Abstract
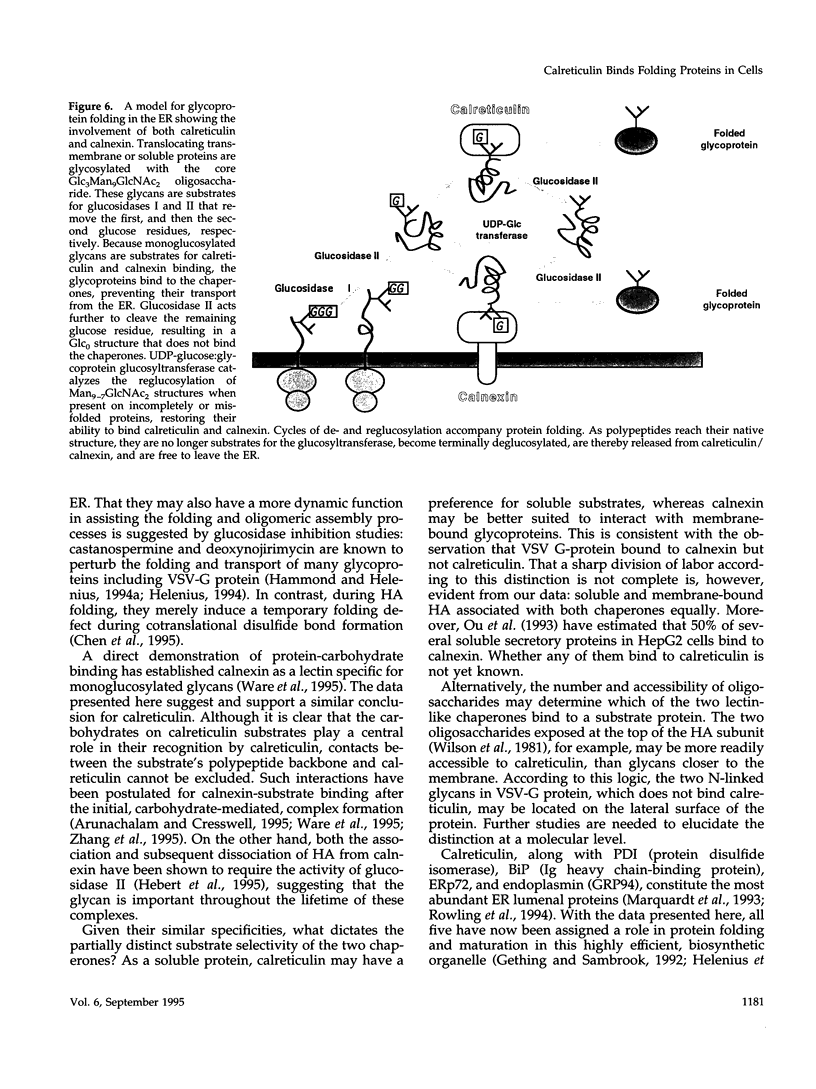
The soluble, calcium-binding protein calreticulin shares high sequence homology with calnexin, a transmembrane chaperone of glycoprotein folding. Our experiments demonstrated that calreticulin, like calnexin, associated transiently with numerous newly synthesized proteins in the endoplasmic reticulum. The population of proteins that bound to calreticulin was partially overlapping with those that bound to calnexin. Hemagglutinin (HA) of influenza virus was shown to associate with both calreticulin and calnexin. Using HA as a model substrate, it was found that both calreticulin- and calnexin-bound HA corresponded primarily to incompletely disulfide-bonded folding intermediates and conformationally trapped forms. Binding of all substrates was oligosaccharide-dependent and required the trimming of glucose residues from asparagine-linked core glycans by glucosidases I and II. In vitro, alpha-mannosidase digestion of calreticulin-bound HA indicated that calreticulin was specific for monoglucosylated glycans. Thus, calreticulin appeared to be a lectin with similar oligosaccharide specificity as its membrane-bound homologue, calnexin. Both are therefore likely to play an important role in glycoprotein maturation and quality control in the endoplasmic reticulum.
