Abstract
Lignin is a complex biopolymer derived primarily from the condensation of three monomeric precursors, the monolignols. The synthesis of monolignols occurs in the cytoplasm. To reach the cell wall where they are oxidized and polymerized, they must be transported across the cell membrane. However, the molecular mechanisms underlying the transport process are unclear. There are conflicting views about whether the transport of these precursors occurs by passive diffusion or is an energized active process; further, we know little about what chemical forms are required. Using isolated plasma and vacuolar membrane vesicles prepared from Arabidopsis, together with applying different transporter inhibitors in the assays, we examined the uptake of monolignols and their derivatives by these native membrane vesicles. We demonstrate that the transport of lignin precursors across plasmalemma and their sequestration into vacuoles are ATP-dependent primary-transport processes, involving ATP-binding cassette-like transporters. Moreover, we show that both plasma and vacuolar membrane vesicles selectively transport different forms of lignin precursors. In the presence of ATP, the inverted plasma membrane vesicles preferentially take up monolignol aglycones, whereas the vacuolar vesicles are more specific for glucoconjugates, suggesting that the different ATP-binding cassette-like transporters recognize different chemical forms in conveying them to distinct sites, and that glucosylation of monolignols is necessary for their vacuolar storage but not required for direct transport into the cell wall in Arabidopsis.
Lignin is a complex and irregular biopolymer that is primarily derived from the condensation of three monomeric precursors, p-hydroxyphenyl, coniferyl, and sinapyl alcohols (termed monolignols). Although lignin affords vital structural support to terrestrial plants and provides hydrophobicity to their vascular elements, its presence in cell walls constitutes a formidable obstacle for digesting forage crops, pulping, and producing renewable biofuels from cellulose and hemicelluloses (1, 2).
Monolignols are synthesized in the cytosol. Thereafter, these monomeric precursors are exported into the cell wall, where they are polymerized and integrated into the wall to form p-hydroxyphenyl, guaiacyl, and syringyl subunits (3). Accordingly, monolignol transport across plasma membranes is a critical step affecting the deposition of lignin and the thickening of the secondary cell wall. Despite the importance of transporting the lignin precursors, the molecular mechanisms underlying their subcellular sequestration and extracellular transportation are sketchy (3). Earlier investigations in gymnosperms and angiosperms provided conflicting interpretations. Using [3H]Phe to label the developing xylem, several studies found that the radiolabel was associated with the rough endoplasmic reticulum (ER) and the Golgi body, and also with some vesicles fused with the plasma membrane (4–6). The potential vesicular trafficking between the cytosol and plasmalemma in differentiating tracheids of xylem tissues was also reported (4). These autoradiographic and ultrastructural analyses engendered the assumption that the lignin precursors were exported to the cell wall through vesicle-mediated exocytosis. However, Kaneda et al. (7) recently adopted a new approach to preparing labeled xylem cells of lodgepole pine for autoradiographic studies. Using cryofixation and freezing substitution techniques, they substantially minimized the damage in sectioned cells, thus preventing the misinterpretation of autoradiography. Then, feeding dissected xylem tissue with a [3H]Phe radiotracer and selectively inhibiting phenylpropanoid and protein biosynthesis by different inhibitors, they discovered that the radiolabel in the ER-Golgi was primarily incorporated into proteins, not the monolignols. Furthermore, they found that the Golgi and Golgi-vesicle clusters abundant in the developing xylem cells were not loaded with phenylpropanoids. These results suggest that ER-Golgi-vesicle-mediated exocytosis does not play a major role in transport of the monolignols (7).
Genetic and chemical analyses demonstrated that lignin biosynthesis displays considerable plasticity. Besides the three classical monolignols, some nontraditional phenolic monomers are incorporated into lignin under certain circumstances (8, 9). For example, in a natural cinnamyl-alcohol dehydrogenase (CAD)-deficient mutant of pine and transgenic tobacco knocked down in CAD, hydroxycinnamaldehydes were incorporated into lignin (10). Similarly, a lack of the caffeic acid O-methyltransferase in a maize bm3 mutant caused the accumulation of 5-hydroxyconiferyl alcohol and the buildup of this unusual precursor in lignin (11). In addition, lignins are frequently acylated with acetate or p-coumarate (12, 13); such acylation implicates the incorporation of acylated lignin monomers. The accommodation of alternative monomers in lignification led to the suggestion of nonspecific passive diffusion of lignin precursors across the plasma membrane (14). This notion was supported by the observation of the in vitro partitioning of lignin monomers or analogs by immobilized liposomes and/or lipid-bilayer discs (15, 16).
Although lignin biosynthesis displays considerable flexibility in incorporating different monomeric precursors, many studies note that these monomers are deposited differentially in discrete regions of particular tissues or cells. For example, lignin in the cell walls of vessels in birch wood is derived mainly from coniferyl alcohol, whereas its fiber wall incorporates both sinapyl and coniferyl alcohols (17). Similarly, in Arabidopsis stems, the lignin of the vascular bundle in vessels primarily contains guaiacyl lignin (from coniferyl alcohol), whereas the interfascicular fibers are enriched in syringyl units (from sinapyl alcohol) (18). Moreover, when feeding the labeled monolignols into the developing xylem, the radiolabeled p-coumaryl alcohol is preferentially laid down in the middle lamella/cell corners, whereas coniferyl alcohol is mainly located within the secondary wall (19). These data suggest that the biosynthesis and deposition of lignin monomers into cell wall is a highly organized, regulated process, and that active transportation mechanisms might selectively permit the deposition of the particular monolignols.
