacuole function and morphology, cell surface integrity, and cell growth. Experiments with a temperature conditional fab1 mutant revealed that their vacuoles rapidly (within 30 min) enlarge to more than double the size upon shifting cells to the nonpermissive temperature. Additional experiments with the fab1 ts mutant together with results obtained with fab1 vps (vacuolar protein sorting defective) double mutants indicate that the nuclear division and cell surface integrity defects observed in fab1 mutants are secondary to the vacuole morphology defects. Based on these data, we propose that Fab1p is a PI(4)P 5-kinase and that the product of the Fab1p reaction, PIP2, functions as an important regulator of vacuole homeostasis perhaps by controlling membrane flux to and/or from the vacuole. Furthermore, a comparison of the phenotypes of fab1 mutants and other yeast mutants affecting PI metabolism suggests that phosphoinositides may serve as general regulators of several different membrane trafficking pathways.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazenet C. E., Ruano A. R., Brockman J. L., Anderson R. A. The human erythrocyte contains two forms of phosphatidylinositol-4-phosphate 5-kinase which are differentially active toward membranes. J Biol Chem. 1990 Oct 15;265(29):18012–18022. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Boronenkov I. V., Anderson R. A. The sequence of phosphatidylinositol-4-phosphate 5-kinase defines a novel family of lipid kinases. J Biol Chem. 1995 Feb 17;270(7):2881–2884. doi: 10.1074/jbc.270.7.2881. [DOI] [PubMed] [Google Scholar]
- Buxeda R. J., Nickels J. T., Jr, Belunis C. J., Carman G. M. Phosphatidylinositol 4-kinase from Saccharomyces cerevisiae. Kinetic analysis using Triton X-100/phosphatidylinositol-mixed micelles. J Biol Chem. 1991 Jul 25;266(21):13859–13865. [PubMed] [Google Scholar]
- Carpenter C. L., Cantley L. C. Phosphoinositide kinases. Biochemistry. 1990 Dec 25;29(51):11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- Cleves A., McGee T., Bankaitis V. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991 Jul;1(1):30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Flanagan C. A., Schnieders E. A., Emerick A. W., Kunisawa R., Admon A., Thorner J. Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science. 1993 Nov 26;262(5138):1444–1448. doi: 10.1126/science.8248783. [DOI] [PubMed] [Google Scholar]
- Flanagan C. A., Thorner J. Purification and characterization of a soluble phosphatidylinositol 4-kinase from the yeast Saccharomyces cerevisiae. J Biol Chem. 1992 Nov 25;267(33):24117–24125. [PubMed] [Google Scholar]
- Flick J. S., Thorner J. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Sep;13(9):5861–5876. doi: 10.1128/mcb.13.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Noumi T., Maeda M. ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annu Rev Biochem. 1989;58:111–136. doi: 10.1146/annurev.bi.58.070189.000551. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Marini F., Stevenson I., Frei C., Hall M. N. PIK1, an essential phosphatidylinositol 4-kinase associated with the yeast nucleus. EMBO J. 1994 May 15;13(10):2352–2361. doi: 10.1002/j.1460-2075.1994.tb06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogarten J. P., Kibak H., Dittrich P., Taiz L., Bowman E. J., Bowman B. J., Manolson M. F., Poole R. J., Date T., Oshima T. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6661–6665. doi: 10.1073/pnas.86.17.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K., Stack J. H., Emr S. D. An essential role for a protein and lipid kinase complex in secretory protein sorting. Trends Cell Biol. 1992 Dec;2(12):363–368. doi: 10.1016/0962-8924(92)90048-r. [DOI] [PubMed] [Google Scholar]
- Huang K. N., Symington L. S. Mutation of the gene encoding protein kinase C 1 stimulates mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1994 Sep;14(9):6039–6045. doi: 10.1128/mcb.14.9.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter K. J., Eipel H. E. Flow cytometric determinations of cellular substances in algae, bacteria, moulds and yeasts. Antonie Van Leeuwenhoek. 1978;44(3-4):269–282. doi: 10.1007/BF00394305. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Gelsolin-polyphosphoinositide interaction. Full expression of gelsolin-inhibiting function by polyphosphoinositides in vesicular form and inactivation by dilution, aggregation, or masking of the inositol head group. J Biol Chem. 1989 Mar 25;264(9):4825–4831. [PubMed] [Google Scholar]
- Jenkins G. H., Fisette P. L., Anderson R. A. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994 Apr 15;269(15):11547–11554. [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller R., Cantley L. C. Phosphatidylinositol 3-kinase. Bioessays. 1994 Aug;16(8):565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specificity of the interaction between phosphatidylinositol 4,5-bisphosphate and the profilin:actin complex. J Cell Biochem. 1988 Jul;37(3):255–267. doi: 10.1002/jcb.240370302. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992 Mar;116(5):1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L. E., Schulz J. T., Cantley L. C. Characterization and purification of membrane-associated phosphatidylinositol-4-phosphate kinase from human red blood cells. J Biol Chem. 1989 Mar 25;264(9):5080–5088. [PubMed] [Google Scholar]
- Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989 Oct;21(5):553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- Palmer R. E., Hogan E., Koshland D. Mitotic transmission of artificial chromosomes in cdc mutants of the yeast, Saccharomyces cerevisiae. Genetics. 1990 Aug;125(4):763–774. doi: 10.1093/genetics/125.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. E., Fitzgerald-Hayes M. A mutation in PLC1, a candidate phosphoinositide-specific phospholipase C gene from Saccharomyces cerevisiae, causes aberrant mitotic chromosome segregation. Mol Cell Biol. 1993 Jul;13(7):4351–4364. doi: 10.1128/mcb.13.7.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston R. A., Reinagel P. S., Jones E. W. Genes required for vacuolar acidity in Saccharomyces cerevisiae. Genetics. 1992 Jul;131(3):551–558. doi: 10.1093/genetics/131.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Raymond C. K., Yamashiro C. T., Stevens T. H. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644–661. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Senior A. E. ATP synthesis by oxidative phosphorylation. Physiol Rev. 1988 Jan;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]
- Sullivan D. S., Huffaker T. C. Astral microtubules are not required for anaphase B in Saccharomyces cerevisiae. J Cell Biol. 1992 Oct;119(2):379–388. doi: 10.1083/jcb.119.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler A. P., Homma M., Irikura V. M., Macnab R. M. Salmonella typhimurium mutants defective in flagellar filament regrowth and sequence similarity of FliI to F0F1, vacuolar, and archaebacterial ATPase subunits. J Bacteriol. 1991 Jun;173(11):3564–3572. doi: 10.1128/jb.173.11.3564-3572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Wickner W. Molecular characterization of VAC1, a gene required for vacuole inheritance and vacuole protein sorting. J Biol Chem. 1992 Jan 5;267(1):618–623. [PubMed] [Google Scholar]
- Yoshida S., Ohya Y., Goebl M., Nakano A., Anraku Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J Biol Chem. 1994 Jan 14;269(2):1166–1172. [PubMed] [Google Scholar]
- Yoshida S., Ohya Y., Nakano A., Anraku Y. Genetic interactions among genes involved in the STT4-PKC1 pathway of Saccharomyces cerevisiae. Mol Gen Genet. 1994 Mar;242(6):631–640. doi: 10.1007/BF00283416. [DOI] [PubMed] [Google Scholar]
- Yoshikawa A., Isono K. Construction of an ordered clone bank and systematic analysis of the whole transcripts of chromosome VI of Saccharomyces cerevisiae. Nucleic Acids Res. 1991 Mar 25;19(6):1189–1195. doi: 10.1093/nar/19.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]