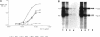Abstract
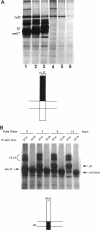
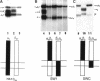
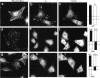
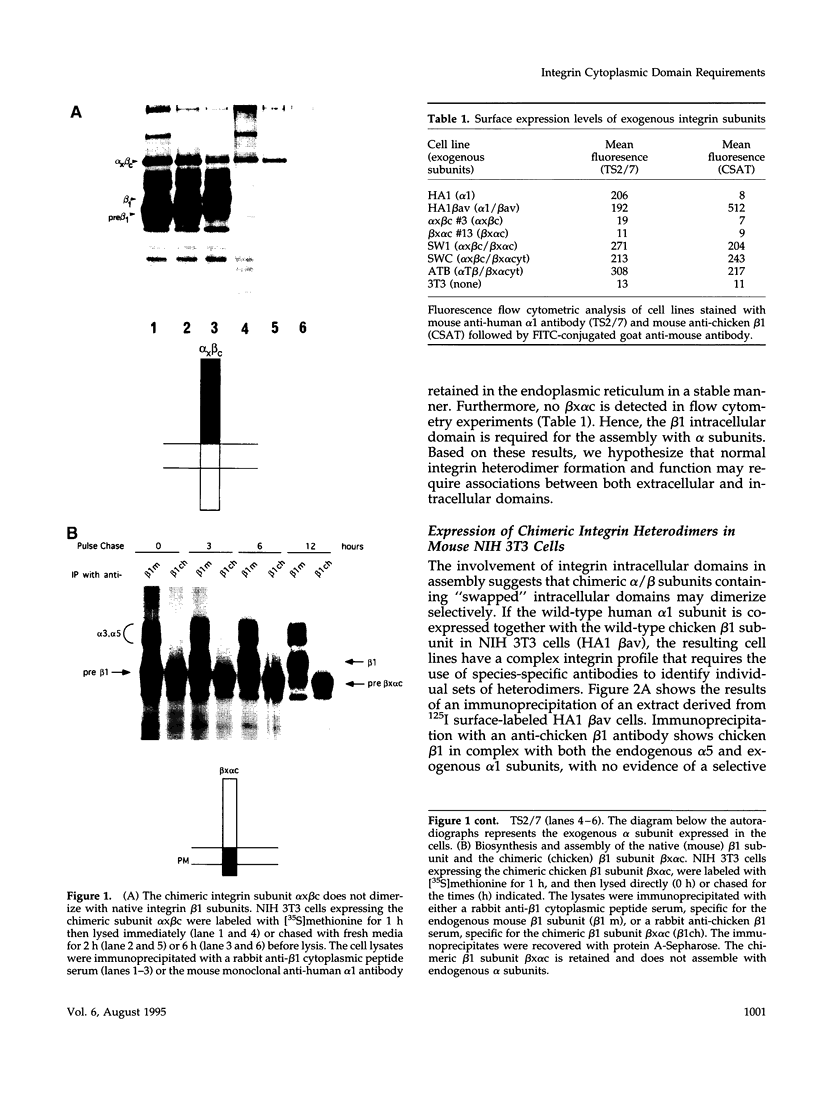
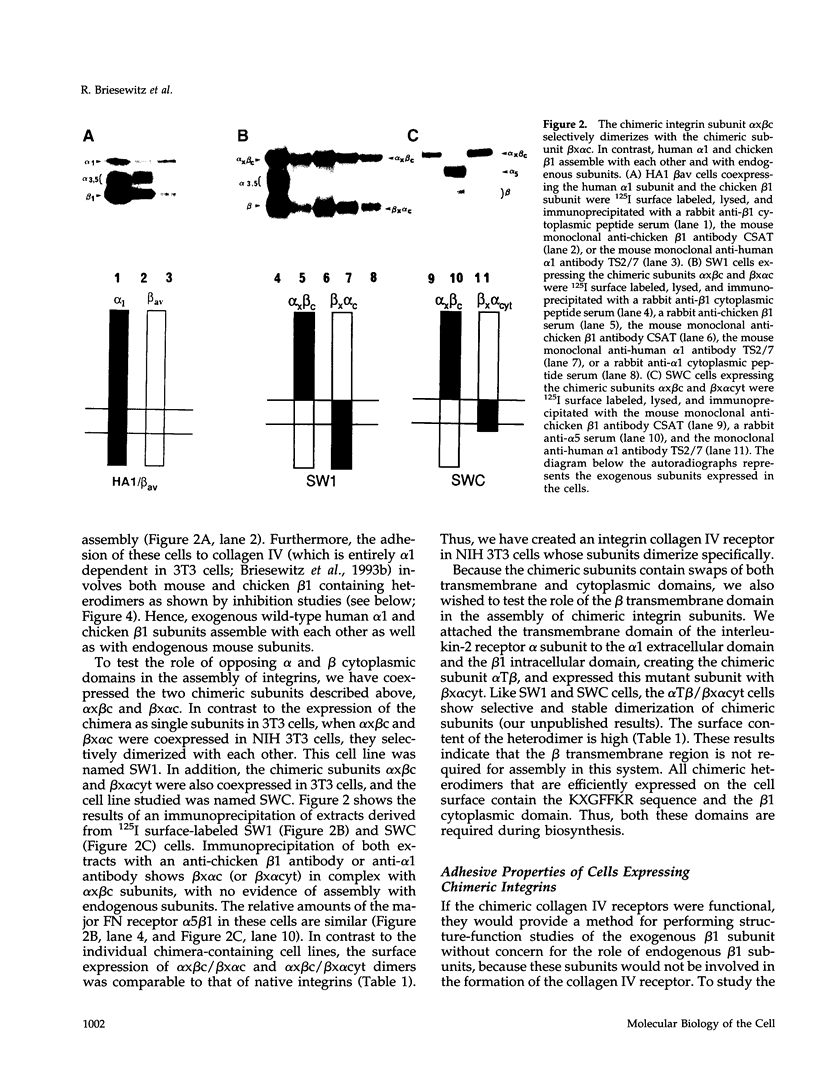
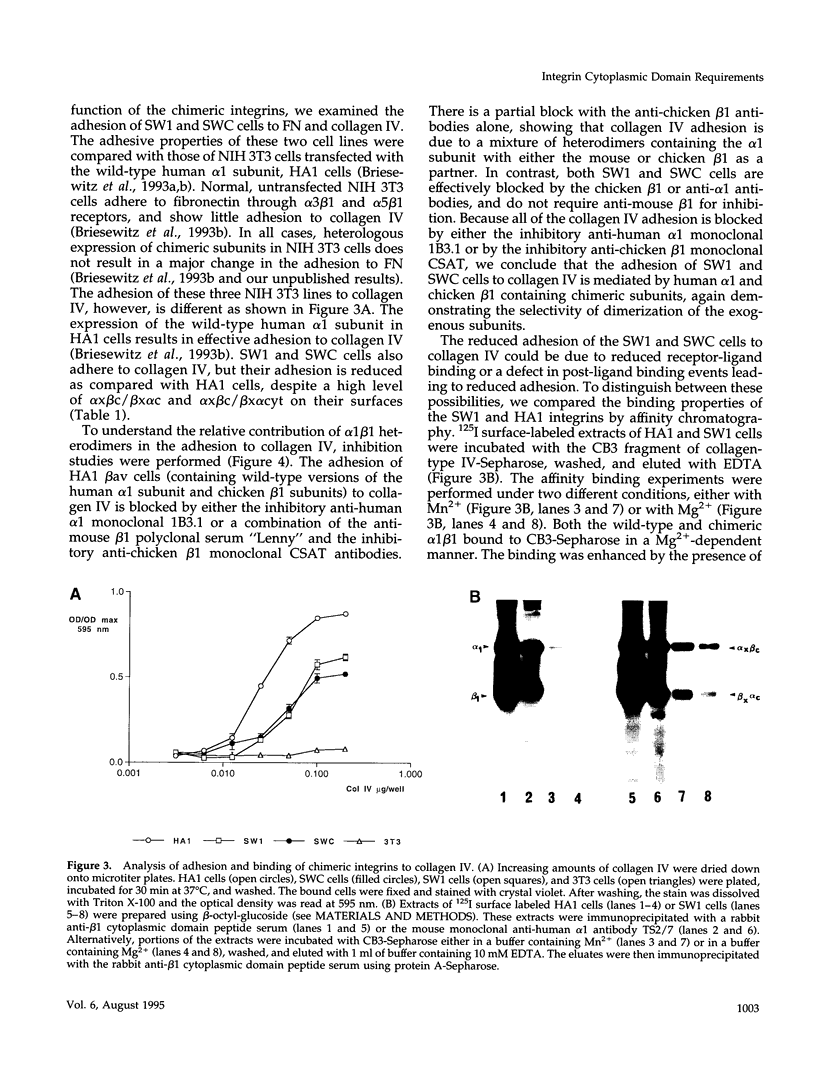
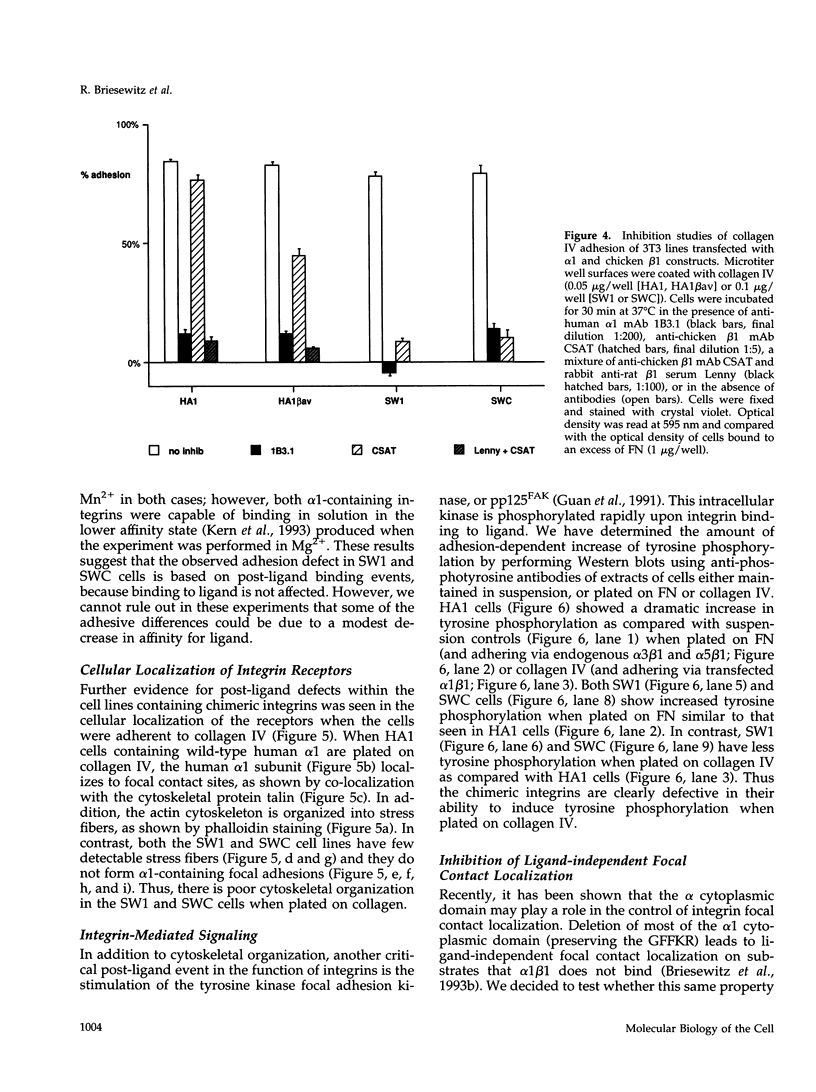
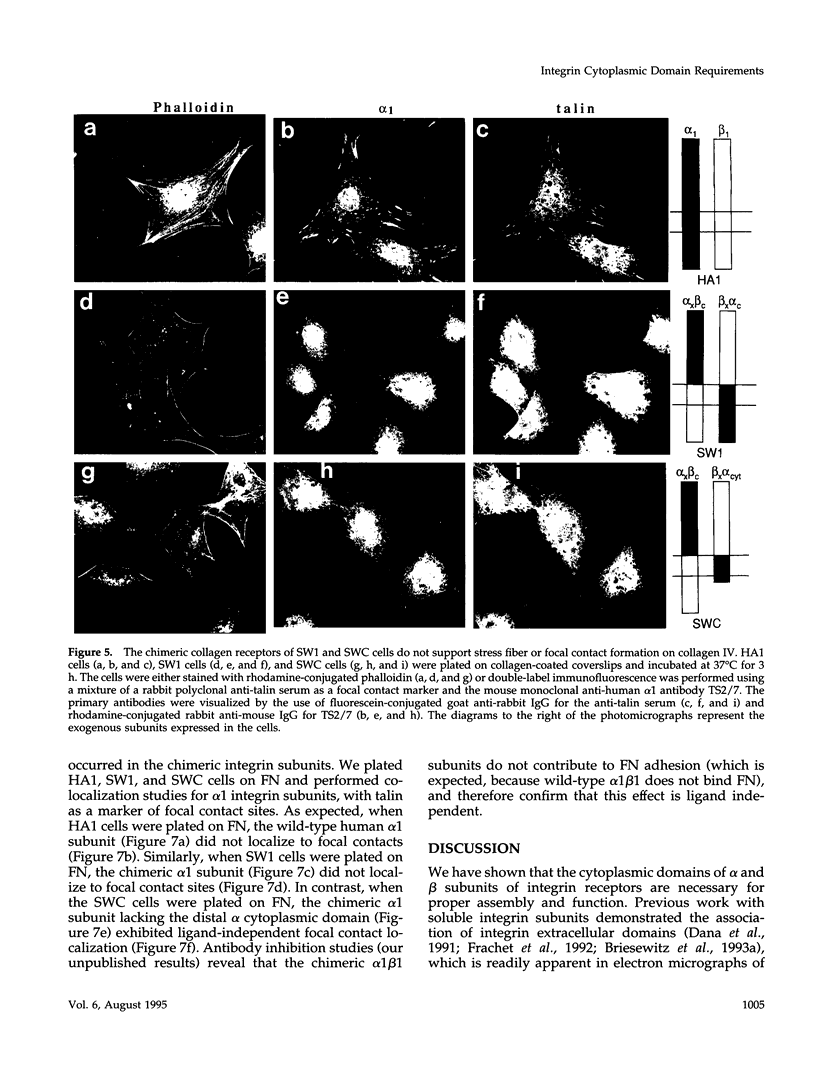
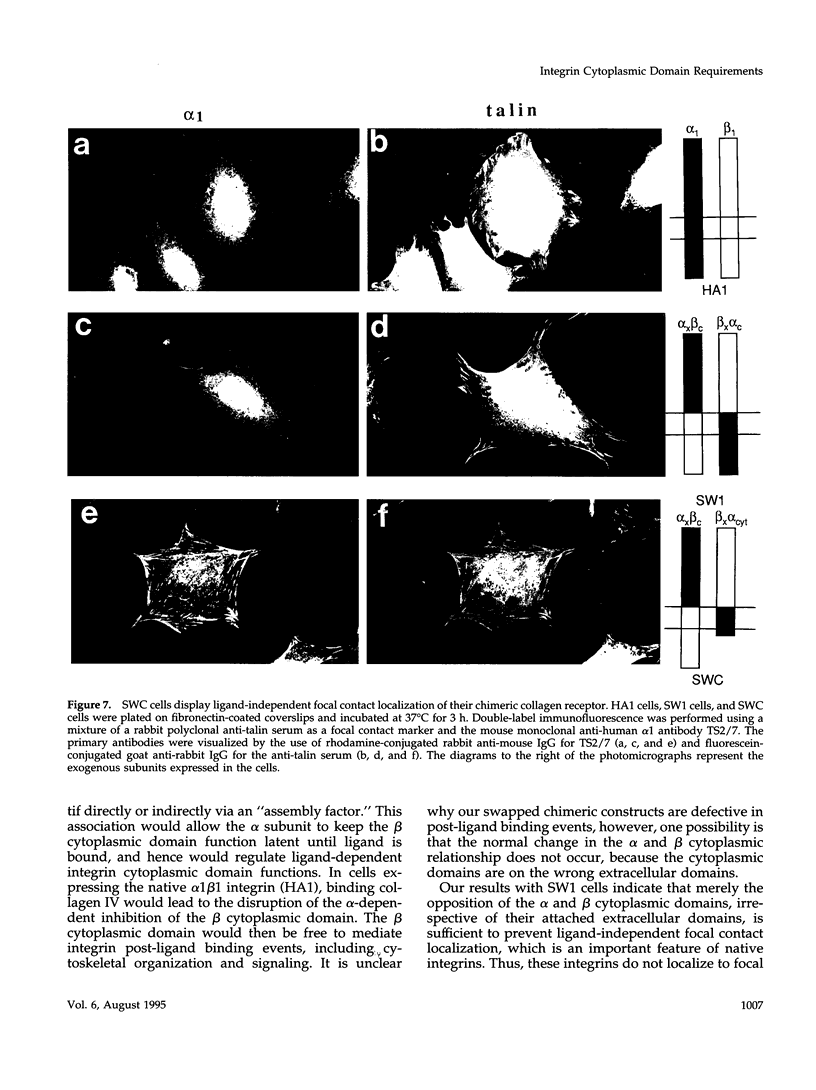
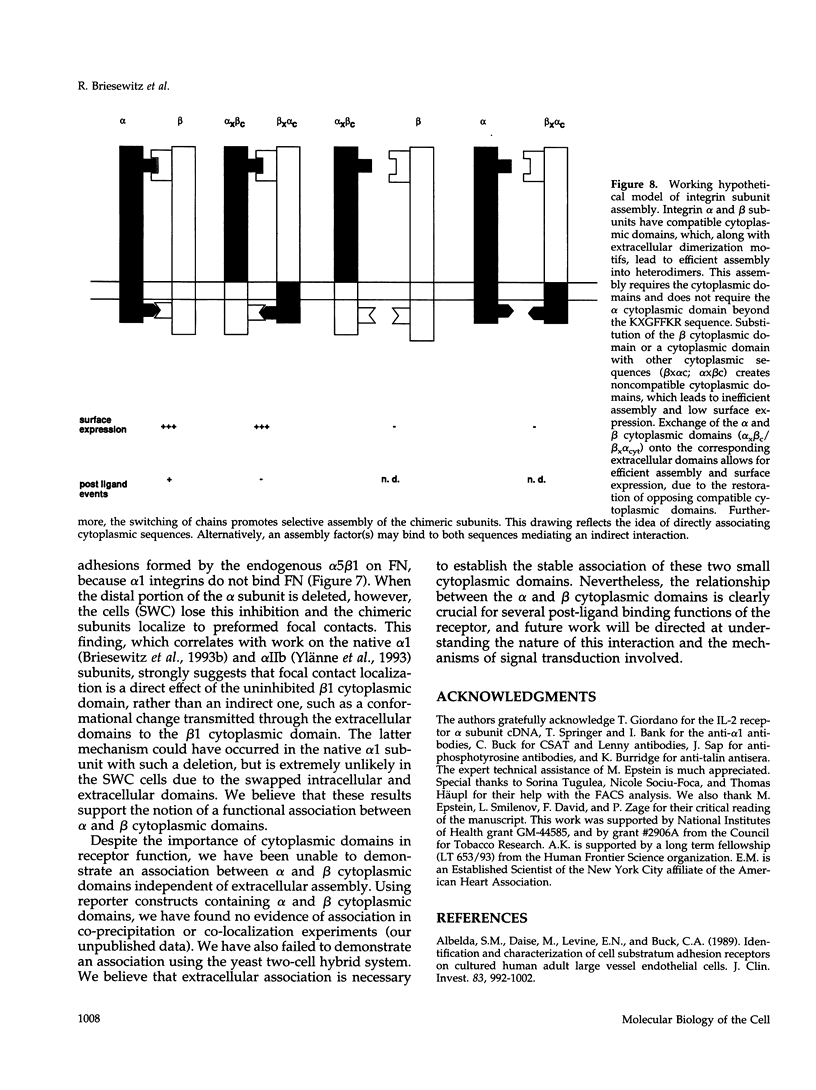
The membrane proximal regions of integrin alpha and beta subunits are highly conserved in evolution. In particular, all integrin alpha subunits share the KXGFFKR sequence at the beginning of their cytoplasmic domains. Previous work has shown that this domain is important in integrin receptor assembly. Using chimeric integrin alpha and beta subunits, we show that the native cytoplasmic domains of both subunits must be present for efficient assembly. Most strikingly, chimeric alpha 1 and beta 1 subunits with reciprocally swapped intracellular domains dimerize selectively into collagen IV receptors expressed at high levels on the surface. However, these receptors, which bind ligand efficiently, are deficient in a variety of post-ligand binding events, including cytoskeletal association and induction of tyrosine phosphorylation. Furthermore, deletion of the distal alpha cytoplasmic domain in the swapped heterodimers leads to