Besides depositing lignin monomers into the cell wall, gymnosperms and some angiosperms store a significant fraction of monolignol 4-O-glucosides within the cytoplasm, presumably in the cell's vacuoles (3). The ability of those plants to divert monolignols to a storage compartment rather than directly incorporating them into cell-wall polymers also implies that the plant contains proteins that can transport monolignols or their derivatives across lipid bilayers into particular compartments.
Recently, several families of plant membrane transporters, including ATP-binding cassette (ABC) transporters and multidrug and toxic compound extrusion (MATE) transporters, were shown to be involved in sequestering intracellularly a variety of small molecular compounds, including phenolics (20, 21). Global transcriptomic and proteomic studies in gymnosperms and angiosperms frequently reveal the presence and high expression of some membrane transporters in lignified wood tissues (22, 23). All such studies suggest that these membrane transporters may be active in sequestration and transport of the monolignols. However, direct biochemical evidence has been lacking.
In this study, we isolated plasma and vacuolar membrane vesicles from Arabidopsis young rosette leaves and the roots of poplar (Populus tremuloides). With the prepared membrane vesicles, we undertook in vitro uptake assays for monolignols, their glucosides, and related phenolics. Together with the application of different transporter inhibitors, we reveal that the transport of lignin monomeric precursors across both plasma and vacuolar membranes is an active ATP-dependent process. Omitting ATP or including specific ABC-type transporter inhibitors severely impaired the transport activity of plasma or vacuolar membrane vesicles to monolignols or their glucosides. In the presence of ATP, plasma membrane vesicles selectively transport monolignol aglycones, whereas vacuolar vesicles prefer monolignol 4-O-glucosides, implying that different ABC-like transporters recognize and convey distinct chemical forms of lignin precursors to particular sites.
Results
ATP-Dependent Transport of Monolignols by Arabidopsis Plasma Membrane Vesicles.
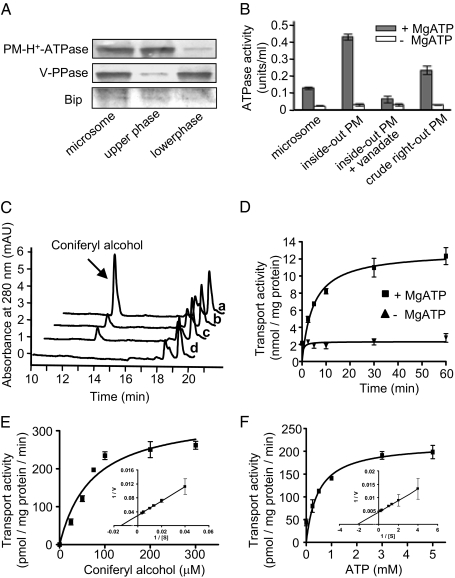
To mimic the in vivo efflux of lignin precursors across plasmalemma, we prepared inside-out (inverted) plasma membrane vesicles from Arabidopsis rosette leaves. We first used an aqueous polymer two-phase partitioning procedure (24) to isolate right-side-out plasma vesicles from an Arabidopsis microsomal fraction. Subsequently, we treated the vesicles with the detergent Brij 58 (25) to convert the right-side-out vesicles to the inside-out ones (cytoplasmic-side-out). We monitored the quality of our membrane preparation by Western blots using antibodies against plasma membrane H+-ATPase, vacuolar H+-pyrophosphatase (V-PPase), and ER luminal-binding protein (Bip) of Arabidopsis thaliana. The gel blot showed that the plasma membrane vesicles were predominantly enriched in the upper phase of the two-phase partition (Fig. 1A). The inverted vesicles showed high H+-ATPase activity, exceeding by approximately three- to fivefold the latent activities of the crude microsomes and the right-side-out vesicles; this activity was inhibited severely by sodium ortho-vanadate, a suppressor of ATPase activity (26) (Fig. 1B). These data verify the high quality of our prepared inverted plasma membrane vesicles.
Fig. 1.
Preparation and characterization of plasma membrane (PM) vesicles and the transport of monolignols by inside-out plasma membrane vesicles. (A) Protein gel-blot analysis of membrane fractions by probing with antibodies against Arabidopsis plasma membrane H+-ATPase (PM-H+-ATPase), vacuolar H+-pyrophosphatase (V-PPase, tonoplast marker), and endoplasmic reticulum-binding protein (Bip, ER marker). (B) H+-ATPase activities of different membrane preparations in the presence or absence of MgATP. The results are from three replicates. (C) Portions of HPLC traces showing the recovered coniferyl alcohol from the inverted vesicles in the uptake assay in the presence (a) and absence (b) of ATP, the presence of sodium vanadate and ATP (c), and the absence of phenolic substrate (d). (D) Time course of the transport of coniferyl alcohol into the inside-out vesicles. The results are the mean and SD of two replicates. (E and F) The kinetics of transport of coniferyl alcohol into Arabidopsis inside-out plasma membrane vesicles for coniferyl alcohol (E) and MgATP (F) concentrations. The data are plotted by a nonlinear regression analysis fit to the Michaelis–Menten equation. The insets in E and F show Lineweaver–Burk plots. The data are the means and SD of two or three replicates.