Full text
PDF


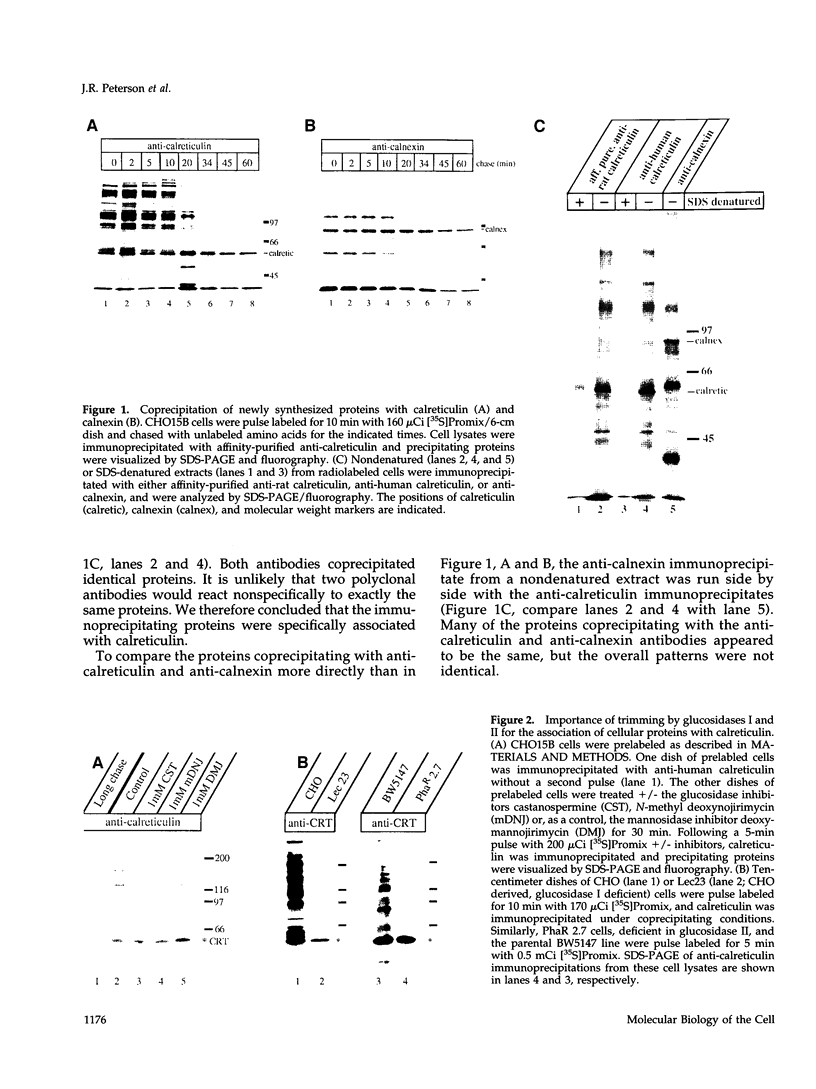


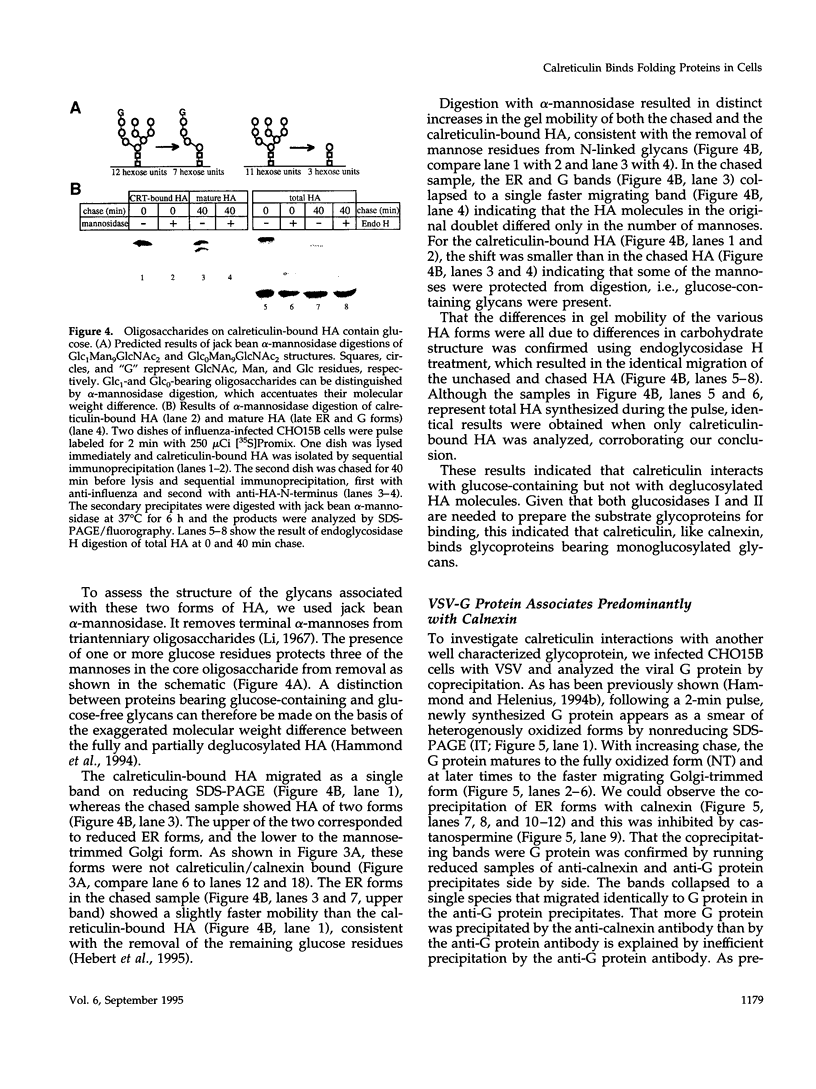




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arunachalam B., Cresswell P. Molecular requirements for the interaction of class II major histocompatibility complex molecules and invariant chain with calnexin. J Biol Chem. 1995 Feb 10;270(6):2784–2790. doi: 10.1074/jbc.270.6.2784. [DOI] [PubMed] [Google Scholar]
- Baksh S., Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem. 1991 Nov 15;266(32):21458–21465. [PubMed] [Google Scholar]
- Balch W. E., Wagner K. R., Keller D. S. Reconstitution of transport of vesicular stomatitis virus G protein from the endoplasmic reticulum to the Golgi complex using a cell-free system. J Cell Biol. 1987 Mar;104(3):749–760. doi: 10.1083/jcb.104.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Brenner M. B., Thomas D. Y., Williams D. B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994 Mar;19(3):124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Braakman I., Hoover-Litty H., Wagner K. R., Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991 Aug;114(3):401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Helenius J., Braakman I., Helenius A. Cotranslational folding and calnexin binding during glycoprotein synthesis. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V., Hochstenbach F., Rajagopalan S., Brenner M. B. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin). J Biol Chem. 1993 May 5;268(13):9585–9592. [PubMed] [Google Scholar]
- Dedhar S., Rennie P. S., Shago M., Hagesteijn C. Y., Yang H., Filmus J., Hawley R. G., Bruchovsky N., Cheng H., Matusik R. J. Inhibition of nuclear hormone receptor activity by calreticulin. Nature. 1994 Feb 3;367(6462):480–483. doi: 10.1038/367480a0. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Doxsey S. J., Sambrook J., Helenius A., White J. An efficient method for introducing macromolecules into living cells. J Cell Biol. 1985 Jul;101(1):19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis M., Schaerer E., Krause K. H., Tschopp J. The calcium-binding protein calreticulin is a major constituent of lytic granules in cytolytic T lymphocytes. J Exp Med. 1993 Jan 1;177(1):1–7. doi: 10.1084/jem.177.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E. H., Sprague E. A., Kelley J. L., Kerbacher J. J., Schwartz C. J., Elbein A. D. Castanospermine inhibits the function of the low-density lipoprotein receptor. Biochemistry. 1989 Sep 19;28(19):7679–7687. doi: 10.1021/bi00445a024. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991 Dec;5(15):3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hammond C., Braakman I., Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994 Oct 21;266(5184):456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- Hammond C., Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994 Jul;126(1):41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D. N., Foellmer B., Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995 May 5;81(3):425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994 Mar;5(3):253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Cohen-Doyle M. F., Peterson P. A., Williams D. B. Regulation of MHC class I transport by the molecular chaperone, calnexin (p88, IP90). Science. 1994 Jan 21;263(5145):384–387. doi: 10.1126/science.8278813. [DOI] [PubMed] [Google Scholar]
- Jethmalani S. M., Henle K. J., Kaushal G. P. Heat shock-induced prompt glycosylation. Identification of P-SG67 as calreticulin. J Biol Chem. 1994 Sep 23;269(38):23603–23609. [PubMed] [Google Scholar]
- Kearse K. P., Williams D. B., Singer A. Persistence of glucose residues on core oligosaccharides prevents association of TCR alpha and TCR beta proteins with calnexin and results specifically in accelerated degradation of nascent TCR alpha proteins within the endoplasmic reticulum. EMBO J. 1994 Aug 15;13(16):3678–3686. doi: 10.1002/j.1460-2075.1994.tb06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy T. E., Kuhl D., Barzilai A., Sweatt J. D., Kandel E. R. Long-term sensitization training in Aplysia leads to an increase in calreticulin, a major presynaptic calcium-binding protein. Neuron. 1992 Dec;9(6):1013–1024. doi: 10.1016/0896-6273(92)90062-i. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Simmerman H. K., Jones L. R., Campbell K. P. Sequence similarity of calreticulin with a Ca2(+)-binding protein that co-purifies with an Ins(1,4,5)P3-sensitive Ca2+ store in HL-60 cells. Biochem J. 1990 Sep 1;270(2):545–548. doi: 10.1042/bj2700545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry S. J., Gierasch L. M. Polypeptide interactions with molecular chaperones and their relationship to in vivo protein folding. Annu Rev Biophys Biomol Struct. 1994;23:645–669. doi: 10.1146/annurev.bb.23.060194.003241. [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn C. Y., Milankov K., Michalak M., Wilkins J., Dedhar S. Cell attachment to extracellular matrix substrates is inhibited upon downregulation of expression of calreticulin, an intracellular integrin alpha-subunit-binding protein. J Cell Sci. 1994 Mar;107(Pt 3):589–600. [PubMed] [Google Scholar]
- Li Y. T. Studies on the glycosidases in jack bean meal. I. Isolation and properties of alpha-mannosidase. J Biol Chem. 1967 Dec 10;242(23):5474–5480. [PubMed] [Google Scholar]
- Macer D. R., Koch G. L. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J Cell Sci. 1988 Sep;91(Pt 1):61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- Malhotra R., Willis A. C., Jensenius J. C., Jackson J., Sim R. B. Structure and homology of human C1q receptor (collectin receptor). Immunology. 1993 Mar;78(3):341–348. [PMC free article] [PubMed] [Google Scholar]
