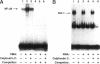Abstract
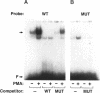
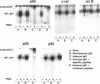
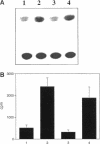
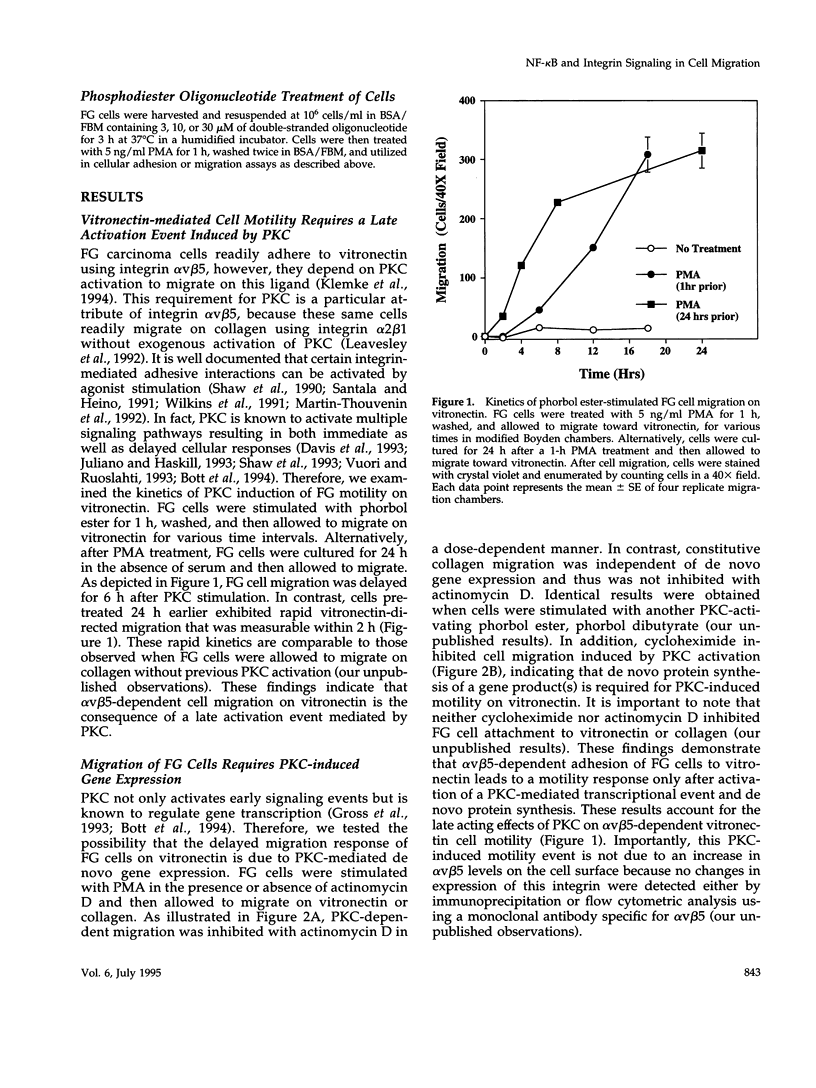
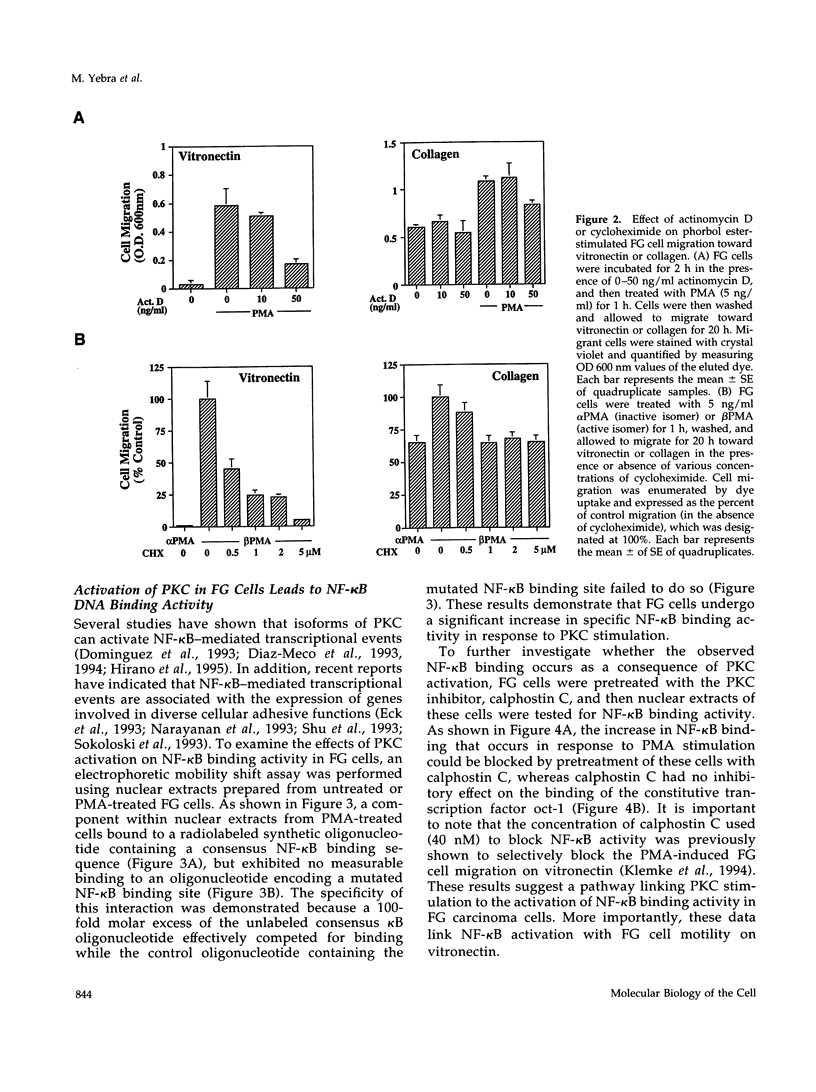
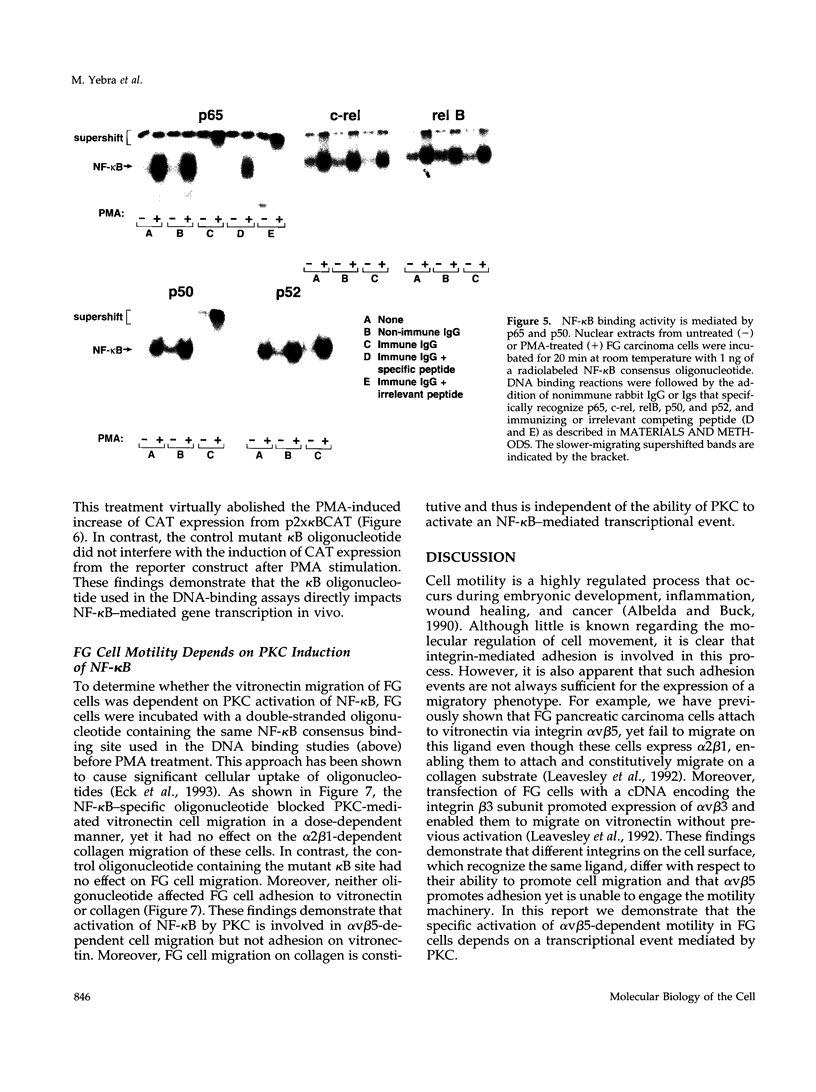
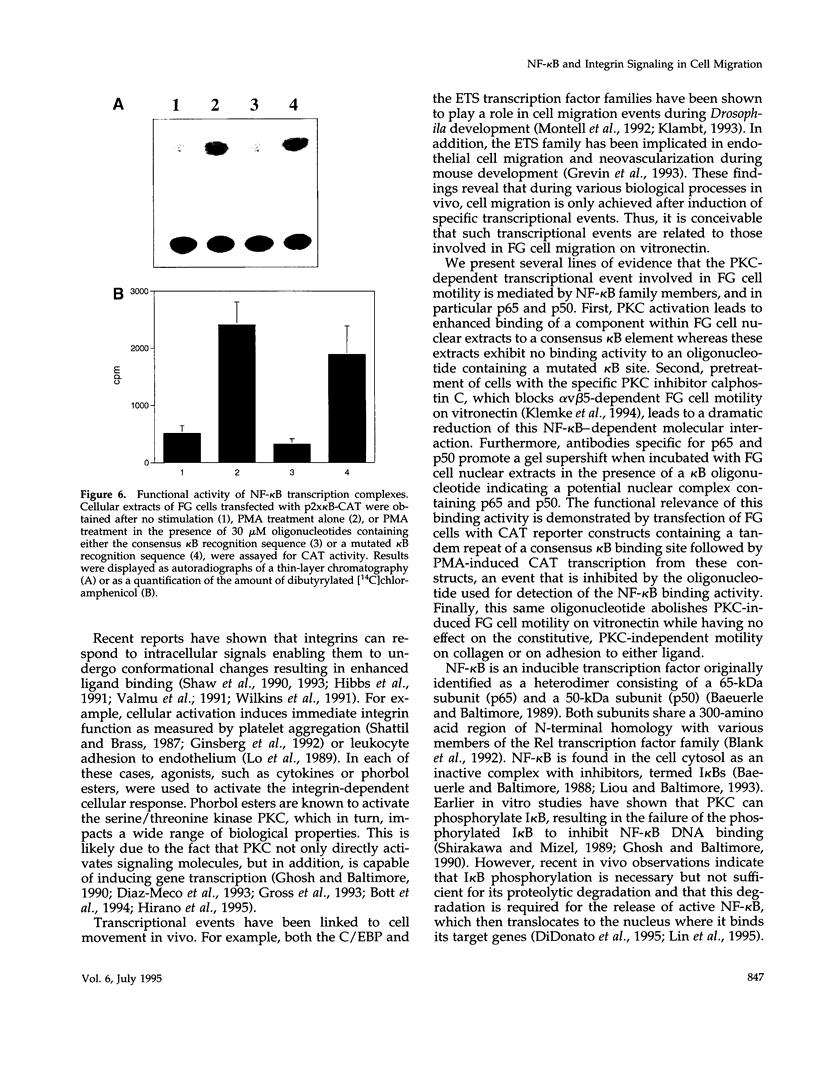
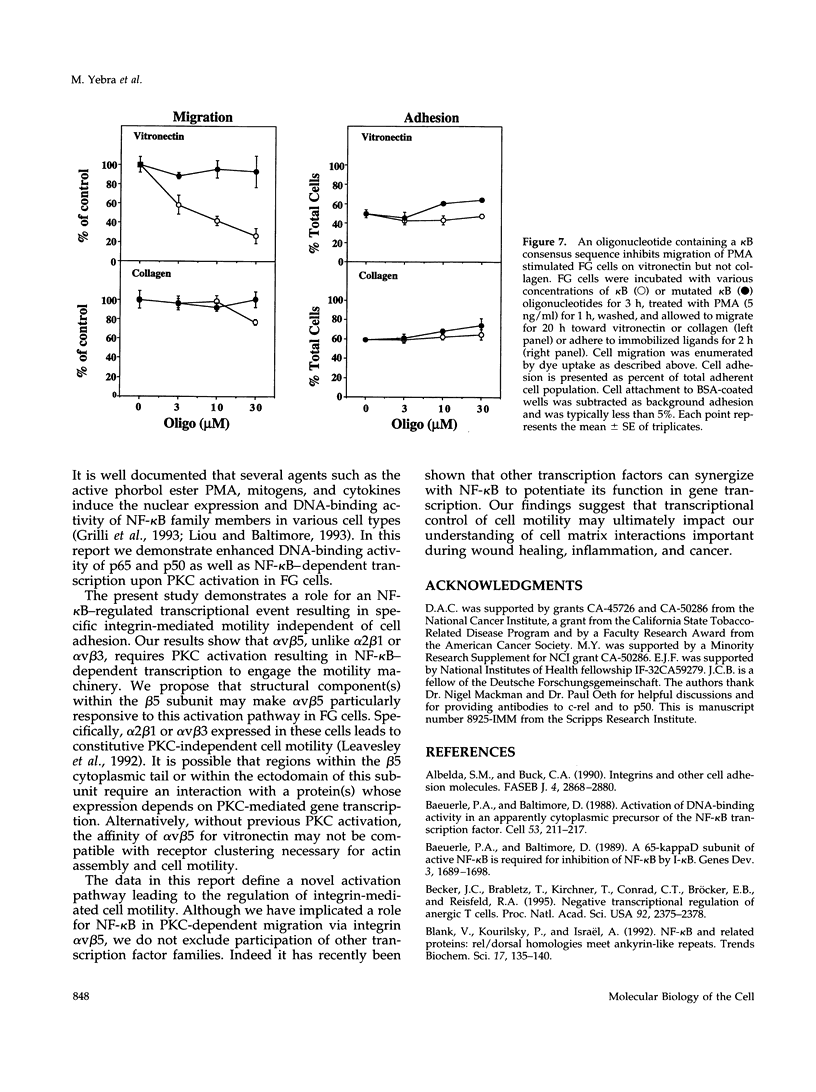
Integrin alpha v beta 5 promotes FG carcinoma cell adhesion to vitronectin yet requires protein kinase C (PKC) activation for migration on this ligand. Here we report that this PKC-dependent cell motility event requires NF-kappaB-dependent transcription. Specifically, a component within nuclear extracts prepared from PKC-stimulated FG cells exhibited a significant increase in binding activity to a synthetic oligonucleotide containing a consensus kappa B sequence. These nuclear DNA-binding complexes were shown to be comprised of p65 and p50 NF-kappaB/rel family members and appeared functionally active because they promoted transcription of a reporter construct containing a kappa B site. The NF-kappa B activation event was directly linked to the alpha v beta 5 motility response because the NF-kappa B-binding oligonucleotide, when introduced into FG cells, inhibited cell migration on vitronectin but not on collagen and had no effect on cell adhesion to either ligand. These results suggest that the detected DNA-binding complexes interact with kappa B transcriptional elements to regulate gene expression required for alpha v beta 5-dependent cell motility on vitronectin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Becker J. C., Brabletz T., Kirchner T., Conrad C. T., Bröcker E. B., Reisfeld R. A. Negative transcriptional regulation in anergic T cells. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2375–2378. doi: 10.1073/pnas.92.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank V., Kourilsky P., Israël A. NF-kappa B and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem Sci. 1992 Apr;17(4):135–140. doi: 10.1016/0968-0004(92)90321-y. [DOI] [PubMed] [Google Scholar]
- Bott C. M., Doshi J. B., Li L. L., McMurtry S. A., Sanders J. L., Fox D. A. Transcriptional regulation of CD6 expression on human T lymphocytes by phorbol ester. J Immunol. 1994 Jul 1;153(1):1–9. [PubMed] [Google Scholar]
- Chen J. D., Kim J. P., Zhang K., Sarret Y., Wynn K. C., Kramer R. H., Woodley D. T. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993 Dec;209(2):216–223. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- Davis C. M., Danehower S. C., Laurenza A., Molony J. L. Identification of a role of the vitronectin receptor and protein kinase C in the induction of endothelial cell vascular formation. J Cell Biochem. 1993 Feb;51(2):206–218. doi: 10.1002/jcb.240510213. [DOI] [PubMed] [Google Scholar]
- DiDonato J. A., Mercurio F., Karin M. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol Cell Biol. 1995 Mar;15(3):1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Berra E., Municio M. M., Sanz L., Lozano J., Dominguez I., Diaz-Golpe V., Lain de Lera M. T., Alcamí J., Payá C. V. A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol. 1993 Aug;13(8):4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Dominguez I., Sanz L., Dent P., Lozano J., Municio M. M., Berra E., Hay R. T., Sturgill T. W., Moscat J. zeta PKC induces phosphorylation and inactivation of I kappa