ligand-independent focal contact localization, which also occurs in wild-type subunits when the distal cytoplasmic domain is deleted. These results show that proper integrin assembly requires opposed alpha and beta cytoplasmic domains, and this opposition prevents ligand-independent focal contact localization. Our working hypothesis is that these two domains may associate during receptor assembly and provide the mechanism for integrin receptor latency.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank I., Hemler M., Brenner M. B., Cohen D., Levy V., Belko J., Crouse C., Chess L. A novel monoclonal antibody, 1B3.1, binds to a new epitope of the VLA-1 molecule. Cell Immunol. 1989 Sep;122(2):416–423. doi: 10.1016/0008-8749(89)90088-9. [DOI] [PubMed] [Google Scholar]
- Bauer J. S., Varner J., Schreiner C., Kornberg L., Nicholas R., Juliano R. L. Functional role of the cytoplasmic domain of the integrin alpha 5 subunit. J Cell Biol. 1993 Jul;122(1):209–221. doi: 10.1083/jcb.122.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briesewitz R., Epstein M. R., Marcantonio E. E. Expression of native and truncated forms of the human integrin alpha 1 subunit. J Biol Chem. 1993 Feb 5;268(4):2989–2996. [PubMed] [Google Scholar]
- Briesewitz R., Kern A., Marcantonio E. E. Ligand-dependent and -independent integrin focal contact localization: the role of the alpha chain cytoplasmic domain. Mol Biol Cell. 1993 Jun;4(6):593–604. doi: 10.1091/mbc.4.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell N. A., Fitzgerald L. A., Steiner B., Erickson H. P., Phillips D. R. Structure of human platelet membrane glycoproteins IIb and IIIa as determined by electron microscopy. J Biol Chem. 1985 Feb 10;260(3):1743–1749. [PubMed] [Google Scholar]
- Chen W. T., Hasegawa E., Hasegawa T., Weinstock C., Yamada K. M. Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol. 1985 Apr;100(4):1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb B. S., Schaller M. D., Leu T. H., Parsons J. T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994 Jan;14(1):147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana N., Fathallah D. M., Arnaout M. A. Expression of a soluble and functional form of the human beta 2 integrin CD11b/CD18. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3106–3110. doi: 10.1073/pnas.88.8.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone D. W., Hynes R. O. Xenopus laevis integrins. Structural conservation and evolutionary divergence of integrin beta subunits. J Biol Chem. 1988 Apr 15;263(11):5333–5340. [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frachet P., Duperray A., Delachanal E., Marguerie G. Role of the transmembrane and cytoplasmic domains in the assembly and surface exposure of the platelet integrin GPIIb/IIIa. Biochemistry. 1992 Mar 3;31(8):2408–2415. doi: 10.1021/bi00123a028. [DOI] [PubMed] [Google Scholar]