The inverted vesicles were incubated with monolignols, represented by coniferyl alcohol, in the presence or absence of MgATP. We then collected the vesicles by vacuum filtration through a wet cellulose-nitrate membrane filter. After thoroughly rinsing the filters, we re-extracted the compounds retained within the vesicles and examined them by HPLC. The amount of monolignols taken up by and recovered from the vesicles clearly depended upon the ATP molecules added during incubation. In the absence of MgATP, only low amounts of monolignols were detected (Fig. 1C; Fig. S1). Adding MgATP in the assay medium increased the uptake of coniferyl alcohol more than threefold (Fig. 1C). We observed a similar ATP-dependent uptake when the inverted plasma membrane vesicles from Populus were used (Fig. S2A).
We explored further the uptake of coniferyl alcohol by the inverted plasma vesicles in a time-course experiment. The transport of monolignols in the presence of MgATP rose with incubation time, and gradually reached its maximum after 1 h (Fig. 1D). This behavior likely coincides with the gradual depletion of ATP molecules.
To determine whether the transport of monolignols across plasma membranes specifically depends on ATP, we replaced ATP with other nucleotides such as MgGTP, -TTP, and -CTP. ATP was the most effective nucleotide triphosphate in driving monolignol transport, whereas GTP, TTP, and CTP slightly promoted the transport of coniferyl alcohol (Table S1).
Together, these data suggest that the transport of the monolignol coniferyl alcohol across plasmalemma is primarily an energy-dependent process, although some non-energy-dependent transport was evident in the in vitro assay.
Kinetics of Monolignol Uptake by Plasma Membrane Vesicles.
The uptake of coniferyl alcohol by the inverted plasma membrane vesicles exhibited typical Michaelis–Menten-type kinetics. At a fixed concentration of MgATP, we calculated the apparent Km and Vmax values for transport of coniferyl alcohol as, respectively, 71.4 μM and 344 pmol·mg protein−1·min−1 (Fig. 1E), and the calculated Km value for ATP was 468 μM (Fig. 1F). These numbers are close to those observed for other ABC-type transporters involved in the transport of low-molecular-weight organic compounds (27, 28).
Effects of Transport Inhibitors on the Uptake of Monolignols Across the Plasma Membrane.
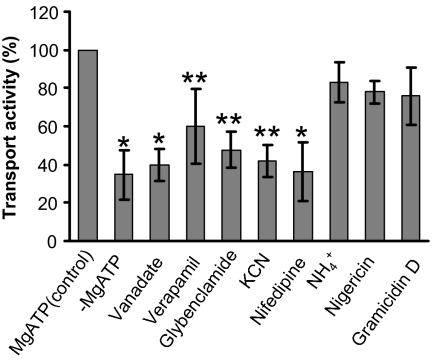
The inverted plasma membrane vesicles were pretreated with the following inhibitors. Sodium ortho-vanadate is a typical suppressor of ATPase activity and inhibitor of ABC transporters that acts as a phosphate analog (26). Verapamil and nifedipine are general Ca2+-channel blockers, also known to preferentially inhibit ABCB-type ABC transporters (27, 29). Glybenclamide is a sulfonylurea derivative acting as an effective inhibitor of especially ABCC-type ABC transporters (27, 30). All impaired the ATP-dependent uptake of coniferyl alcohol. In particular, vanadate and nifedipine reduced transport activity by ≈60%, reaching the baseline level of uptake in the absence of ATP (Fig. 2). In contrast, ionophores/protonophores, such as gramicidin D which dissipates both the pH gradient and membrane potential, and nigericin and NH4Cl which destroy the pH gradient across membranes (31), displayed little effect on monolignol uptake (Fig. 2).
Fig. 2.
Inhibition of ATP-dependent uptake of plasma membrane vesicles to coniferyl alcohol by inhibitors of membrane transporters. Inside-out vesicles were incubated with 100 μM coniferyl alcohol, to which we added different inhibitors at varied concentrations as described in Materials and Methods. The average transport activity under MgATP and monolignol only was set to 100%. The data are the means and SD of three replicates. *Significant changes at P < 0.01 and **significant changes at P < 0.05, compared with the control, under Student's t test.
Selective Transport of Phenolics by Plasma Membrane Vesicles.
We tested various phenolics as potential substrates in uptake assays of plasma membrane vesicles. These include hydroxycinnamic acids, hydroxycinnamyl aldehydes, alcohols, and/or their glucosides (Table 1). The inverted vesicles showed a base level and unselective transport activity to a range of phenolic aglycones in the absence of ATP, indicating potential intrinsic, nonselective permeability of the plasmalemma to those hydrophobic compounds. When we added ATP, the transport activity toward hydroxycinnamyl alcohols and aldehydes profoundly increased. However, the inverted membrane vesicles did not display any measurable transport activity for the monolignol glucosides coniferin and syringin, and only showed a negligible uptake of ferulic acid, either in the absence or presence of MgATP (Table 1).
Table 1.
