Abstract
Many organisms incorporate inorganic solids in their tissues to enhance their functional, primarily mechanical, properties. These mineralized tissues, also called biominerals, are unique organo-mineral nanocomposites, organized at several hierarchical levels, from nano- to macroscale. Unlike man made composite materials, which often are simple physical blends of their components, the organic and inorganic phases in biominerals interface at the molecular level. Although these tissues are made of relatively weak components at ambient conditions, their hierarchical structural organization and intimate interactions between different elements lead to superior mechanical properties. Understanding basic principles of formation, structure and functional properties of these tissues might lead to novel bioinspired strategies for material design and better treatments for diseases of the mineralized tissues. This review focuses on general principles of structural organization, formation and functional properties of biominerals on the example the bone tissues.
Keywords: Bone, Nanoscale objects, Biomimetic materials, Self-assembly, Proteins
Biominerals are biogenic hierarchical composite materials, comprised of organic and mineral phases 1–5. In its strict form, the term biomineral defines biogenic mineral phases, however, in the recent years it is used in a broader sense to describe organo-mineral nanocomposites comprising mineralized tissues and organs 6, 7. We use here this later broader definition of biominerals, since the mineral and organic phases are intimately associated at the molecular level, and are practically inseparable both structurally and in terms of their functional properties.
Biominerals are produced by living organisms for different purposes, such as support of the body, protection of the vital organs, defense against predators, and others. Biominerals are found among all kingdoms of life and more than 50 mineral phases associated with them are identified so far 8; although the majority of the organisms produce their mineralized tissues from calcium phosphates and carbonates or amorphous silica. Biominerals vary greatly in terms of their organic content, structural organization and functional, primarily mechanical, properties. At the same time a number of common principles of biomineralization are found throughout different phyla which distinguish them from geological mineral forms and synthetic minerals 9.
One of the major characteristics of the biomineralization strategies is the use of bottom up approaches, in which the material is built starting at atomic and molecular scales, leading to the formation of nanostructured building blocks, which in turn organize into complex hierarchical structures 1, 4, 10. The basis of any biomineralization process is interplay between assemblies of biomacromolecules and forming mineral phases. The macromolecules play a number of important functions in biomineralization processes, including the control over mineral phases formed 11–14, shape of the mineral particles 15, 16 and their organization 17. Furthermore, the macromolecules are extremely important for maintaining unique mechanical properties of mature mineralized tissues 4, 18–20.
There are two major mechanisms by which biominerals form; one is via templating of the mineral in the preformed organic matrix 10, 21, 22, and another via co-operative interactions between macromolecular assemblies and forming mineral phases 17, 23. These two mechanisms are not mutually exclusive and most likely they are present simultaneously in many biomineralization processes. Another important feature of many biomineralization processes in different phyla is the use of transient metastable mineral phases, which has a number of advantages over classical solution crystal growth 24–28.
Unlike modern man-made materials, which are usually made under extreme conditions, the biominerals are formed under ambient temperatures and pressures from relatively weak individual components, yet they are remarkably resilient, thanks to their unique structural organization, and interactions of their organic and mineral components at atomic and molecular levels. The unique characteristics of biominerals and their formation processes attracted attention of researchers interested in exploring the principles of biomineralization in design of novel materials for a variety of applications 29–35. More specifically, these so called biomimetic or bioinspired approaches to the formation of nanostructured hierarchical composite materials promise to revolutionize the fields of materials for hard tissue repair and regeneration 35–38.
The present review will focus on the state of our understanding of the biomineralization processes and the structure-function relationships in biominerals at several hierarchical levels, starting from nanoscale, using as examples the bone type tissues, the major family of mineralized tissues in human body.
Biomineralization in bone tissues: structure, formation and function
The family of bone tissues include different bone types, dentin, cementum and mineralized tendon 4. All bone tissues are comprised primarily of collagen type I fibrous matrix and carbonated apatite crystals. They share a basic building block- the mineralized collagen fibril, however the structural organization of the fibrils at the meso- and micro-scales is different in different bone types 4.
Composition of bone tissues
Bone is the major mineralized tissue of the human body, containing ~60–70% w/w of calcium phosphate mineral, ~20–30% w/w of organic matrix and 10% of water. It is a nano-composite with unique mechanical properties, determined by its chemical composition and structural organization 4. Fibrillar collagen type I is the major organic component of bone and comprises close to 90% of its total organic content with dozens of other so-called noncollagenous macromolecules which account for the remaining 10%. The noncollagenous fraction consists mainly of extremely acidic proteins which are believed to play crucial roles in the formation and function of these tissues 18, 39 Several hierarchical levels of organization are recognized in bone starting from its individual components, such as mineral crystallites and collagen fibrils up to the organ level 4, 40 (Figure 1).
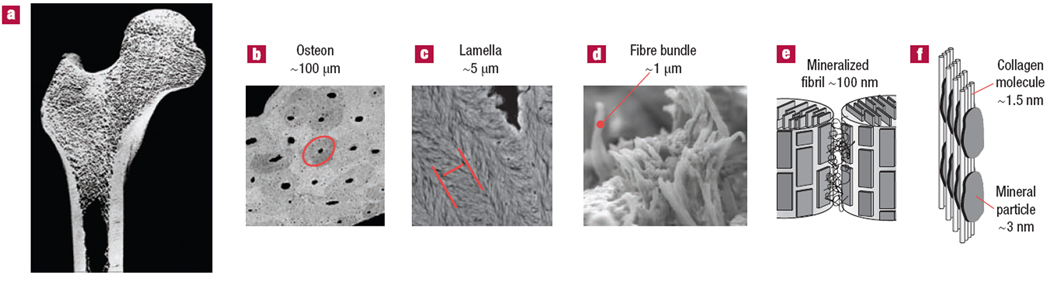
Figure 1.
Hierarchical organization of bone from macro to nanoscale. a) Organ level- femoral bone. b) Tissue level- haversian (osteonal) compact bone; red ellipse outlines an individual osteon. c) Microscopic level- bone lamellae are the structural elements of lamellar bone tissues; red parallel lines outline one lamella. d) Mesoscopic level arrays (buldles of mineralized collagen fibrils. e) nanoscale level- mineralized collagen fibrils. f) Molecular level- arrangements of collagen molecules and mineral crystallites in the mineralized collagen fibril. The figure is reprinted with permission from reference 40.
