Abstract
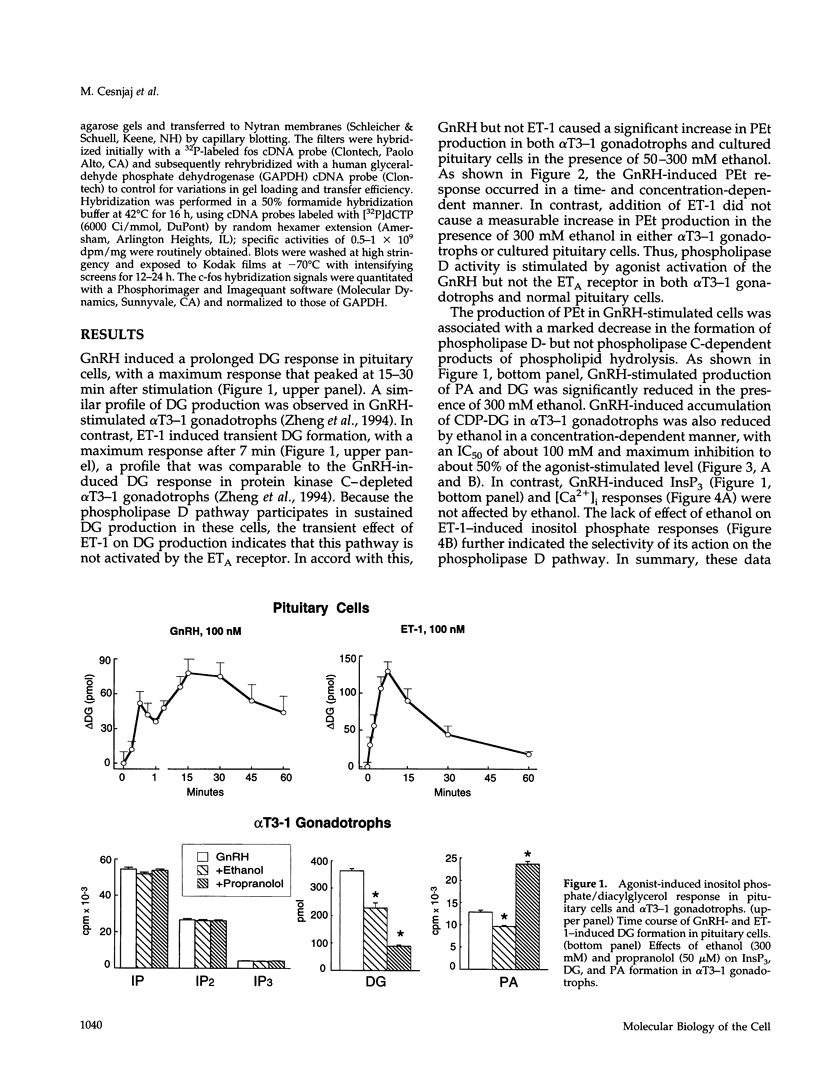
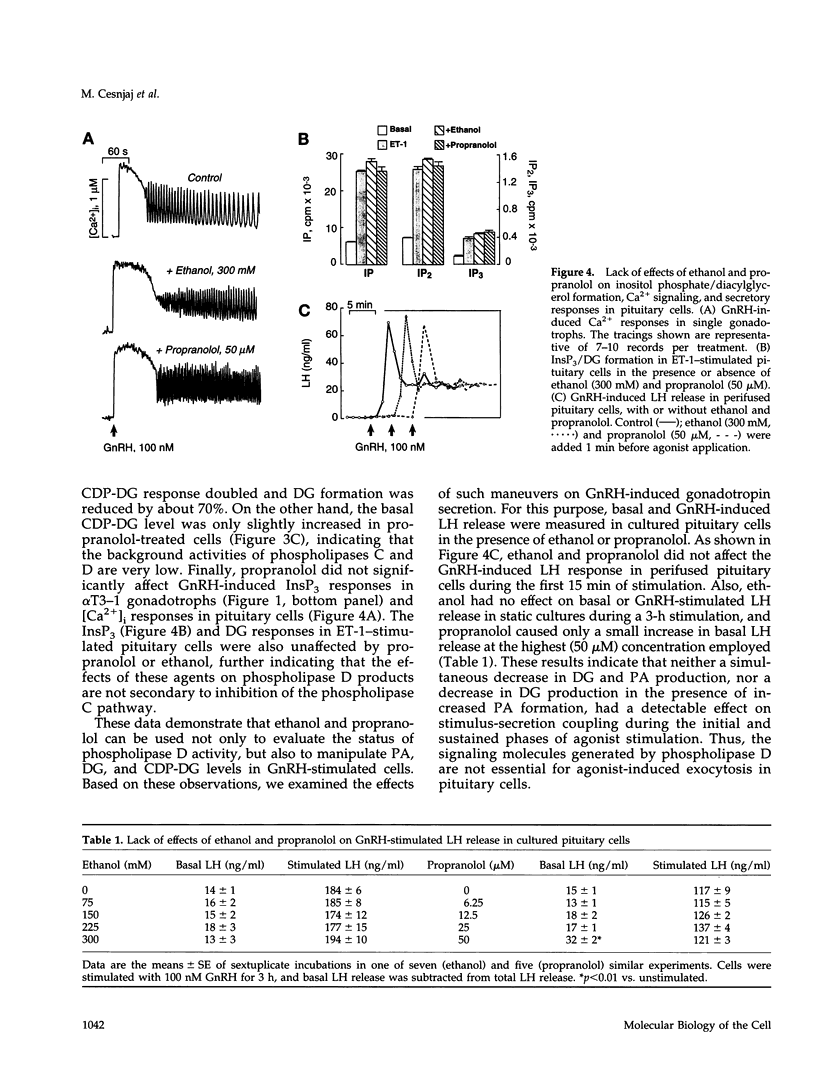
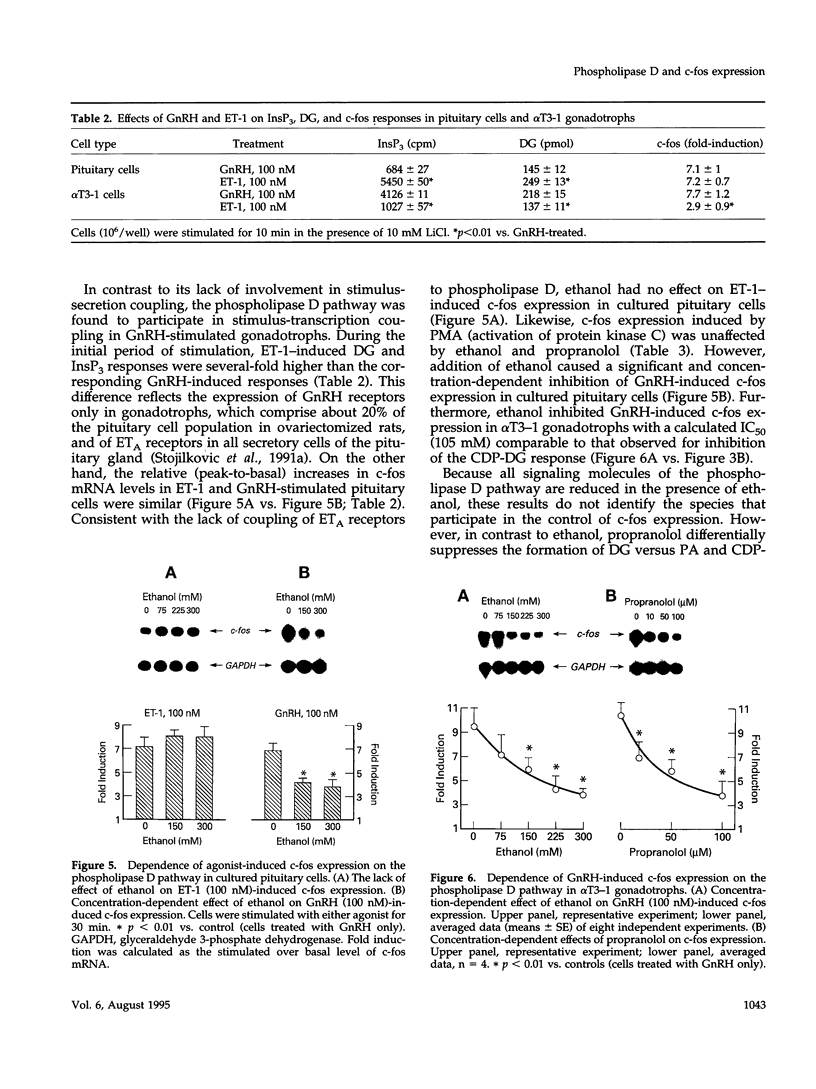
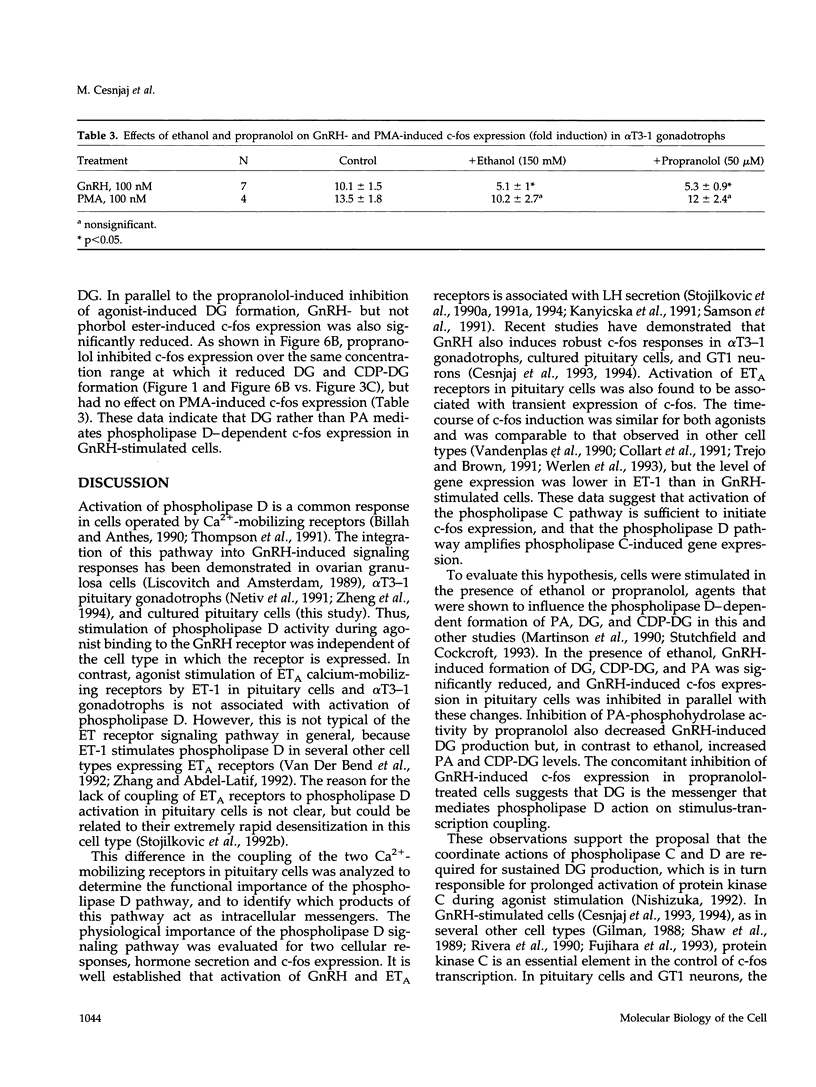
Stimulation of phospholipase D activity is frequently observed during agonist activation of Ca(2+)-mobilizing receptors, but the cellular functions of this signaling pathway are not well defined. Pituitary gonadotrophs express Ca(2+)-mobilizing receptors for gonadotropin-releasing hormone (GnRH) and endothelin (ET), activation of which stimulates luteinizing hormone secretion and transient expression of c-fos. In pituitary cells and alpha T3-1 gonadotrophs, GnRH action was associated with both initial and sustained diacylglycerol (DG) production, whereas ET-1 induced only a transient DG response. Also, phospholipase D activity, estimated by the production of phosphatidylethanol from phosphatidylcholine in the presence of ethanol, was stimulated by GnRH but not ET-1. Such formation of phosphatidylethanol at the expense of phosphatidic acid (PA) during GnRH-induced activation of phospholipase D significantly reduced the production of PA, DG, and cytidine diphosphate diacylglycerol. Inhibition of PA-phosphohydrolase activity by propranolol also decreased GnRH-induced DG production and, in contrast to ethanol, increased PA and cytidine diphosphate diacylglycerol levels. The fall in DG production caused by ethanol and propranolol was accompanied by inhibition of GnRH-induced c-fos expression, whereas agonist-induced luteinizing hormone release was not affected. In contrast to their inhibitory actions on GnRH-induced early gene expression, neither ethanol nor propranolol affected ET-1-induced c-fos expression, or GnRH- and ET-1-induced inositol trisphosphate/Ca2+ signaling. These findings demonstrate that phospholipase D participates in stimulus-transcription but not stimulus-secretion coupling, and indicate that DG is the primary signal for this action.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Balboa M. A., Firestein B. L., Godson C., Bell K. S., Insel P. A. Protein kinase C alpha mediates phospholipase D activation by nucleotides and phorbol ester in Madin-Darby canine kidney cells. Stimulation of phospholipase D is independent of activation of polyphosphoinositide-specific phospholipase C and phospholipase A2. J Biol Chem. 1994 Apr 8;269(14):10511–10516. [PubMed] [Google Scholar]