B-alpha in vitro. EMBO J. 1994 Jun 15;13(12):2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I., Sanz L., Arenzana-Seisdedos F., Diaz-Meco M. T., Virelizier J. L., Moscat J. Inhibition of protein kinase C zeta subspecies blocks the activation of an NF-kappa B-like activity in Xenopus laevis oocytes. Mol Cell Biol. 1993 Feb;13(2):1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck S. L., Perkins N. D., Carr D. P., Nabel G. J. Inhibition of phorbol ester-induced cellular adhesion by competitive binding of NF-kappa B in vivo. Mol Cell Biol. 1993 Oct;13(10):6530–6536. doi: 10.1128/mcb.13.10.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Du X., Plow E. F. Inside-out integrin signalling. Curr Opin Cell Biol. 1992 Oct;4(5):766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Grevin D., Chen J. H., Raes M. B., Stehelin D., Vandenbunder B., Desbiens X. Involvement of the proto-oncogene c-ets 1 and the urokinase plasminogen activator during mouse implantation and placentation. Int J Dev Biol. 1993 Dec;37(4):519–529. [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Gross V., Zhang B., Geng Y., Villiger P. M., Lotz M. Regulation of interleukin-6 (IL-6) expression: evidence for a tissue-specific role of protein kinase C. J Clin Immunol. 1993 Sep;13(5):310–320. doi: 10.1007/BF00920239. [DOI] [PubMed] [Google Scholar]
- Hibbs M. L., Xu H., Stacker S. A., Springer T. A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science. 1991 Mar 29;251(5001):1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- Hirano M., Hirai S., Mizuno K., Osada S., Hosaka M., Ohno S. A protein kinase C isozyme, nPKC epsilon, is involved in the activation of NF-kappa B by 12-O-tetradecanoylphorbol-13-acetate (TPA) in rat 3Y1 fibroblasts. Biochem Biophys Res Commun. 