Collagen Fibrils
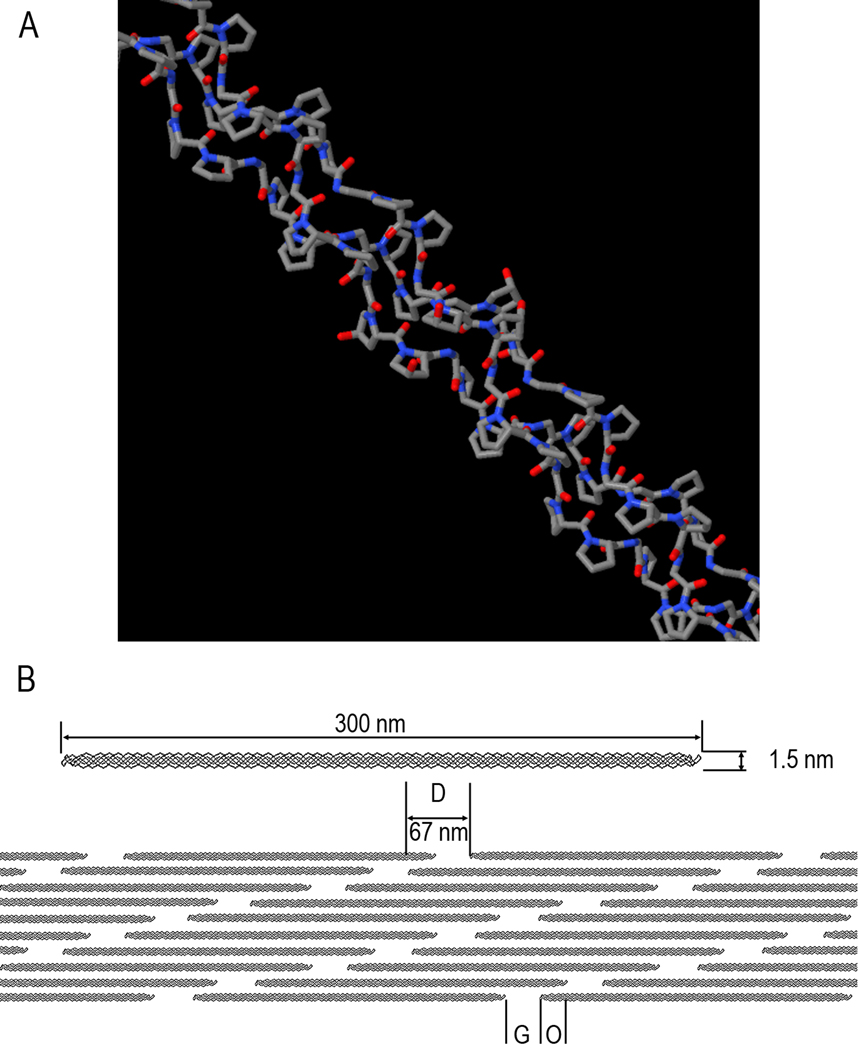
Type I collagen is the most abundant fibril forming protein in the body. It is also the most thoroughly studied extracellular protein, yet many aspects of its assembly and fibril structure remain unclear 41. Type I collagen molecules are supercoiled assemblies of three polypeptide chains, each with over 1000 amino acid residues. Type I collagen molecules, also called triple helices (Figure. 2A), consist of two identical α1-chains and one α2-chain which has a different sequence. The main part of each collagen polypeptide chain is a helical domain, comprised of Gly-X-Y repeats, where amino acids proline (Pro) and hydroxyproline (Hyp) predominantly occupy X and Y positions respectively. All three chains in the helical portion of the molecule adopt polyproline II (PPII)-like structure, which is stabilized by direct and water mediated inter- and intra- chain hydrogen bonds 42, 43. Procollagen, a full length collagen molecule, contains two large pro-domains on both N and C terminal ends of each polypeptide chain, which prevent any spontaneous self-assembly inside the cells. After secretion the pro-domains are proteolitically cleaved in the extracellular space, allowing assembly of individual collagen molecules into the fibrils 44. The cleaved form of the collagen molecule called tropocollagen is roughly 300 nm long and 1.5 nm across. Besides the main helical part collagen triple helices contain short non-helical end sequences called telopeptides on both N and C ends of the molecule. The telopeptides are thought to play a role in the assembly process however the exact mode of their action is unclear 45. Tropocollagen molecules aggregate with their long axes in parallel to form fibrils 41, 44, 46. Within the fibril there is a linear shift of some 67 nm (the D-period) between neighboring molecules. This staggered fibril assembly leaves gaps of 0.6D between the ends of co-linear collagen molecules and 0.4D overlap region for every 67-nm fibril repeat 44, 47 (Figure 2B).
Figure 2.
A. Triple-helical structural motif of collagen molecules. B. Organization of collagen fibril. Three hundred nm long and 1.5 nm wide triple-helical collagen molecules assemble into the fibril in a staggered arrangement, where each molecule is shifted in respect to its neighbor. The neighboring molecules in the axial dimension are 40 nm apart. This structural organization of the fibril gives rise to the 67 nm periodic pattern- D-period (D), which consists of Gap (G) and overlap (O) region.
Mineral Phase
Mineral phase of mature bone tissues primarily consists of poorly crystalline non-stoichiometric carbonated hydroxyapatite (dahlite), with hexagonal crystal structure. The carbonate concentration varies between 4 and 7% with the carbonate ions substituting both phosphate (B-type substitution) and hydroxyl ions (A-type substitution) in the lattice 48. Bone crystallites are the smallest biogenic crystals known. They are only 2–6 nm thick, 30–50 nm wide and 60–100 nm long 49–53, which implies that, at least in one dimension, these crystallites contain only a few unit cells thick and hence have extremely high surface to bulk ratio. Optical axes of these crystallites (c-axes) are usually aligned with the longest dimension, while a- and b-axes are aligned along two other dimensions; it is not clear however, if there is a preferred association of these crystallographic axes with a specific dimension. Recent NMR studies clearly demonstrate that the surface layer of the crystallites in bone tissues is hydrated and highly disordered 54–56. Despite their extremely small size the bone crystallites are very stable and resistant to dissolution. It has been proposed that that the mineral particles become insensitive to the dissolution at nanoscale, when their sizes become smaller than the critical dissolution pit size 57, 58.