- Geiger B., Salomon D., Takeichi M., Hynes R. O. A chimeric N-cadherin/beta 1-integrin receptor which localizes to both cell-cell and cell-matrix adhesions. J Cell Sci. 1992 Dec;103(Pt 4):943–951. doi: 10.1242/jcs.103.4.943. [DOI] [PubMed] [Google Scholar]
- Giordano T., Howard T. H., Coleman J., Sakamoto K., Howard B. H. Isolation of a population of transiently transfected quiescent and senescent cells by magnetic affinity cell sorting. Exp Cell Res. 1991 Jan;192(1):193–197. doi: 10.1016/0014-4827(91)90175-t. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Haimovich B., Reszka A., Boettiger D., Horwitz A. Expression and function of chicken integrin beta 1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990 Jan;110(1):175–184. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528–535. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Marcantonio E. E., Stepp M. A., Urry L. A., Yee G. H. Integrin heterodimer and receptor complexity in avian and mammalian cells. J Cell Biol. 1989 Jul;109(1):409–420. doi: 10.1083/jcb.109.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M. E., Theler J. M., Schlegel W., Appel R. D., Wright S. D., Lew P. D. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991 Mar;112(6):1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S., Hemler M. E. Role of the alpha subunit cytoplasmic domain in regulation of adhesive activity mediated by the integrin VLA-2. J Biol Chem. 1993 Aug 5;268(22):16279–16285. [PubMed] [Google Scholar]