- Ben-Menahem D., Shraga Z., Lewy H., Limor R., Hammel I., Stein R., Naor Z. Dissociation between release and gene expression of gonadotropin alpha-subunit in gonadotropin-releasing hormone-stimulated alpha T3-1 cell line. Biochemistry. 1992 Dec 29;31(51):12893–12898. doi: 10.1021/bi00166a026. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C., Mullmann T. J. Receptor-coupled phospholipase D: regulation and functional significance. Biochem Soc Trans. 1991 Apr;19(2):324–329. doi: 10.1042/bst0190324. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989 Oct 15;264(29):17069–17077. [PubMed] [Google Scholar]
- Boarder M. R. A role for phospholipase D in control of mitogenesis. Trends Pharmacol Sci. 1994 Feb;15(2):57–62. doi: 10.1016/0165-6147(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Bollag W. B., Barrett P. Q., Isales C. M., Liscovitch M., Rasmussen H. A potential role for phospholipase-D in the angiotensin-II-induced stimulation of aldosterone secretion from bovine adrenal glomerulosa cells. Endocrinology. 1990 Sep;127(3):1436–1443. doi: 10.1210/endo-127-3-1436. [DOI] [PubMed] [Google Scholar]
- Carnero A., Cuadrado A., del Peso L., Lacal J. C. Activation of type D phospholipase by serum stimulation and ras-induced transformation in NIH3T3 cells. Oncogene. 1994 May;9(5):1387–1395. [PubMed] [Google Scholar]
- Carnero A., Dolfi F., Lacal J. C. ras-p21 activates phospholipase D and A2, but not phospholipase C or PKC, in Xenopus laevis oocytes. J Cell Biochem. 1994 Apr;54(4):478–486. doi: 10.1002/jcb.240540415. [DOI] [PubMed] [Google Scholar]
- Cesnjaj M., Catt K. J., Stojilkovic S. S. Coordinate actions of calcium and protein kinase-C in the expression of primary response genes in pituitary gonadotrophs. Endocrinology. 1994 Aug;135(2):692–701. doi: 10.1210/endo.135.2.7518388. [DOI] [PubMed] [Google Scholar]