Structural organization of bone tissues
Mineralized collagen fibril
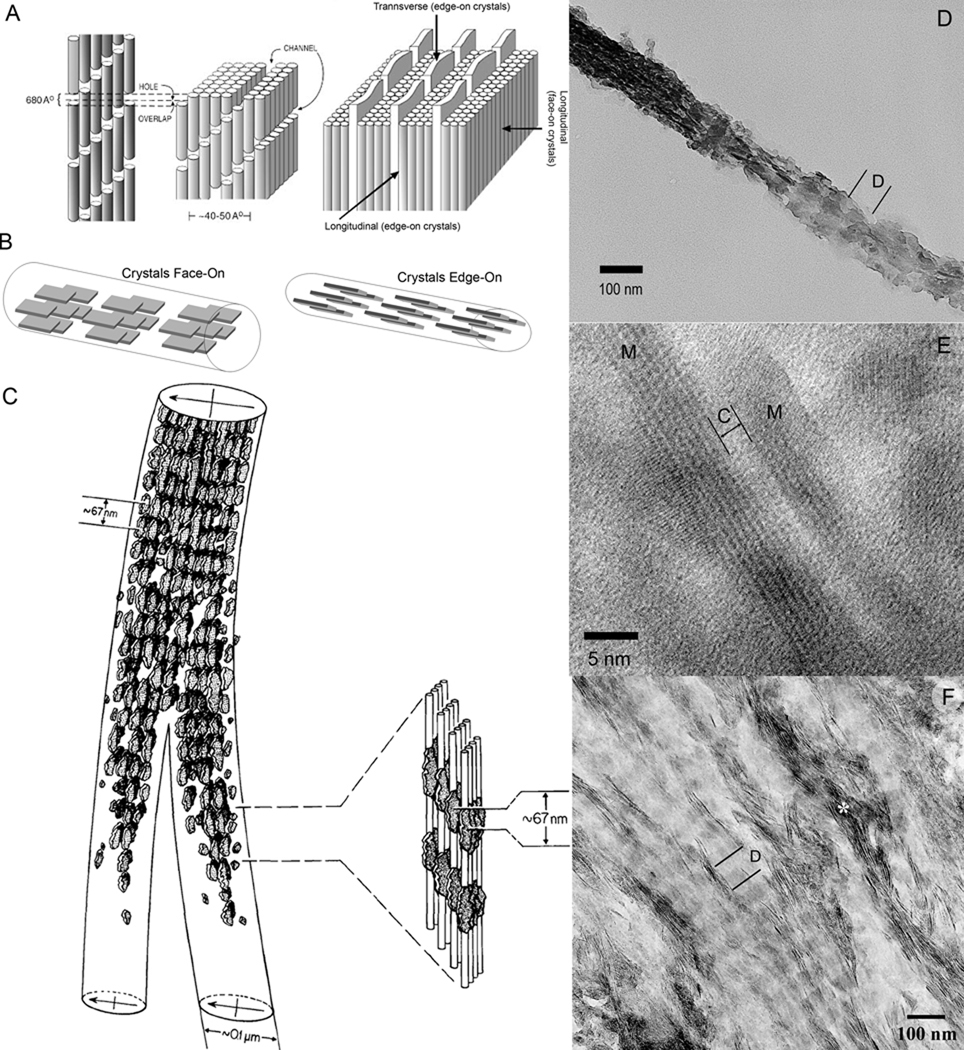
Mineralized collagen fibrils are the basic building blocks of bone tissues and determine their mechanical properties at nanoscale 4. In the mineralized collagen fibrils tiny plate-shaped crystallites of carbonated apatite are organized into parallel arrays with their c-axes co-aligned with the long axes of the collagen fibril 49, 59, 60 (Figure 3). The crystals localize in the gap regions, as well as between the layers of collagen molecules in the overlap region (Figure 3D). There are more mineral crystals in the gap than in the overlap regions of the mineralized fibrils leading to the banded periodic appearance, with the repeat motif corresponding to the D-period of the fibrils (Figure 3B,C), see 61 for example. Taking into account the large surface area of the mineral platelets and the fact that their thickness is only marginally greater than the diameter of collagen molecules, the mineralized collagen fibril can be viewed as a composite nano-structure in which mineral and organic phases interface at the atomic and molecular level. Although the nature of collagen-mineral interactions are still not well understood it is likely that they involve ionic bonds between side chain carboxyls of the protein and calcium ions in the mineral particles 62, 63. It is also possible that the backbone carbonyls of prolines residues form complexes with calcium ions in the mineral phase 64, 65.
Figure 3.
Structural organization of the mineralized collagen fibril. A. Schematic representation of collagen fibril showing channels in which crystals start to form (left side); on the right, arrays of plate like mineral crystals sandwiched between layers of collagen triple-helices (depicted as cylinders), note that the structure of the mineralized collagen fibril is orthogonally anisotropic. Reprinted with permission from 4 B. Face-on and Edge-on projections of the crystals in the mineralized fibril. C. The drawing of two mineralized collagen fibrils obtained from the electron tomographic reconstruction of the normally calcified mineralized avian tendon. Note that the density of the crystals is higher in the gap regions, leading to a periodic mineral density profile with 67 nm spacing. Also note that the periodic patterns in the neighboring fibrils are in register. Reprinted with permission from 49. D. TEM micrograph of an isolated mineralized collagen fibril from human dentin. The fibril is twisted and both edge-on (left) and face-on (right) crystals present in the same fibril. Note the periodic pattern of mineral density (D-period). E. HRTEM micrograph of two edge-on crystals (M) in the mineralized fibril from human dentin. The narrow space between them is filled with collagen molecules (C). F. An array of the mineralized fibrils from human dentin with crystals in face-on orientation, with the exception of the fibril marked with asterisk with crystals in edge-on orientation. Note the periodic changes in mineral density, corresponding to D-period. Also note that the periodic patterns in neighboring fibrils are in register.
Extrafibrillar Material
In addition to the crystallites associated with the collagen fibrils (intrafibrillar mineralization) 2, 10, 66 a significant amount of mineral is located in the spaces between the fibrils (extrafibrillar mineralization) 61, 67–70. These are two distinct fractions of mineral and vary in terms of their formation, structure and functional properties.
Spaces between mineralized collagen fibrils are filled with matrix made of noncollagenous macromolecules and mineral crystals. It has been reported that the extrafibrillar mineral fraction can account to as much as 75% of total mineral in bone tissues 67, 70, 71. Like intrafibrillar mineral particles, extrafibrillar crystals are plate-like, although a number of reports suggest that they are longer and thicker than their intrafibrillar counterparts 70–72. These extrafibrillar crystals are oriented with their broad surface facing collagen fibril surface 70 and are organized into parallel arrays with their c-axes co-aligned with the axes of the adjacent mineralized collagen fibrils 61, 68
Fibrillar arrays
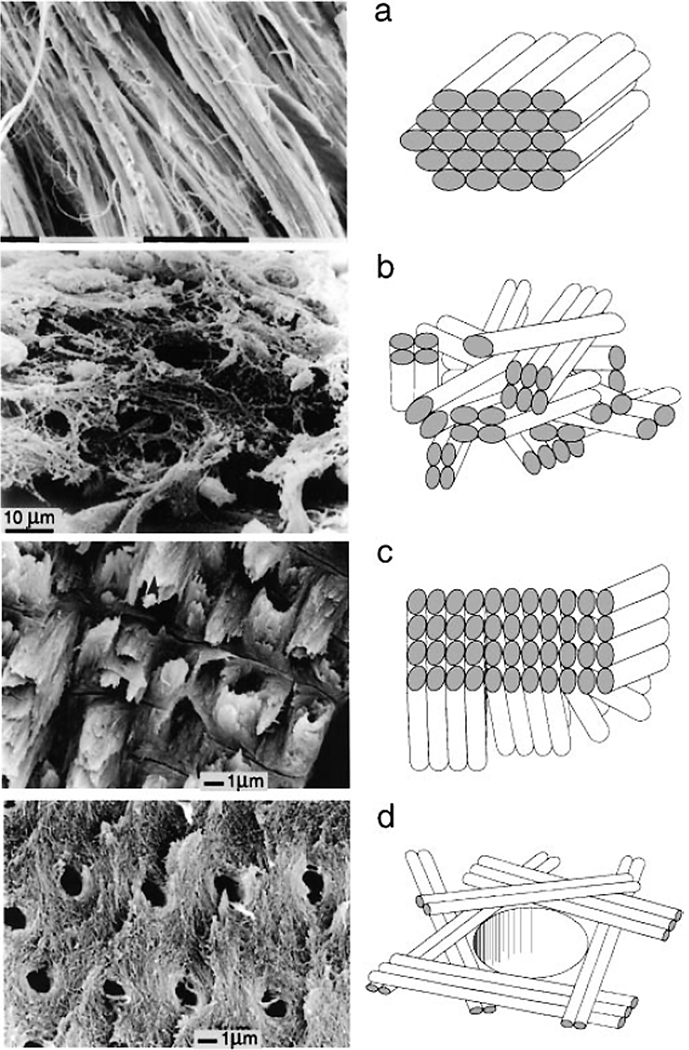
Although the mineralized collagen fibrils provide a basic building block of all bone tissues, the organization of these fibrils in different bone types varies significantly, reflecting the differences in their functional properties 4 (Figure 4).