Uptake of different phenolics by plasma and vacuolar membrane vesicles
| Plasma membrane |
Vacuolar membrane |
|||
| +MgATP |
−MgATP |
+MgATP |
−MgATP |
|
| Substrate | (nmol/mg protein) (10 min) | |||
| Coniferyl alcohol | 7.9 ± 2.0 | 2.2 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Coniferaldehyde | 3.0 ± 0.9 | 1.1 ± 0.3 | ND | ND |
| Coniferin | ND | ND | 3.2 ± 0.9 | 0.6 ± 0.1 |
| Sinapyl alcohol | 5.8 ± 1.3 | 1.8 ± 0.3 | 0.2 ± 0.05 | 0.2 ± 0.01 |
| Sinapaldehyde | 1.7 ± 0.6 | 0. 5 ± 0.1 | ND | ND |
| Syringin | ND | ND | 2.1 ± 0.2 | 0. 7 ± 0.1 |
| Ferulic acid | Trace | Trace | ND | ND |
Uptake was measured in standard uptake medium containing various monolignols in the presence or absence of 5 mM MgATP. Values shown are mean ± SD (n = 3). ND, not detectable.
Uptake of Monolignol Glucosides into Arabidopsis Vacuolar Vesicles.
Arabidopsis accumulates soluble monolignol 4-O-glucosides in its root tissues, presumably in the vacuoles of cells (32). We asked whether the vacuolar sequestration and accumulation of monolignol glucosides involve active transport.
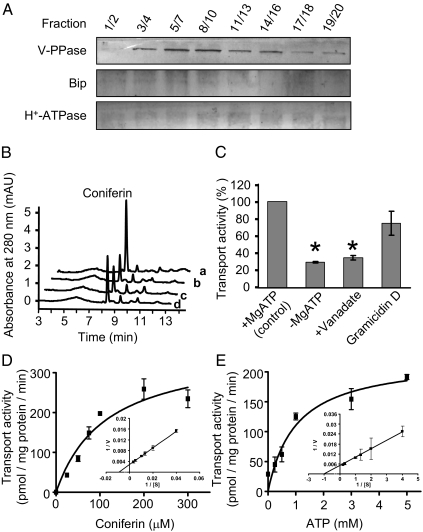
We prepared vacuolar membrane vesicles from intracellular membranes, derived from two-phase partitioning, by sucrose differential-density centrifugation. The quality of membrane separation was monitored by examining marker proteins with immunoblots against V-PPase, H+-ATPase, and Bip antibodies for the selected centrifugation fractions (Fig. 3A), and by measuring the activities of V-PPase for PPi hydrolysis and PPi-dependent H+ translocation and of H+-ATPase (Fig. S3). We collected the fractions enriched with vacuolar membranes that showed high activity and strong immune signals for V-PPase, and used them for the uptake assays.
Fig. 3.
Preparation of vacuolar membranes and vacuolar sequestration assay. (A) Protein gel-blot analysis of selected fractions probed with antibodies against V-PPase, Bip, and plasma membrane H+-ATPase. (B) Portions of HPLC traces showing recovered coniferyl alcohol 4-O-glucoside (coniferin) from the vacuolar vesicles in the sequestering assay in the presence (a) and absence (b) of ATP, the presence of sodium vanadate and ATP (c), and the presence of gramicidin D and ATP (d). (C) Effects of the membrane transporter inhibitors sodium vanadate (1 mM) and gramicidin D (5 μM) on the ATP-dependent uptake of coniferin by vacuolar vesicles. Data were from three replicates. *Significant changes at P < 0.01 under t test. (D and E) The kinetics of transport of coniferin into vacuolar vesicles for coniferin (D) and MgATP (E) concentrations. The data are from two or three replicates and are plotted by nonlinear regression analysis fit to the Michaelis–Menten equation. Insets show Lineweaver–Burk plots.
Monolignol 4-O-glucosides were prepared by enzymatic reactions catalyzed by a UDP-glucose (UDPG):4-O-glucosyltransferase, UGT72E2, one of the functionally characterized glycosyltransferases from Arabidopsis (33) (Fig. S4).
We found that when monolignol glucosides were incubated with Arabidopsis vacuolar vesicles, uptake of coniferin was active in the presence of MgATP (Fig. 3B). The ABC transporter inhibitor vanadate impaired this transport activity severely, but gramicidin D, a compound potentially inhibiting MATE-transporter activity (31), had little effect (Fig. 3C).
In contrast to the relaxed specificity of plasma membranes for phenolics, the uptake of vacuolar vesicles showed strict substrate specificity. ATP-dependent vacuolar sequestration predominantly occurred for the 4-O-glucosides coniferin and syringin. The vacuolar membranes exhibited only minor transport activity for monolignol aglycones, whether ATP was present or absent; no activity was detected toward other phenolics (Table 1).
Similar to the kinetic behavior of plasma membranes transporting monolignol aglycones, the vacuolar vesicles sequestering monolignol glucosides also displayed Michaelis–Menten-type kinetics. The apparent Km for coniferin was 113.9 μM, and was 1 mM for ATP (Fig. 3 D and E).
Discussion
ABC-like Transporters Export Lignin Precursors Across the Plasmalemma and Sequester Them into Vacuoles.