- Cesnjaj M., Krsmanovic L. Z., Catt K. J., Stojilkovic S. S. Autocrine induction of c-fos expression in GT1 neuronal cells by gonadotropin-releasing hormone. Endocrinology. 1993 Dec;133(6):3042–3045. doi: 10.1210/endo.133.6.8243334. [DOI] [PubMed] [Google Scholar]
- Chang J. P., Morgan R. O., Catt K. J. Dependence of secretory responses to gonadotropin-releasing hormone on diacylglycerol metabolism. Studies with a diacylglycerol lipase inhibitor, RHC 80267. J Biol Chem. 1988 Dec 15;263(35):18614–18620. [PubMed] [Google Scholar]
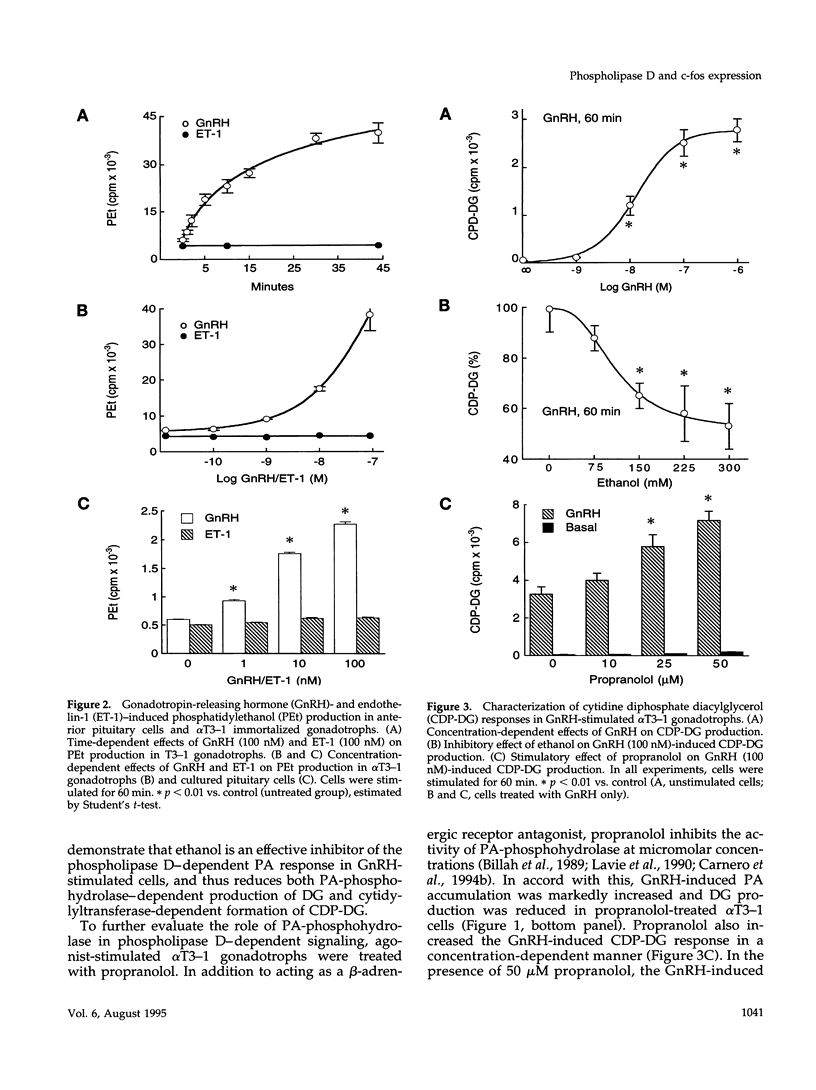
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Thomas G. M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N. F., Truong O., Hsuan J. J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994 Jan 28;263(5146):523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Tourkine N., Belin D., Vassalli P., Jeanteur P., Blanchard J. M. c-fos gene transcription in murine macrophages is modulated by a calcium-dependent block to elongation in intron 1. Mol Cell Biol. 1991 May;11(5):2826–2831. doi: 10.1128/mcb.11.5.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conricode K. M., Brewer K. A., Exton J. H. Activation of phospholipase D by protein kinase C. Evidence for a phosphorylation-independent mechanism. J Biol Chem. 1992 Apr 15;267(11):7199–7202. [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Phospholipases C and D in mitogenic signal transduction. Rev Physiol Biochem Pharmacol. 1992;119:13–45. doi: 10.1007/3540551921_2. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Squinto S. P., Bazan N. G. Fos-jun and the primary genomic response in the nervous system. Possible physiological role and pathophysiological significance. Mol Neurobiol. 1990 Spring-Summer;4(1-2):27–55. doi: 10.1007/BF02935584. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Fujihara M., Muroi M., Muroi Y., Ito N., Suzuki T. Mechanism of lipopolysaccharide-triggered junB activation in a mouse macrophage-like cell line (J774). J Biol Chem. 1993 Jul 15;268(20):14898–14905. [PubMed] [Google Scholar]