Figure 4.
Different types of fibril array patterns. A. Parallel fiber array, found in mineralized turkey tendons and parallel fiber bone. B. Woven array. Found in woven bone. C. Lamellae. Found in lamellar bone. D. Radial array. Found in dentin. The figure is reprinted with permission from reference 4.
Woven bone is the bone type in which individual mineralized collagen fibrils or small bundles of the fibrils are randomly organized (Figure 4A) 61, 73. Woven bone is fast-forming and mechanically weakest bone type; it appears transiently during the embryonic development and in the repair sites, and is substituted by other more organized bone types 74, 75.
In parallel-fiber structures all the fibrils are parallel to each other over large areas of the tissue (Figure 4B). Such organization of the mineralized fibrils is found in bovine long bones and mineralized tendons 76, 77 (Figure 4B).
Lamellar bone consists of lamellae, made of one or more parallel fibrillar arrays varying in thickness from a few hundred nanometers to a few microns 78. The simplest type of lamellar organization is found in tooth cementum, in which the parallel arrays of the fibrils in the neighboring lamellae are rotated 30° in respect to each other 79. In bones lamellar organization is much more complex with individual lamellae made of sublayers of varying thickness, with different orientation of collagen fibrils 78, 80 (Figure 4C). The thicknesses and the main fibril direction in neighboring lamellae can vary leading to the formation of unique patterns which are often species specific and can even vary among different bone types in the same species 78, 80.
Tooth dentin, another tissue which also belongs to the bone-like materials, has so called radial fibril array organization 4 (Figure 4D). In dentin the mineralized collagen fibrils are organized in layers which are roughly parallel to the plane of the pulp cavity wall, however inside the layers the fibrils are randomly or poorly oriented.
The fibrillar arrays are organized into much more complex structures at the 10 to 100 micron scales, such as secondary osteons, formed during remodeling of lamellar bone (Figure 1). These are cylindrical structures with a central channel, containing blood supply and bone cells in the middle, with the walls made of lamellar arrays. Finally the whole bone organ is made of several bone types with different porosity and organization at the submilimeter scale, i.e. compact cortical bone comprising the outer region of the long bones vs. spongy trabecular bone found in the core (Figure 1).
Formation of the Bone Tissues
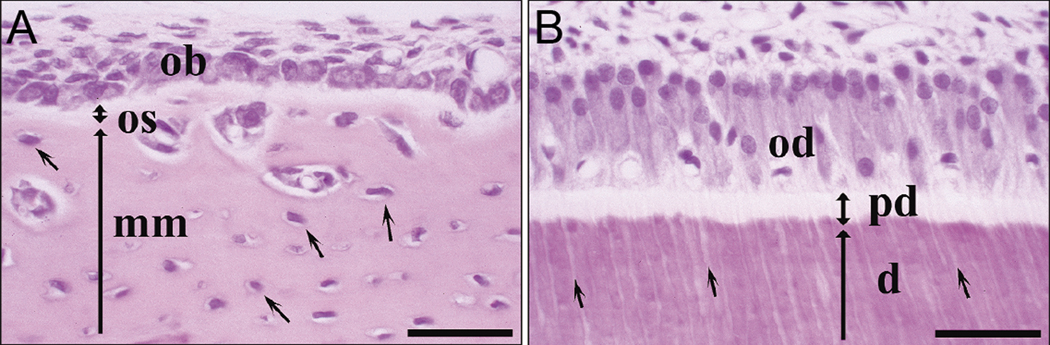
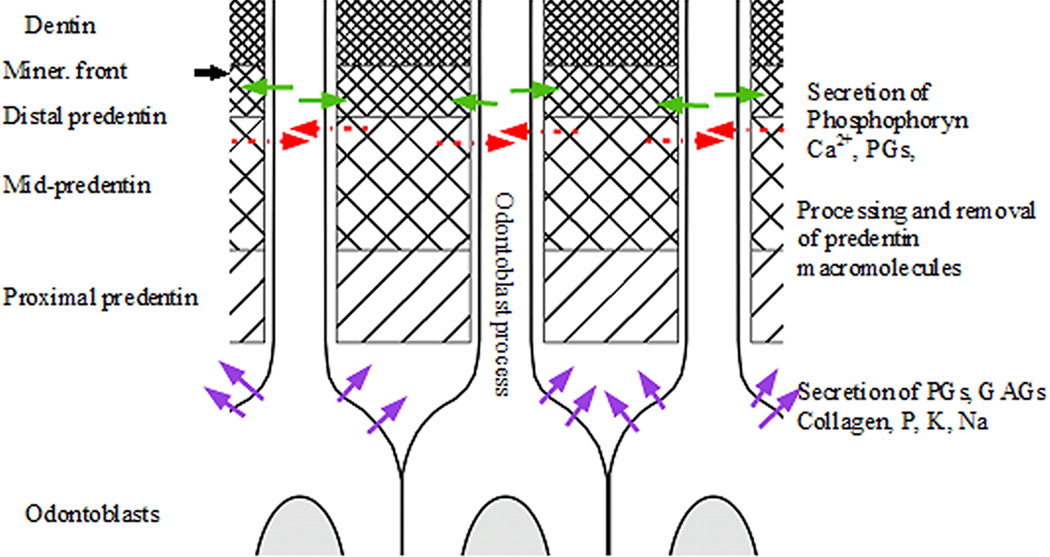
Bone and dentin are produced by specialized cell types, osteoblasts and odontoblasts, respectively 81 (Figure 5). These cells deposit a layer of nonmineralized extracellular matrix, called osteoid in bone and predentin in dentin, consisting of collagen fibrils and noncollagenous macromolecules, which eventually mineralizes a few microns away from the cells. In bone some osteoblasts become entrapped the mineralizing matrix and differentiate into osteocytes 82,83. Osteocytes develop long processes, which form a network connecting them to each other and to the osteoblasts outside of the bone matrix. They are believed to play an important role in the regulation of bone homeostasis. Similarly to osteocytes, odontoblasts also form long processes which penetrate dentin matrix; these cell processes are believed to be responsible for regulation of mineralization in dentin 83, 84. The structural organization of collagen fibrillar arrays in the bone tissues is determined by the cells during the matrix deposition stage. It has been shown in predenin that its biochemical composition as well as structural organization of the collagen fibrils undergo significant changes from the proximal portion adjacent to the cells toward the distal layer next to the mineralization front 85–88. A number of macromolecules, such as proteins, proteoglycans (PG) and glycosaminoglycans (GAG) enter the proximal predentin together with collagen, while others are secreted into the distal predentin via odontablast processes, in a close proximity to the mineralization front (Figure 6). Furthermore, some of the macromolecules, specifically GAGs secreted into proximal predentin are enzymatically degraded in mid and distal layers of predentin. It is believed that the molecules secreted into the proximal predentin are responsible for regulation of collagen assembly and maturation, while the macromolecules entering predentin at the mineralization front are responsible for its mineralization. In particular, small leucine rich PGs, such as biglycan and decorin, were shown to play essential roles in collagen fibrillogenesis and the lack of these macromolecules leads to severe abnormalities in bone tissues 86, 89–92. PGs specifically bind to collagen triple helices and these interactions influence both kinetics of the fibrillogenesis and control the fibril diameter 90, 93–96. It is not just the changes in macromolecular composition which take place during the predentin maturation; its inorganic makeup also undergoes significant changes. For example, while phosphate ions are evenly distributed throughout predentin, elevated levels of calcium are found in distal dentin, with its highest concentration at predentin-dentin boundary where the mineralization occurs 97 which suggest that the distribution of inorganic ions is tightly controlled by the cells. These data indicate that the extracellular matrix of predentin undergoes a cell regulated maturation process, and mineralization is triggered by the increase in calcium concentration and secretion of specific macromolecules at the mineralization front. At the same time the questions remain in respect to the mechanisms by which odontoblasts control the matrix maturation and the onset of mineralization in bone. Since osteoblasts do not have cell processes and there is a nonmineralized osteoid between them and the mineralization front they cannot exert a direct control over mineralization. It is likely that other bone cell type, osteocytes, are involved in the regulation of matrix maturation and mineralization 98, 99.