1995 Jan 5;206(1):429–436. doi: 10.1006/bbrc.1995.1059. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke R. L., Yebra M., Bayna E. M., Cheresh D. A. Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. J Cell Biol. 1994 Nov;127(3):859–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klämbt C. The Drosophila gene pointed encodes two ETS-like proteins which are involved in the development of the midline glial cells. Development. 1993 Jan;117(1):163–176. doi: 10.1242/dev.117.1.163. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Rosen C. A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993 Oct;13(10):6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavesley D. I., Ferguson G. D., Wayner E. A., Cheresh D. A. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992 Jun;117(5):1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavesley D. I., Schwartz M. A., Rosenfeld M., Cheresh D. A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993 Apr;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis H., Kaszubska W., DeLamarter J. F., Whelan J. Cooperativity between two NF-kappa B complexes, mediated by high-mobility-group protein I(Y), is essential for cytokine-induced expression of the E-selectin promoter. Mol Cell Biol. 1994 Sep;14(9):5701–5709. doi: 10.1128/mcb.14.9.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. C., Brown K., Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H. C., Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993 Jun;5(3):477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Lo S. K., Detmers P. A., Levin S. M., Wright S. D. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989 May 1;169(5):1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Thouvenin V., Gendron M. C., Hogervorst F., Figdor C. G., Lanotte M. Phorbol ester-induced promyelocytic leukemia cell adhesion to marrow stromal cells involves fibronectin specific alpha 5 beta 1 integrin receptors. J Cell Physiol. 1992 Oct;153(1):95–102. doi: 10.1002/jcp.1041530113. [DOI] [PubMed] [Google Scholar]
- Matthay M. A., Thiery J. P., Lafont F., Stampfer F., Boyer B. Transient effect of epidermal growth factor on the motility of an immortalized mammary epithelial cell line. J Cell Sci. 1993 Nov;106(Pt 3):869–878. doi: 10.1242/jcs.106.3.869. [DOI] [PubMed] [Google Scholar]
- Montell D. J., Rorth P., Spradling A. C. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992 Oct 2;71(1):51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Higgins K. A., Perez J. R., Coleman T. A., Rosen C. A. Evidence for differential functions of the p50 and p65 subunits of NF-kappa B with a cell adhesion model. Mol Cell Biol. 1993 Jun;13(6):3802–3810. doi: 10.1128/mcb.13.6.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeth P. A., Parry G. C., Kunsch C., Nantermet P., Rosen C. A., Mackman N. Lipopolysaccharide induction of tissue factor gene expression in monocytic cells is mediated by binding of c-Rel/p65 heterodimers to a kappa B-like site. Mol Cell Biol. 1994 Jun;14(6):3772–3781. doi: 10.1128/mcb.14.6.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Ernst M. K. In vivo control of NF-kappa B activation by I kappa B alpha. EMBO J. 1993 Dec;12(12):4685–4695. doi: 10.1002/j.1460-2075.1993.tb06157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santala P., Heino J. Regulation of integrin-type cell adhesion receptors by cytokines. J Biol Chem. 1991 Dec 5;266(34):23505–23509. [PubMed] [Google Scholar]