- Fukami K., Takenawa T. Phosphatidic acid that accumulates in platelet-derived growth factor-stimulated Balb/c 3T3 cells is a potential mitogenic signal. J Biol Chem. 1992 Jun 5;267(16):10988–10993. [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gustavsson L., Moehren G., Torres-Marquez M. E., Benistant C., Rubin R., Hoek J. B. The role of cytosolic Ca2+, protein kinase C, and protein kinase A in hormonal stimulation of phospholipase D in rat hepatocytes. J Biol Chem. 1994 Jan 14;269(2):849–859. [PubMed] [Google Scholar]
- Haslam R. J., Coorssen J. R. Evidence that activation of phospholipase D can mediate secretion from permeabilized platelets. Adv Exp Med Biol. 1993;344:149–164. doi: 10.1007/978-1-4615-2994-1_11. [DOI] [PubMed] [Google Scholar]
- Horn F., Bilezikjian L. M., Perrin M. H., Bosma M. M., Windle J. J., Huber K. S., Blount A. L., Hille B., Vale W., Mellon P. L. Intracellular responses to gonadotropin-releasing hormone in a clonal cell line of the gonadotrope lineage. Mol Endocrinol. 1991 Mar;5(3):347–355. doi: 10.1210/mend-5-3-347. [DOI] [PubMed] [Google Scholar]
- Jenkins G. H., Fisette P. L., Anderson R. A. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994 Apr 15;269(15):11547–11554. [PubMed] [Google Scholar]
- Kanyicska B., Burris T. P., Freeman M. E. Endothelin-3 inhibits prolactin and stimulates LH, FSH and TSH secretion from pituitary cell culture. Biochem Biophys Res Commun. 1991 Jan 15;174(1):338–343. doi: 10.1016/0006-291x(91)90525-c. [DOI] [PubMed] [Google Scholar]
- Kusner D. J., Schomisch S. J., Dubyak G. R. ATP-induced potentiation of G-protein-dependent phospholipase D activity in a cell-free system from U937 promonocytic leukocytes. J Biol Chem. 1993 Sep 25;268(27):19973–19982. [PubMed] [Google Scholar]
- Lavie Y., Piterman O., Liscovitch M. Inhibition of phosphatidic acid phosphohydrolase activity by sphingosine. Dual action of sphingosine in diacylglycerol signal termination. FEBS Lett. 1990 Dec 17;277(1-2):7–10. doi: 10.1016/0014-5793(90)80796-l. [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Kim H. S., Pai J. K., Ryu S. H., Suh P. G. Activation of phospholipase D induced by platelet-derived growth factor is dependent upon the level of phospholipase C-gamma 1. J Biol Chem. 1994 Oct 28;269(43):26842–26847. [PubMed] [Google Scholar]