Figure 5.
A. Forming bone. OB-osteoblasts, OS-osteoid (nonmineralized matrix), MM-mineralized matrix. B. Forming dentin. OD-odontoblasts. PD-predentin, D-dentin. The figure is reprinted with permission from reference 81.
Figure 6.
Schematic representation of changes in organic matrix composition in predentin prior to mineralization. Green arrows indicate the secretion of dentin macromolecules and Ca2+ ions by the odontoblast processes; red arrows indicate removal of processesed predentin molecules by odontoblast processes. Purple arrows indicate the secretion of collagen, predentin macromolecules and phosphate ions by odontoblast cell bodies.
A number of studies indicate that the mineralization begins outside of the collagen fibrils and eventually progresses into the fibrils 68, 100. These initial extrafibrillar mineral particles are comprised of ACP26, 100, 101 and might be associated with matrix vesicles 102, although crystalline mineral particles at the sites of initial extrafibrillar mineralization have been reported as well 68.
Noncollagenous Acidic Proteins
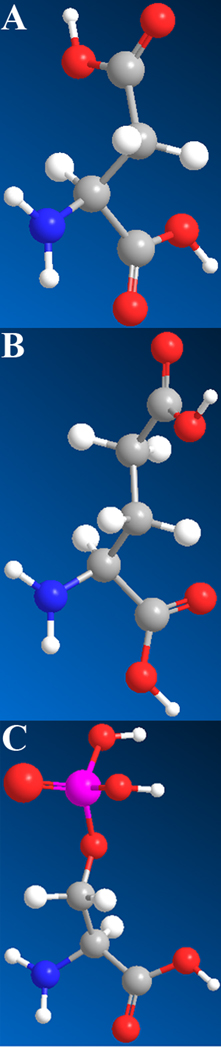
The mineralization in bone and dentin is a well orchestrated multi-step process, which requires a number of components introduced into the system in an appropriate sequence. Although collagen fibrils are the major component of the bone-type tissues, they alone are not able to initiate oriented calcium phosphate nucleation and growth 103–105. At the same time a number of proteins expressed in bone and dentin are involved in different aspects of calcium phosphate mineralization in these tissues. These, so called non-collagenous proteins of bone tissues, share a number of unique characteristics which are essential for their function in the control of mineralization process 39, 106, 107. The vast majority of these proteins belong to SIBLING (Small Integrin Binding Ligand N-Linked Glycoprotein) family and are believed to play essential roles in the formation of bone tissues 81, 108. Specifically these proteins, are extremely rich in acidic amino such as aspartic (Asp) and glutamic acid (Glu), which contain carboxyl group in their side chain, and phosphorylated serine (Ser) 109–111 (Figure 7). For example, 30% of all amino acids in dentin phosphoprotein, the major noncollagenous protein of dentin, are Asp, and 60% are Ser, with up to 90% of all serine residues phosphorylated 110. The extreme acidity is a common thread of proteins found in biominerals. For example the members of Asprich protein family found in mollusk shells contain up to 53% of Asp 112. High levels of acidic amino acids in these proteins determine their high binding capacity for polyvalent metal ions, such as calcium, which important for their function in mineralization processes 107, 113–115.
Figure 7.
Acidic amino acids prominent in noncollagenous acidic proteins: A. Aspartic acid. B. Glutamic acid, C. Phosphoserine
Another important feature of many biomineralization proteins, including acidic noncollagenous proteins of bone tissues, is that in solution they adopt extended intrinsically disordered conformation 108, 116–120. This structural characteristic is believed to play important role in the function of these proteins, since the intrinsically disordered proteins (IDPs) tend to participate in intermolecular interactions in protein assemblies and at the protein-mineral interface 119.
Recent studies indicate that SIBLING proteins are able to form supramolecular assemblies 121, 122. These reports demonstrate that DMP1 and phosphophoryn, two major phosphoproteins of dentin and bone can form filamentous supramolecular assemblies in the presence of calcium ions. It is likely that the assembly is driven by formation inermolecular salt bridges between side chain acidic moieties and Ca2+. It is believed that the proteins in such assemblies adopt β-sheet conformation, in a way similar to filamentous assemblies of synthetic peptides and amyloid-like protein fibrils 123–125. Formation of β-sheet assemblies would require hydrogen bonds between the protein molecules, hence calcium driven assembly of acidic noncollagenous proteins is most likely a 2-step process. Initially, divalent ions reduce the repulsive electrostatic forces between the protein molecules and bring them into a close proximity via salt bridges, which will help to align the molecules and induce the formation of β-sheets. Similar assembly of synthetic acidic peptides into β-sheets was described in the literature 126. The supramolecular assemblies of noncollagenous proteins might play a critical role in the regulation of mineral formation and organization in bone tissues 121 as well as in the mechanical performance of bone tissues 127.