- Shattil S. J., Brass L. F. Induction of the fibrinogen receptor on human platelets by intracellular mediators. J Biol Chem. 1987 Jan 25;262(3):992–1000. [PubMed] [Google Scholar]
- Shaw L. M., Messier J. M., Mercurio A. M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1 integrin. J Cell Biol. 1990 Jun;110(6):2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. B., Agranoff A. B., Nabel E. G., Leung K., Duckett C. S., Neish A. S., Collins T., Nabel G. J. Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol. 1993 Oct;13(10):6283–6289. doi: 10.1128/mcb.13.10.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloski J. A., Sartorelli A. C., Rosen C. A., Narayanan R. Antisense oligonucleotides to the p65 subunit of NF-kappa B block CD11b expression and alter adhesion properties of differentiated HL-60 granulocytes. Blood. 1993 Jul 15;82(2):625–632. [PubMed] [Google Scholar]
- Valmu L., Autero M., Siljander P., Patarroyo M., Gahmberg C. G. Phosphorylation of the beta-subunit of CD11/CD18 integrins by protein kinase C correlates with leukocyte adhesion. Eur J Immunol. 1991 Nov;21(11):2857–2862. doi: 10.1002/eji.1830211130. [DOI] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993 Oct 15;268(29):21459–21462. [PubMed] [Google Scholar]
- Wilkins J. A., Stupack D., Stewart S., Caixia S. Beta 1 integrin-mediated lymphocyte adherence to extracellular matrix is enhanced by phorbol ester treatment. Eur J Immunol. 1991 Feb;21(2):517–522. doi: 10.1002/eji.1830210239. [DOI] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]