- Kern A., Eble J., Golbik R., Kühn K. Interaction of type IV collagen with the isolated integrins alpha 1 beta 1 and alpha 2 beta 1. Eur J Biochem. 1993 Jul 1;215(1):151–159. doi: 10.1111/j.1432-1033.1993.tb18017.x. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme S. E., Akiyama S. K., Yamada K. M. Regulation of fibronectin receptor distribution. J Cell Biol. 1992 Apr;117(2):437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Guan J. L., Trevithick J. E., Hynes R. O. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990 Jul;1(8):597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Green N. M., Eason P., Yamada S. S., Yamada K. M. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988 Dec 20;7(13):4093–4099. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Katagiri Y., Faull R. J., Peter K., Tamura R., Quaranta V., Loftus J. C., Shattil S. J., Ginsberg M. H. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994 Mar;124(6):1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Mandelman D., Forsyth J., Shattil S. J., Plow E. F., Ginsberg M. H. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991 Nov 8;254(5033):845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reszka A. A., Hayashi Y., Horwitz A. F. Identification of amino acid sequences in the integrin beta 1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992 Jun;117(6):1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994 Mar;14(3):1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994 Dec 22;372(6508):786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Ingber D. E., Lawrence M., Springer T. A., Lechene C. Multiple integrins share the ability to induce elevation of intracellular pH. Exp Cell Res. 1991 Aug;195(2):533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C., Ingber D. E. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993 Feb;120(4):1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Haimovich B., Cunningham M., Lipfert L., Parsons J. T., Ginsberg M. H., Brugge J. S. Tyrosine phosphorylation of pp125FAK in platelets requires coordinated signaling through integrin and agonist receptors. J Biol Chem. 1994 May 20;269(20):14738–14745. [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Kazazis D. M., Gailit J., Ruoslahti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988 Jun;106(6):2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska J., Guan J. L., Marcantonio E. E., Trevithick J. E., Buck C. A., Hynes R. O. Expression of normal and mutant avian integrin subunits in rodent cells. J Cell Biol. 1989 Aug;109(2):853–861. doi: 10.1083/jcb.109.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg P., Kern A., Ries A., Luckenbill-Edds L., Mann K., Kühn K. Characterization of a type IV collagen major cell binding site with affinity to the alpha 1 beta 1 and the alpha 2 beta 1 integrins. J Cell Biol. 1991 Jun;113(6):1475–1483. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994 Dec 2;266(5190):1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- Williams M. J., Hughes P. E., O'Toole T. E., Ginsberg M. H. The inner world of cell adhesion: integrin cytoplasmic domains. Trends Cell Biol. 1994 Apr;4(4):109–112. doi: 10.1016/0962-8924(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J. R., Johansson S., Hök M. Adhesion and cytoskeletal organisation of fibroblasts in response to fibronectin fragments. EMBO J. 1986 Apr;5(4):665–670. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Protein kinase C involvement in focal adhesion formation. J Cell Sci. 1992 Feb;101(Pt 2):277–290. doi: 10.1242/jcs.101.2.277. [DOI] [PubMed] [Google Scholar]
- Xing Z., Chen H. C., Nowlen J. K., Taylor S. J., Shalloway D., Guan J. L. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994 Apr;5(4):413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylänne J., Chen Y., O'Toole T. E., Loftus J. C., Takada Y., Ginsberg M. H. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993 Jul;122(1):223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]