The molecular mechanisms for depositing monolignols or their derivatives from the cytoplasm into the cell wall and for sequestering them intracellularly have been long-standing issues. Because of its amphiphilic property, the lipid-bilayer membrane may be permeable to hydrophobic phenolic compounds. An in vitro partitioning assay readily detected lignin monomeric analogs absorbed into immobilized liposomes or lipid-bilayer discs (15, 16), supporting a nonselective, passive diffusion mechanism for monolignols (14).
However, using plasma and vacuolar membranes from Arabidopsis and Populus, which retain many physiological properties of the native lipid bilayers, to mimic the efflux of lignin precursors across plasmalemma and their influx into vacuoles, we demonstrated that significant movement of monolignols or their glucosides into the inverted plasma or vacuolar vesicles, respectively, occurred under energized conditions (Figs. 1 and 3; Table 1). In the absence of ATP, lignin precursors were transported by plasma or vacuolar membrane vesicles only at a low level (Figs. 1 and 3; Table 1). This base level of transport activity might reflect a passive diffusion that may function as a component in transporting lignin monomeric precursors across membranes. However, passive diffusion is unlikely to play a major role; instead, transport across plasma and vacuolar membranes is dominated by ATP-dependent primary transport. Several lines of evidence corroborated our conclusion. First, the uptake of monolignols or their glucosides into vesicles depended upon nucleotide phosphates, particularly ATP (Table S1). Second, uptake showed selectivity for different substrates. In the presence of ATP, plasma vesicles preferentially transport monolignol aglycones, whereas vacuolar sequestration favors glucoconjugates (Table 1). Third, the uptake of monolignols or their glucosides displays typical Michaelis–Menten kinetics (Figs. 1 E and F and 3 D and E), or a cooperative ligand-binding behavior observed in the uptake of Populus membrane vesicles (Fig. S2B), indicating a membrane-protein-mediated biochemical process rather than passive diffusion. Last, a set of ABC transporter inhibitors severely reduced the energy-dependent transport (Figs. 2 and 3). These in vitro pieces of evidence suggest that lignin precursors transported across the plasmalemma and sequestered into vacuoles require membrane-protein-mediated active transport that involves ATP-binding cassette-like transporters.
Among the multidrug resistance protein inhibitors/blockers used in the uptake assays, KCN also displayed an inhibitory effect on the uptake of monolignols by the plasma membrane vesicles in the presence of ATP (Fig. 2). Although cyanide is known as an inhibitor of the respiratory electron-transport chain that depletes ATP (34), it also can directly damage cell membranes by altering the membrane's resistance (increasing its permeability) (35). We tested this possibility by monitoring the potential changes of the latent ATP-hydrolyzing activity of the right-side-out plasma membrane vesicles after KCN treatment. In the presence of ATP, we observed that KCN treatment increased the measurable ATP-hydrolyzing activity of the right-side-out membrane vesicles but did not affect the activity presented in the inside-out membranes (Fig. S5), indicating the treatment did not directly affect ATPase but might enhance the permeability of the membranes (for ATP) or damage the integrity of the membrane vesicles. Although the underlying mechanism of the inhibition by KCN on monolignol uptake may be complex, one possibility for the observed effect might reflect the action of KCN on the membrane's physical properties, thereby interfering with the retention of transported phenolics in the vesicles.
Compared with vacuolar vesicles, plasma membranes displayed notable promiscuity in conveying different phenolics in the presence or absence of ATP molecules (Table 1). The lesser selectivity of plasma membranes might explain the observed plasticity of lignin biosynthesis. The promiscuous active transport and/or the low level of intrinsic diffusion may lead to the deposition of nonclassic lignin precursors into the cell wall.
Different Structural Forms of Monolignols Are Required for Transport Across Plasmalemma and Sequestration into Vacuoles.
In gymnosperms and some angiosperm species, monolignols often are glucosylated on the phenolic hydroxyl group to form 4-O-β-d-glucosides, namely coniferin and syringin (3, 36). The possible presence of monolignol glucosides in the vacuoles of differentiating conifer xylem cells led to the hypothesis that monolignol glucosides might be a storage and transport form of monolignols (3, 36), and that the UDPG:coniferyl alcohol glucosyltransferase, together with coniferin-β-glucosidase, which releases the sugar moiety from glucoconjugates in the cell wall, may regulate the storage and mobilization of monolignols for lignin biosynthesis (37).
Arabidopsis accumulates monolignol glucosides in the cells of its root and leaf tissues (32, 33). The vacuolar membrane vesicles prepared from Arabidopsis rosette leaves displayed considerable activity in sequestering coniferin and syringin in the presence of ATP (Fig. 3; Table 1). In contrast, the plasma membrane vesicles were inactive to the glucoconjugated monolignols in either the presence or absence of ATP (Table 1). These data suggest that glucosylation of monolignols is a prerequisite for their vacuolar storage but not for the direct transport into cell walls of Arabidopsis. These results complement previous genetic studies wherein down- or up-regulating the expression of its UDPG:monolignol glucosyltransferases entailed the corresponding reduction or accumulation of the soluble monolignol glucosides in transgenic roots or leaves (32), but a change in lignin content or composition was not observed.
The different chemical forms of monolignols required in ATP-dependent transport also implicate the distinct classes of ABC transporters involved in diverting and partitioning the polarized “storage-form” glucosides of monolignols into vacuoles, and the hydrophobic aglycones across plasmalemma.