- Liscovitch M., Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J Biol Chem. 1989 Jul 15;264(20):11762–11767. [PubMed] [Google Scholar]
- Liscovitch M. Signal-dependent activation of phosphatidylcholine hydrolysis: role of phospholipase D. Biochem Soc Trans. 1991 Apr;19(2):402–407. doi: 10.1042/bst0190402. [DOI] [PubMed] [Google Scholar]
- Llahi S., Fain J. N. Alpha 1-adrenergic receptor-mediated activation of phospholipase D in rat cerebral cortex. J Biol Chem. 1992 Feb 25;267(6):3679–3685. [PubMed] [Google Scholar]
- Martin T. F., Hsieh K. P., Porter B. W. The sustained second phase of hormone-stimulated diacylglycerol accumulation does not activate protein kinase C in GH3 cells. J Biol Chem. 1990 May 5;265(13):7623–7631. [PubMed] [Google Scholar]
- Martinson E. A., Trilivas I., Brown J. H. Rapid protein kinase C-dependent activation of phospholipase D leads to delayed 1,2-diglyceride accumulation. J Biol Chem. 1990 Dec 25;265(36):22282–22287. [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Morgan R. O., Chang J. P., Catt K. J. Novel aspects of gonadotropin-releasing hormone action on inositol polyphosphate metabolism in cultured pituitary gonadotrophs. J Biol Chem. 1987 Jan 25;262(3):1166–1171. [PubMed] [Google Scholar]
- Moritz A., De Graan P. N., Gispen W. H., Wirtz K. W. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992 Apr 15;267(11):7207–7210. [PubMed] [Google Scholar]
- Naor Z., Dan-Cohen H., Hermon J., Limor R. Induction of exocytosis in permeabilized pituitary cells by alpha- and beta-type protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4501–4504. doi: 10.1073/pnas.86.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netiv E., Liscovitch M., Naor Z. Delayed activation of phospholipase D by gonadotropin-releasing hormone in a clonal pituitary gonadotrope cell line (alpha T3-1). FEBS Lett. 1991 Dec 16;295(1-3):107–109. doi: 10.1016/0014-5793(91)81396-p. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Samson W. K., Skala K. D., Alexander B., Huang F. L. Possible neuroendocrine actions of endothelin-3. Endocrinology. 1991 Mar;128(3):1465–1473. doi: 10.1210/endo-128-3-1465. [DOI] [PubMed] [Google Scholar]