Finally, a number of noncollagenous proteins of bone tissues were found to specifically bind collagen 128, 129. These interactions are based on the molecular recognition mechanisms between the noncollagenous proteins and certain domains of the collagen sequence. Hence, these proteins bind to specific locations on the triple-helices, which leads to the spatial specificity of the protein distribution along the collagen fibrils 85, 130–133.
Although the effects of noncollagenous acidic proteins on calcium phosphate formation have been extensively studied over the years, the exact mechanisms of mineralization in bone tissues and the roles of individual proteins in this process still remain unclear. Here we will try to analyze the results of these studies and discuss the generally accepted models as well as current hypotheses based on the recent data.
In vitro mineralization experiments using the noncollagenous acidic proteins, carried over the years by a number of groups, provided invaluable insights into the potential function of these molecules in the bone tissue mineralization 35, 106, 115, 134, 135. It is broadly accepted that the noncollagenous proteins of bone tissue can affect calcium phosphate mineralization in a number of different ways, namely they can induce crystal nucleation, control crystal shape and size, inhibit mineralization and stabilize transient mineral phases, i.e. ACP. Interestingly enough, the same protein can affect mineralization in several different ways, depending on its concentration, degree of phosphorylation and either it is bound to the surface or in solution 21, 105, 115, 121, 131, 134, 136. Such poly-functionality is not very surprising considering the physicochemical and structural properties of these proteins.
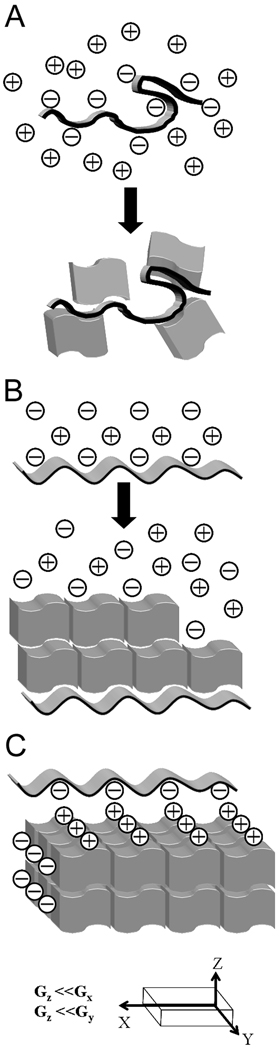
As has been mentioned in the preceding paragraphs the noncollagenous proteins are acidic molecules, which can interact with metal ions in solution or on the mineral surface via their anionic side chains. It was shown that when these proteins are present in solution at high enough concentrations they can completely inhibit mineral formation, while at lower concentrations they can influence the mineral particles size 105. There are several possible mechanisms of inhibition, which are employed by different proteins 115. At high enough concentration the proteins can bind large amounts of calcium ions, decreasing the degree of saturation and reducing the driving force toward mineral precipitation 105, 136. Alternatively, these proteins can inhibit mineral nucleation by binding to the nascent mineral clusters and arresting their further growth 115. The noncollagenous proteins in solution were shown to bind crystals via the interactions between their acidic side chains and calcium ions at the crystal surface and slow down crystal growth in the concentration dependant manner 105, 134, 137, 138. Furthermore, the biomineralization proteins can control the crystal shape by preferably binding to certain crystallographic planes and selectively slowing down growth in the direction normal to the plane 15, 22, 137, 139 (Figure 8).
Figure 8.
The classical models of regulation of mineralization by acidic proteins. A. Randomly organized acidic macromolecules (folded ribbon) can induce crystal nucleation by attracting metal ions and increasing a local supersaturation. This mechanism, however, does not provide means to control the crystals orientation. Irregular blocks represent crystals. B. Acidic proteins adopting regular conformation can promote an oriented crystal nucleation and growth via epitaxy. A nucleating macromolecule is represented as a ribbon with periodic structure; epitaxially growing crystals depicted as blocks forming a “brick wall” structure. C. Shape modifying macromolecules are thought to adopt regular conformation, matching lattice parameters of certain crystallographic planes, which results in the preferred binding of the macromolecules to specific crystalline faces. As a result, the crystal growth is inhibited in a direction normal to the affected crystalline face. G is the rate of growth in different crystallographic directions. It is slower in the along Z-axis. Unit cell (smallest repeating unit) of a crystal is represented by blocks forming a “brick wall”. A periodic ribbon represents a shape-modifying macromolecule.
The same proteins when immobilized on the surface can induce mineralization and promote oriented crystal nucleation 21, 134. Again there are several possible mechanisms of such action. The negatively charged macromolecules bound to the surface can attract positively charged metal ions leading to local supersaturation and hence creating higher driving force toward mineral precipitation in the close proximity to the protein (Figure 8), however such mechanism is not sufficient for oriented nucleation of mineral crystals on the surface as observed in many mineralization systems. It is believed that the immobilized proteins can organize on the surface with their acidic side chains arranged in a periodic pattern which matches lattice parameters of certain planes in the forming crystal. Such relationships provide the basis for templated nucleation of the crystals on the protein surface, creating energetically preferred conditions for oriented crystal nucleation 21, 22, 140, 141 (Figure 8).
Use of in vitro diffusion mineralization assays mineralization provided a wealth of information about the possible functions of noncollagenous acidic proteins 134. Specifically they demonstrate that some proteins, such as osteocalcin and osteopontin inhibit mineralization, while others, i.e. DPP, BSP and DMP1 increase the amount of mineral formed 115, 134, 142, 143. These studies indicate that the interplay between mineralization inhibitors and promoters can be the major factor in the regulation of mineralization process in the bone tissues.
A number of biomineralization proteins from different tissues were also shown to transiently stabilize amorphous mineral phases 14, 21, 136, 144, 145. This recently discovered function of the biomineralization proteins is significant, since in many biominerals including bone tissues the initial mineral phase is amorphous 25–27, 100, 146. Although the mechanisms by which the biomineralization proteins stabilize amorphous phases are not yet understood, there are several reports, which provide some limited insights. It has been reported in a number of studies of both calcium phosphate and calcium carbonate biomineralization, that phosphorylation can play a role in the ability of proteins to stabilize amorphous mineral phases 14, 147–149. It was also found that the proteins associated with amorphous calcium carbonate are richer in glutamic acid, while aspartic rich proteins are found in crystalline calcium carbonate 150. Asp and Glu are almost identical amino acids, the only difference is that Glu side chain has one more methyl group than Asp (Figure 7), and hence has more degrees of freedom. This longer and more flexible Glu side chain might be the key to its role in the stabilization of amorphous mineral, by binding to mineral clusters and disrupting their organization into crystalline lattices. Furthermore, as was mentioned in the preceding paragraphs many biomineralization proteins are intrinsically disordered. It is therefore feasible that the disordered proteins will bind multiple initial mineral nanoclusters, forming larger composite protein-mineral aggregates and preventing or slowing down their transformation into crystalline periodic structures.