- Marquardt T., Hebert D. N., Helenius A. Post-translational folding of influenza hemagglutinin in isolated endoplasmic reticulum-derived microsomes. J Biol Chem. 1993 Sep 15;268(26):19618–19625. [PubMed] [Google Scholar]
- McCauliffe D. P., Yang Y. S., Wilson J., Sontheimer R. D., Capra J. D. The 5'-flanking region of the human calreticulin gene shares homology with the human GRP78, GRP94, and protein disulfide isomerase promoters. J Biol Chem. 1992 Feb 5;267(4):2557–2562. [PubMed] [Google Scholar]
- Michalak M., Milner R. E., Burns K., Opas M. Calreticulin. Biochem J. 1992 Aug 1;285(Pt 3):681–692. doi: 10.1042/bj2850681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. E., Baksh S., Shemanko C., Carpenter M. R., Smillie L., Vance J. E., Opas M., Michalak M. Calreticulin, and not calsequestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum. J Biol Chem. 1991 Apr 15;266(11):7155–7165. [PubMed] [Google Scholar]
- Opas M., Dziak E., Fliegel L., Michalak M. Regulation of expression and intracellular distribution of calreticulin, a major calcium binding protein of nonmuscle cells. J Cell Physiol. 1991 Oct;149(1):160–171. doi: 10.1002/jcp.1041490120. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993 Aug 26;364(6440):771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Pal R., Hoke G. M., Sarngadharan M. G. Role of oligosaccharides in the processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3384–3388. doi: 10.1073/pnas.86.9.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys J. B., McPherson S. M., Mathews L., Campbell K. P., Longo F. J. Presence of inositol 1,4,5-trisphosphate receptor, calreticulin, and calsequestrin in eggs of sea urchins and Xenopus laevis. Dev Biol. 1994 Feb;161(2):466–476. doi: 10.1006/dbio.1994.1045. [DOI] [PubMed] [Google Scholar]
- Peter F., Nguyen Van P., Söling H. D. Different sorting of Lys-Asp-Glu-Leu proteins in rat liver. J Biol Chem. 1992 May 25;267(15):10631–10637. [PubMed] [Google Scholar]
- Plakidou-Dymock S., McGivan J. D. Calreticulin--a stress protein induced in the renal epithelial cell line NBL-1 by amino acid deprivation. Cell Calcium. 1994 Jul;16(1):1–8. doi: 10.1016/s0143-4160(05)80002-8. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Xu Y., Brenner M. B. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994 Jan 21;263(5145):387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- Ray M. K., Yang J., Sundaram S., Stanley P. A novel glycosylation phenotype expressed by Lec23, a Chinese hamster ovary mutant deficient in alpha-glucosidase I. J Biol Chem. 1991 Dec 5;266(34):22818–22825. [PubMed] [Google Scholar]
- Reitman M. L., Trowbridge I. S., Kornfeld S. A lectin-resistant mouse lymphoma cell line is deficient in glucosidase II, a glycoprotein-processing enzyme. J Biol Chem. 1982 Sep 10;257(17):10357–10363. [PubMed] [Google Scholar]