- Shaw P. E., Frasch S., Nordheim A. Repression of c-fos transcription is mediated through p67SRF bound to the SRE. EMBO J. 1989 Sep;8(9):2567–2574. doi: 10.1002/j.1460-2075.1989.tb08395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Stojilkovic S. S., Reinhart J., Catt K. J. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994 Aug;15(4):462–499. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Balla T., Fukuda S., Cesnjaj M., Merelli F., Krsmanović L. Z., Catt K. J. Endothelin ETA receptors mediate the signaling and secretory actions of endothelins in pituitary gonadotrophs. Endocrinology. 1992 Jan;130(1):465–474. doi: 10.1210/endo.130.1.1309344. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Iida T., Cesnjaj M., Catt K. J. Differential actions of endothelin and gonadotropin-releasing hormone in pituitary gonadotrophs. Endocrinology. 1992 Dec;131(6):2821–2828. doi: 10.1210/endo.131.6.1446620. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Iida T., Merelli F., Catt K. J. Calcium signaling and secretory responses in endothelin-stimulated anterior pituitary cells. Mol Pharmacol. 1991 Jun;39(6):762–770. [PubMed] [Google Scholar]
- Stojilković S. S., Iida T., Merelli F., Torsello A., Krsmanović L. Z., Catt K. J. Interactions between calcium and protein kinase C in the control of signaling and secretion in pituitary gonadotrophs. J Biol Chem. 1991 Jun 5;266(16):10377–10384. [PubMed] [Google Scholar]
- Stojilković S. S., Merelli F., Iida T., Krsmanović L. Z., Catt K. J. Endothelin stimulation of cytosolic calcium and gonadotropin secretion in anterior pituitary cells. Science. 1990 Jun 29;248(4963):1663–1666. doi: 10.1126/science.2163546. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Stutzin A., Izumi S., Dufour S., Torsello A., Virmani M. A., Rojas E., Catt K. J. Generation and amplification of the cytosolic calcium signal during secretory responses to gonadotropin-releasing hormone. New Biol. 1990 Mar;2(3):272–283. [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Correlation between secretion and phospholipase D activation in differentiated HL60 cells. Biochem J. 1993 Aug 1;293(Pt 3):649–655. doi: 10.1042/bj2930649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. T., Bonser R. W., Garland L. G. Receptor-coupled phospholipase D and its inhibition. Trends Pharmacol Sci. 1991 Nov;12(11):404–408. doi: 10.1016/0165-6147(91)90617-2. [DOI] [PubMed] [Google Scholar]
- Trejo J., Brown J. H. c-fos and c-jun are induced by muscarinic receptor activation of protein kinase C but are differentially regulated by intracellular calcium. J Biol Chem. 1991 Apr 25;266(12):7876–7882. [PubMed] [Google Scholar]
- Vandenplas M. L., Mouton W. L., Vandenplas S., Bester A. J., Ricketts M. H. Increased intracellular Ca2+ is necessary for maximal expression of the proto-oncogene c-jun in the Jurkat T-cell line. Biochem J. 1990 Apr 15;267(2):349–351. doi: 10.1042/bj2670349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., Godfrey P. P. The role of receptor-stimulated inositol phospholipid hydrolysis in the autonomic nervous system. Pharmacol Ther. 1988;38(3):387–417. doi: 10.1016/0163-7258(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Werlen G., Belin D., Conne B., Roche E., Lew D. P., Prentki M. Intracellular Ca2+ and the regulation of early response gene expression in HL-60 myeloid leukemia cells. J Biol Chem. 1993 Aug 5;268(22):16596–16601. [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Abdel-Latif A. A. Activation of phospholipase D by endothelin-1 and other pharmacological agents in rabbit iris sphincter smooth muscle. Cell Signal. 1992 Nov;4(6):777–786. doi: 10.1016/0898-6568(92)90058-g. [DOI] [PubMed] [Google Scholar]
- Zheng L., Stojilkovic S. S., Hunyady L., Krsmanovic L. Z., Catt K. J. Sequential activation of phospholipase-C and -D in agonist-stimulated gonadotrophs. Endocrinology. 1994 Mar;134(3):1446–1454. doi: 10.1210/endo.134.3.8119185. [DOI] [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992 Jul 1;285(Pt 1):235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]