Mineralization of collagen fibrils
As it was mentioned earlier, mineralized collagen fibrils are essential building blocks of bone tissues with unique structural properties, many aspects of collagen mineralization remain unclear despite numerous extensive studies of this process 39, 66, 105. Nevertheless a significant progress has been made in our understanding of collagen mineralization, and a coherent picture of this process is beginning to emerge. Following is a short summary of the state of our current knowledge of this process.
It is well established that collagen fibrils by themselves cannot induce organized calcium phosphate mineralization 103–105, 151 and acidic noncollagenous proteins are required for the proper collagen mineralization. Interestingly, the studies of animal models in which a number of such molecules were selectively knocked out often demonstrate significant changes in bone tissues, yet none of these knockouts lead to complete cessation of mineralization 152–161. These results indicate that there is some redundancy built into the system and the luck of one particular protein can be compensated by others. Furthermore, it appears that these proteins can be divided into 2 groups based on their effects on mineralization. One group, including bone sialoprotein (BSP) 161, dentin matrix protein 1 (DMP1) 152 and dentin phosphoprotein (DPP) 159, promote mineral formation in bone tissues while others, such as osteocalcin and 154, osteopontin 157 and osteonectin 156 inhibit mineral formation and maturation. Yet it is extremely difficult to establish the role of these proteins in collagen mineralization, based solely on the results of these knockout studies, since most of these proteins have other functions beyond the matrix mineralization. For example, DMP1 is involved in the phosphate homeostasis regulation 162, while other proteins such as osteocalcin and BSP are involved in the regulation of osteoclast and osteoblast differentiation and energy metabolism 153, 161, 163. Therefore, lack of these proteins can lead to multiple changes in the organism which can affect directly or indirectly bone tissue mineralization.
Another approach to understanding of the roles of the acidic molecules in mineralization is in vitro mineralization experiments, In recent years several in vitro mineralization studies were conducted using collagen fibrils and noncollagenous proteins or model acidic polypeptides 66, 104, 105, 131, 151, 164. These studies have demonstrated that acidic peptides such as polyaspartic acid can regulate mineralization, in vitro, leading to the formation of mineralized collagen fibrils similar to ones found in vivo with the mineral crystallites co-aligned with the long axis of the fibril (Figure 9). The fact that simple polyanionic peptides can induce the formation of mineralized collagen fibrils indicates that the negative charge of the non-collagenous acidic proteins is the main factor in their role in mineralization of bone tissues. Yet, since some of these proteins act as inhibitors of mineralization, while others promote this process, other factors such as structure and posttranslational modifications must also be important. Initial mineral phase observed in these in vitro experiments was ACP, which transformed into apatitic crystalline phase with time 66, 105. These results are in a good agreement with the data from the in vivo studies demonstrating the presence of transient amorphous mineral in forming bone tissue 26, 100, 146. Furthermore the shape of amorphous mineral particles inside the collagen fibrils was identical to the mature mineral crystals observed at the later stage, suggesting that the shape of mineral particles is determined by the proteins rather than by the physicochemical properties of the crystalline phase 105.
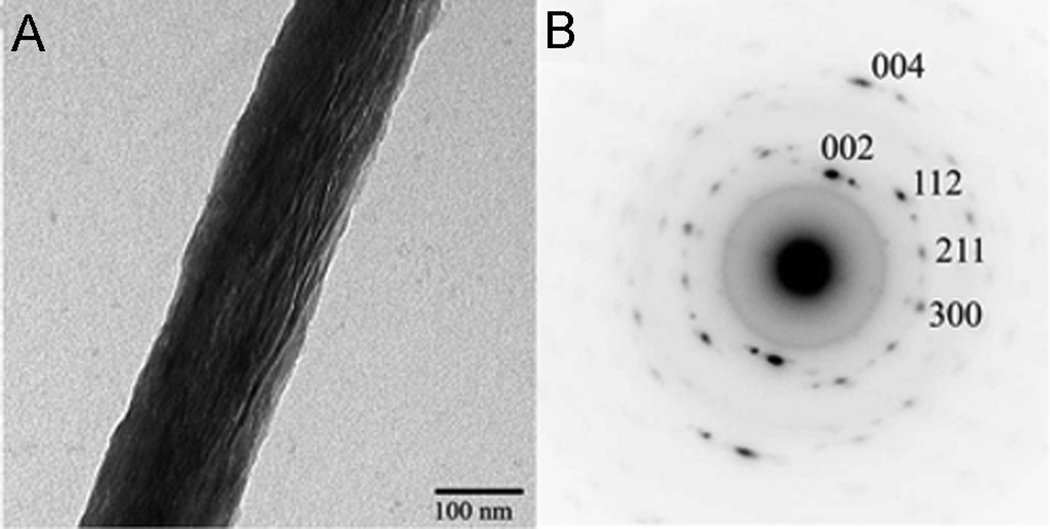
Figure 9.
A. TEM micrograph of collagen fibril mineralized in vitro in the presence of polyaspartic acid. Note that the mineral particles are aligned along the fibril axis. B. Electron diffraction pattern of the mineralized fibril in Figure 9A.; c-axes of the crystals are co-aligned with the fibril axis. The figure is reprinted with permission from reference 105.
Although acidic noncollagenous proteins are essential for the mineralization of the bone tissues, collagen fibrils also play very important role. While, it has been shown in a number of in vitro studies that collagen fibrils alone cannot induce organized calcium phosphate mineralization, after the initial mineral is formed they guide the organization and growth of mineral particles. The role of collagen in the mineralization is clearly demonstrated in the structural studies of bone tissues in osteogenesis imperfecta (OI) 165–167, a disease caused by mutations in the collagen type I genes, which lead to structural defects of the fibrils 168, 169. These studies demonstrate that the structural organization of mineral crystals in the OI bones is significantly altered; suggesting that the structural organization of collagen triple helices and fibrils is an essential prerequisite for their proper mineralization. It is not clear, however, if in collagen fibrils the mineral growth is controlled by physical constrains of triple helical molecules which delineate the spaces in which mineral particles can grow or chemical interactions between collagen molecules and mineral particles can play a role in this process.
Models of collagen mineralization in the bone tissues
Currently, there are several models of collagen mineralization, although none of them can fully explain all the information about this process accumulated up to date.
According to one theory the acidic noncollagenous proteins specifically bind to collagen molecules in the gap region and induce crystal nucleation inside the collagen fibrils 39 (Figure 10A). This theory is supported by the fact that the initial mineral is deposited in the gap regions and that many noncollagenous acidic molecules bind specifically to collagen in this region 85, 131, 132. Furthermore, chains of nanometer size mineral nuclei were reported in the mineralizing collagen fibrils of dentin 170, 171. These initial nuclei fuse together to form elongated initial crystals, with their c-axes aligned with the long axes of the fibrils, which suggest some sort of epitaxial relationships between noncollagenous acidic proteins bound to the collagen fibrils and forming mineral. At the same time this hypothesis does not account for the presence of transient ACP in bone tissues.