- Rowling P. J., McLaughlin S. H., Pollock G. S., Freedman R. B. A single purification procedure for the major resident proteins of the ER lumen: endoplasmin, BiP, calreticulin and protein disulfide isomerase. Protein Expr Purif. 1994 Aug;5(4):331–336. doi: 10.1006/prep.1994.1049. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Singh I., Doms R. W., Wagner K. R., Helenius A. Intracellular transport of soluble and membrane-bound glycoproteins: folding, assembly and secretion of anchor-free influenza hemagglutinin. EMBO J. 1990 Mar;9(3):631–639. doi: 10.1002/j.1460-2075.1990.tb08155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa M. C., Ferrero-Garcia M. A., Parodi A. J. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992 Jan 14;31(1):97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Suh K., Bergmann J. E., Gabel C. A. Selective retention of monoglucosylated high mannose oligosaccharides by a class of mutant vesicular stomatitis virus G proteins. J Cell Biol. 1989 Mar;108(3):811–819. doi: 10.1083/jcb.108.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B., Füllekrug J., Nguyen Van P., Diekmann W., Robinson D. G., Mieskes G. Retention and retrieval: both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J Cell Sci. 1994 Oct;107(Pt 10):2705–2717. doi: 10.1242/jcs.107.10.2705. [DOI] [PubMed] [Google Scholar]
- Tatu U., Hammond C., Helenius A. Folding and oligomerization of influenza hemagglutinin in the ER and the intermediate compartment. EMBO J. 1995 Apr 3;14(7):1340–1348. doi: 10.1002/j.1460-2075.1995.tb07120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta S. E., Bosch M., Parodi A. J. Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry. 1989 Oct 3;28(20):8108–8116. doi: 10.1021/bi00446a022. [DOI] [PubMed] [Google Scholar]
- Ware F. E., Vassilakos A., Peterson P. A., Jackson M. R., Lehrman M. A., Williams D. B. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995 Mar 3;270(9):4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Tector M., Salter R. D. Calnexin recognizes carbohydrate and protein determinants of class I major histocompatibility complex molecules. J Biol Chem. 1995 Feb 24;270(8):3944–3948. doi: 10.1074/jbc.270.8.3944. [DOI] [PubMed] [Google Scholar]
- de Silva A. M., Balch W. E., Helenius A. Quality control in the endoplasmic reticulum: folding and misfolding of vesicular stomatitis virus G protein in cells and in vitro. J Cell Biol. 1990 Sep;111(3):857–866. doi: 10.1083/jcb.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva A., Braakman I., Helenius A. Posttranslational folding of vesicular stomatitis virus G protein in the ER: involvement of noncovalent and covalent complexes. J Cell Biol. 1993 Feb;120(3):647–655. doi: 10.1083/jcb.120.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]