Do Other Multidrug Membrane Transporters Participate in Monolignol Transportation?
Both the ABC and MATE membrane transporters reportedly are involved in transporting a range of small secondary metabolites, including phenolics and polyphenolics (20, 38). For instance, the maize ABC transporter ZmMRP3, encoding a GS-X pump and localized in the tonoplast, was shown, genetically, to be necessary for translocating anthocyanin (39). However, sequestration of proanthocyanidins, a group of polyphenolics structurally related to anthocyanin, requires H+-gradient–dependent transport (31, 40). The Arabidopsis mutant transparent testa12 (tt12), lacking the gene encoding a MATE-family secondary transporter-like protein, exhibited much less deposition of proanthocyanidins in the vacuoles of endothelial cells (41). Adding ABC transporter inhibitors or blockers in uptake assays of plasma and vacuolar membrane vesicles severely disrupted the uptake of monolignols or their glucosylated derivatives (Figs. 2 and 3). However, ionophoric agents, such as gramicidin D, nigericin, and NH4Cl which disturb the activities of MATE-family transporters, had negligible effects on monolignol uptake (Fig. 2). These data imply that the secondary energized MATE transporters may not play vital roles as do the ABC transporters in mediating lignin-precursor transport.
Materials and Methods
Plant Material and Growth Conditions.
Arabidopsis thaliana ecotype Columbia (Col-0) was used in this study. For aerial tissues, seedling plants were grown in soil for 4 wk with a controlled environment of 16/8-h light/dark cycle (light, 100 μmol photons m−2·s−1, 22 °C; dark, 17 °C). Then, the plants were harvested and stored at −80 °C until isolation of the microsomal fractions. Populus tremuloides root tissues were collected from 8-mo-old plants grown in the growth chamber under the same conditions.
Chemicals.
All of the chemicals used were purchased from Sigma-Aldrich, unless otherwise stated.
Preparation of Plasma Membranes and Formation of Inside-Out Vesicles.
The microsomal fractions from Arabidopsis rosette leaves and Populus root tissues were prepared essentially as described by Palmgren et al. (24). The resulting microsomes were suspended in 5 mM potassium phosphate buffer (pH 7.8) containing 330 mM sucrose, 5 mM KCl, 1 mM DTT, and 0.1 mM EDTA (buffer A). Plasma membranes (predominantly right-side-out vesicles) were purified from the microsomal preparation by partitioning in an aqueous polymer two-phase system, as described by Larsson et al. (42). Briefly, the microsomal fraction was added to a phase system with a final weight of 36 g and a final composition of 6.5% (wt/wt) dextran T500 and 6.5% (wt/wt) polyethylene glycol 3350 in buffer A (pH 7.8), and then mixed and partitioned. The final upper phase containing the right-side-out plasma membranes was diluted severalfold with buffer A. The plasma membranes were pelleted and resuspended to 15–20 mg/mL protein in the same buffer, and stored at −80 °C until further use.
The purified right-side-out plasma membrane vesicles in buffer A were mixed with the detergent Brij 58 (Sigma) to a final concentration of 0.05% (wt/vol) and KCl to 50 mM. The preparations were then frozen in liquid N2 and thawed in water at 20 °C four times to produce the sealed, inside-out vesicles as described by Johansson et al. (25). The inverted plasma membranes were pelleted at 100,000 × g for 2 h. The pellets were gently resuspended in 10 mM Mops·KOH buffer (buffer B; pH 7.5) containing 0.33 M sucrose, 0.1 mM EDTA, 1 mM DTT, and 1× protease inhibitor mixture (Sigma). We monitored the quality of the prepared plasma membranes by measuring the vanadate-inhibited Mg2+-ATPase activity with an ATPase assay kit according to the manufacturer's instructions (Innova Biosciences).
Preparation of Vacuolar Membrane Vesicles.
Vacuolar membrane vesicles were extracted from the remaining lower phase of the aqueous polymer two-phase system (see above). They were diluted about 10-fold in buffer A, and then pelleted at 100,000 × g for 2 h. All procedures were performed at 4 °C. The pellet was resuspended in buffer A containing 1× protease inhibitor mixture. The suspension was layered over a discontinuous sucrose gradient [10%, 15%, 20%, 25%, 30%, 40%, and 50% (wt/vol) in 20 mM Tris·HCl buffer (pH 7.6), 1 mM DTT, and 1 mM EDTA] in a 40-mL tube. The tubes were centrifuged at 100,000 × g for 3 h. Successive 2-mL fractions were collected from the top of the centrifuge tube, diluted with buffer B, and again centrifuged at 100,000 × g for another 2 h. The pellets were resuspended in 0.2 mL of buffer B for subsequent assays.
Measurement of Vacuolar ATPase and PPase Activity.
Vacuolar ATPase activity was measured by the method of Ames (43). PPi-dependent H+ translocation by vacuolar membrane vesicles was assayed fluorimetrically at 25 °C using quinacrine as ΔpH indicator. For details, see SI Materials and Methods.
Transport Activity Assay.
For the uptake assay, we modified a method described by Zhao and Dixon (31) and Sugiyama et al. (27). The 500-μL assay mixtures contained 25 mM Tris·Mes (pH 8.0), 0.4 M sorbitol, 50 mM KCl, 5 mM MgATP, 0.1% (wt/vol) BSA, and the indicated concentration of phenolic substrate. ATP was omitted from the nonenergized controls. Assays were started by adding the membrane vesicles (50–100 μg of protein) while briefly agitating the mixture at 25 °C. Batches of the reaction mixture (100 μL) were removed at various times, and their reactions were terminated with 1.0 mL of ice-cold washing solution (25 mM Tris·Mes, pH 8.0, 0.4 M sorbitol). The mixtures underwent vacuum filtration through prewetted nitrocellulose membrane filters (0.22-μm pore diameter; Millipore). The dried filters, transferred to 20-mL glass vials containing 0.5 mL of 50% (vol/vol) methanol, were extracted for 1 h at room temperature in an orbital shaker. The eluate was analyzed by HPLC. The sample was resolved on a Gemini C18 reverse-phase column (Phenomenex) in 0.2% acetic acid (A) with an increasing concentration gradient of acetonitrile containing 0.2% acetic acid (B): 0–20 min, 30% B; 20–25 min, 100% B at a constant rate of 0.8 mL/min. UV absorption was monitored at 254, 280, and 310 nm using a multiple-wavelength photodiode array detector (Agilent).
For our tests of transport inhibitors, we preincubated them with the membrane vesicles for 2 min at the following final concentrations: 1 mM vanadate (in water), 5 μM verapamil (in DMSO), 150 μM glybenclamide (in DMSO), 5 μM gramicidin D (in DMSO), 2 μM nigericin (in DMSO), 50 μM nifedipine (in DMSO), 1 mM potassium cyanide (in water), and 1 mM NH4Cl (in water). Sodium vanadate was depolymerized before use according to Goodno's procedure (26). Then, to each we added MgATP. To avoid unexpected side effects, each inhibitor was used at the concentration given for its specific inhibitory effect on transporters and pumps, or for disrupting membrane potential as well as ΔpH based on values in the literature. To the 250-μL assay mixture, 1–2.5 μL of each stock solution was added, where the concentration of organic solvent was less than 1% (vol/vol). After incubation at 25 °C for 30 min, the transported phenolics were measured as described above. The statistical analyses on the obtained datasets were carried out with a Student's paired t test, using two-tailed distribution and two-sample unequal variances.
Kinetics of Transport of Monolignol or Its Glucoside.
We assessed the kinetics of transport of coniferyl alcohol by plasma membrane vesicles or of coniferin by vacuolar vesicles. For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US Department of Energy (DOE) through Grant DEAC0298CH10886 to C.-J.L. Initially, this work was also partially supported by the Office of Biological and Environmental Research of DOE through the pilot project of biofuel Scientific Focus Area program (BO148).
Footnotes
This article is a PNAS Direct Submission.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007747108/-/DCSupplemental.
References
- 1.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Weng JK, Chapple C. Improvement of biomass through lignin modification. Plant J. 2008;54:569–581. doi: 10.1111/j.1365-313X.2008.03457.x. [DOI] [PubMed] [Google Scholar]
- 3.Whetten R, Sederoff R. Lignin biosynthesis. Plant Cell. 1995;7:1001–1013. doi: 10.1105/tpc.7.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickett-Heaps JD. Xylem wall deposition: Radioautographic investigations using lignin precursors. Protoplasma. 1968;65:181–205. [Google Scholar]
- 5.Fujita M, Harada H. Autoradiographic investigations of cell wall development. II. Tritiated phenylalanine and ferulic acid assimilation in relation to lignification. Mokuzai Gakkaishi. 1979;25:89–94. [Google Scholar]
- 6.Takabe KFM, Harada H, Saiki H. Autoradiographic investigations of lignification in the cell walls of cryptomeria (Cryptomeria japonica D. Don) Mokuzai Gakkaishi. 1985;31:613–619. [Google Scholar]
- 7.Kaneda M, et al. Tracking monolignols during wood development in lodgepole pine. Plant Physiol. 2008;147:1750–1760. doi: 10.1104/pp.108.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ralph J, et al. Abnormal lignin in a loblolly pine mutant. Science. 1997;277:235–239. doi: 10.1126/science.277.5323.235. [DOI] [PubMed] [Google Scholar]
- 9.Sederoff RR, MacKay JJ, Ralph J, Hatfield RD. Unexpected variation in lignin. Curr Opin Plant Biol. 1999;2:145–152. doi: 10.1016/S1369-5266(99)80029-6. [DOI] [PubMed] [Google Scholar]
- 10.Morreel K, et al. Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol. 2004;136:3537–3549. doi: 10.1104/pp.104.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapierre C, Tollier MT, Monties B. A new type of constitutive unit in lignins from the corn bm3 mutant. C R Acad Sci III. 1988;307:723–728. [Google Scholar]
- 12.del Río JC, et al. Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J Agric Food Chem. 2008;56:9525–9534. doi: 10.1021/jf800806h. [DOI] [PubMed] [Google Scholar]
- 13.Lu F, Ralph J, Morreel K, Messens E, Boerjan W. Preparation and relevance of a cross-coupling product between sinapyl alcohol and sinapyl p-hydroxybenzoate. Org Biomol Chem. 2004;2:2888–2890. doi: 10.1039/B411428K. [DOI] [PubMed] [Google Scholar]
- 14.Vanholme R, Morreel K, Ralph J, Boerjan W. Lignin engineering. Curr Opin Plant Biol. 2008;11:278–285. doi: 10.1016/j.pbi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Boija E, Johansson G. Interactions between model membranes and lignin-related compounds studied by immobilized liposome chromatography. Biochim Biophys Acta. 2006;1758:620–626. doi: 10.1016/j.bbamem.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Boija E, Lundquist A, Edwards K, Johansson G. Evaluation of bilayer disks as plant cell membrane models in partition studies. Anal Biochem. 2007;364:145–152. doi: 10.1016/j.ab.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Fergus BJ, Goring DAI. The distribution of lignin in birch wood as determined by ultraviolet microscopy. Holzforschung. 1970;24:118–124. [Google Scholar]
- 18.Chapple CC, Vogt T, Ellis BE, Somerville CR. An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell. 1992;4:1413–1424. doi: 10.1105/tpc.4.11.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terashima N, Fukushima K, He L-F, Takabe K. Comprehensive model of the lignified plant cell wall. In: Jung HG, Buxton DR, Hatfield RD, Ralph J, editors. Forage Cell Wall Structure and Digestibility. Madison, WI: ASA-CSSA-SSSA; 1993. pp. 247–270. [Google Scholar]
- 20.Yazaki K. Transporters of secondary metabolites. Curr Opin Plant Biol. 2005;8:301–307. doi: 10.1016/j.pbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Dixon RA. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010;15:72–80. doi: 10.1016/j.tplants.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Ehlting J, et al. Global transcript profiling of primary stems from Arabidopsis thaliana identifies candidate genes for missing links in lignin biosynthesis and transcriptional regulators of fiber differentiation. Plant J. 2005;42:618–640. doi: 10.1111/j.1365-313X.2005.02403.x. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson R, et al. Proteomics of plasma membranes from poplar trees reveals tissue distribution of transporters, receptors, and proteins in cell wall formation. Mol Cell Proteomics. 2010;9:368–387. doi: 10.1074/mcp.M900289-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmgren MG, et al. Sealed inside-out and right-side-out plasma membrane vesicles: Optimal conditions for formation and separation. Plant Physiol. 1990;92:871–880. doi: 10.1104/pp.92.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson F, Olbe M, Sommarin M, Larsson C. Brij 58, a polyoxyethylene acyl ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J. 1995;7:165–173. doi: 10.1046/j.1365-313x.1995.07010165.x. [DOI] [PubMed] [Google Scholar]
- 26.Goodno CC. Inhibition of myosin ATPase by vanadate ion. Proc Natl Acad Sci USA. 1979;76:2620–2624. doi: 10.1073/pnas.76.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiyama A, Shitan N, Yazaki K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol. 2007;144:2000–2008. doi: 10.1104/pp.107.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangne N, et al. Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H(+)-antiport and ATP-binding cassette-type mechanisms. Plant Physiol. 2002;128:726–733. doi: 10.1104/pp.010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornwell MM, Pastan I, Gottesman MM. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987;262:2166–2170. [PubMed] [Google Scholar]
- 30.Payen L, et al. The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells. Br J Pharmacol. 2001;132:778–784. doi: 10.1038/sj.bjp.0703863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Dixon RA. MATE transporters facilitate vacuolar uptake of epicatechin 3′-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell. 2009;21:2323–2340. doi: 10.1105/tpc.109.067819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanot A, et al. The glucosyltransferase UGT72E2 is responsible for monolignol 4-O-glucoside production in Arabidopsis thaliana. Plant J. 2006;48:286–295. doi: 10.1111/j.1365-313X.2006.02872.x. [DOI] [PubMed] [Google Scholar]
- 33.Lim EK, et al. Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J Biol Chem. 2001;276:4344–4349. doi: 10.1074/jbc.M007263200. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima H. Effects of respiratory inhibitors on respiration, ATP contents, and the circadian conidiation rhythm of Neurospora crassa. Plant Physiol. 1984;76:612–614. doi: 10.1104/pp.76.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson WP, Hendrix DL, Higinbotham N. The effect of cyanide and carbon monoxide on the electrical potential and resistance of cell membranes. Plant Physiol. 1974;54:712–716. doi: 10.1104/pp.54.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savidge RA. Coniferin, a biochemical indicator of commitment to tracheid differentiation in conifers. Can J Bot. 1989;67:2663–2668. [Google Scholar]
- 37.Samuels AL, et al. Cellular machinery of wood production: Differentiation of secondary xylem in Pinus contorta var. latifolia. Planta. 2002;216:72–82. doi: 10.1007/s00425-002-0884-4. [DOI] [PubMed] [Google Scholar]
- 38.Yazaki K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006;580:1183–1191. doi: 10.1016/j.febslet.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell. 2004;16:1812–1826. doi: 10.1105/tpc.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marinova K, et al. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell. 2007;19:2023–2038. doi: 10.1105/tpc.106.046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debeaujon I, Peeters AJM, Léon-Kloosterziel KM, Koornneef M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–871. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson C, Sommarin M, Widell S. Isolation of highly purified plant plasma membranes and the separation of inside-out and right-side-out vesicles. Methods Enzymol. 1994;228:451–469. [Google Scholar]
- 43.Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.