Figure 10.
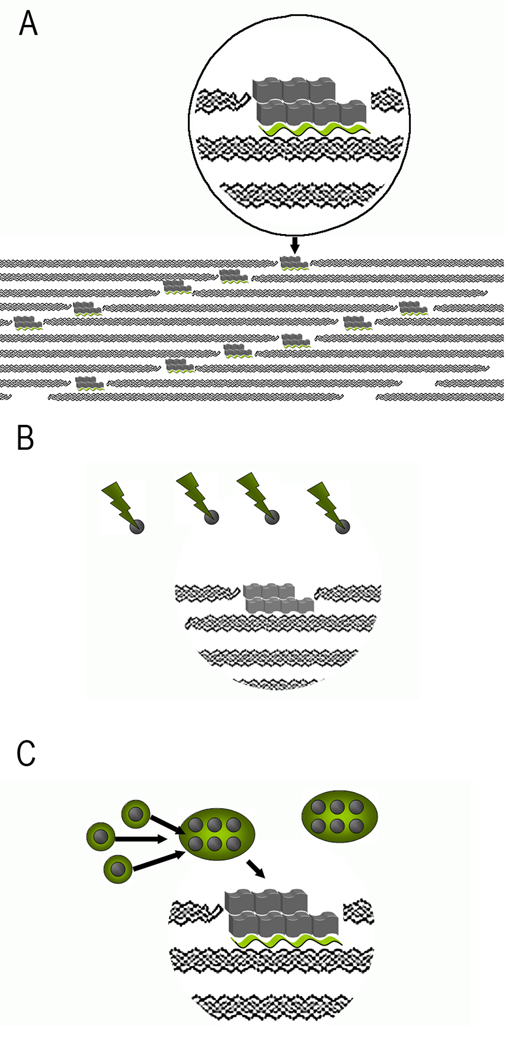
Models of collagen mineralization. A. Templating of mineral crystals, represented by gray blocks by acidic noncollagenous macromolecules (green wavy ribbons) bound to collagen molecules in the gap region. B. Size exclusion model. Large acidic proteins (green lightening bolts) prevent mineral nucleation (nuclei represented by grey spheres) outside of the fibrils. However, the proteins are too big to fit into the gap regions of the fibrils, where mineral crystals start to grow. C. Protein assemblies (green circles) stabilize the mineral nuclei outside of the fibrils. Upon binding to collagen molecules in the fibril proteins change their conformation, leading to the templated nucleation of mineral crystals in the gap regions.
Another, so called size exclusion model suggests that large acidic non-collagenous molecules inhibit mineralization outside of the collagen fibrils, however their large size prevents them from penetrating into the gap regions of the collagen fibrils 172 (Figure 10B). In this situation the crystal growth is inhibited everywhere except inside the collagen fibril, leading to initial mineral precipitation in the gap regions of the fibrils. Although this is a very straightforward elegant hypothesis it does not explain the fact that the crystallites in the fibril are all co-aligned. Furthermore, this model cannot explain the presence of extrafibrillar crystallites, which comprise a large fraction of mineral in bone tissues 67, 68.
Another recently developed model suggests that mineral-protein complexes can form outside the fibrils and then diffuse inside where they transform into the organized crystalline arrays guided by the collagen structure 111, 121. This model proposes that the initial mineral formed is transient amorphous calcium phosphate stabilized in supramolecular assemblies of noncollagenous acidic proteins. These aggregates bind to collagen fibrils, which leads to the transformation of amorphous to crystalline phase. It is possible that the noncollagenous proteins in these protein-mineral aggregates interact with collagen molecules. Such interactions can affect the protein conformation, leading to changes its function from stabilizing ACP to control of crystal nucleation (Figure 10C). A variation of this hypothesis was proposed 66, 173, in which a liquid protein-ACP phase infiltrates the collagen fibrils via capillary forces, filling the gap regions and the spaces between the triple helices and then transforms into oriented crystals. In this case it was proposed that the collagen molecules themselves guide the oriented crystal nucleation. However, the later hypothesis is unable to account for the fact that the mineral particles in vivo first form inside the gap region of collagen fibrils and then slowly propagate into the overlap 49, 59, 60, 174.
Most likely the collagen mineralization process involves a combination of aforementioned mechanisms, as our recent study of collagen mineralization using polyaspartic acid as a mimic of the acidic noncollagenous proteins suggests 105. Aspartic acid is the major amino acid of the noncollagenous acidic proteins and has been used as their mimic in in vitro mineralization studies. As many noncollagenous acidic proteins it strongly binds to calcium phosphate mineral crystals, affects crystal growth kinetics and stabilizes ACP 137, 175. Our in vitro mineralization experiments demonstrate that at high polyaspartic acid concentrations at which spontaneous mineral precipitation is completely inhibited, collagen fibrils were mineralized 105. These fibrils contained plate or ribbon-shaped crystallites organized into parallel arrays with their c-axes aligned with the fibril axis (Figure 9); the organization which closely resembles the mineralized collagen fibrils of bone tissues. Interestingly, the initial mineral deposits formed in the fibrils were amorphous and transformed into crystals with time; however their shape and organization were identical to the crystals found in the mature mineralized fibrils. The results of this study can be explained using a combination of different models of collagen mineralization. The fact that mineralization was effectively inhibited outside of the collagen fibrils suggests that the mechanisms outlined in the size exclusion model 176 are at work here. At the same time polyaspartic acid is too small to be effectively excluded from the fibrils 172. It is possible that polyaspartic acid binds to the collagen fibrils in the gap regions or at the fibril surface and induces mineral formation in these regions. The fact that the initial amorphous mineral particles in collagen fibrils had the same shape and organization as the mature crystals suggests that the mineral morphology is determined primarily by the matrix molecules. It cannot be ruled out that the collagen molecules in the fibrils influence the mineral particles morphology and organization. However, since the collagen fibrils alone cannot initiate organized calcium phosphate mineralization 103–105, 151 it is likely that the polyaspatate-collagen complexes regulate mineral formation, morphology and organization.
Mechanical properties of bone tissues at different hierarchical levels
As it was mentioned earlier bone tissues are hierarchical organo-mineral composite nanomaterials. Bones are made of weak individual components yet their mechanical properties are orders of magnitude better than a simple sum of their individual components and extremely well adopted to their function 177. These unique functional properties of the bone tissues at the macroscale are determined by their organization, composition and spatial distribution of its elements at the atomic, nano-, meso- and micro-scales 10. Following is a brief review of the mechanical properties of bone tissues at different hierarchical levels.
One of the major parameters determining the mechanical properties of bone tissues is mineral to organic ratio, or mineral content, in these tissues. As it was clearly demonstrated by John Currey, who has tested dozens of bone types, the mineral content is directly related to the materials stiffness and reversely related to its strain 178. Such relationships are quite typical for composite materials. At the same time the mechanical properties of the bone tissues are not determined solely by their composition. For example, artificial collagen/calcium phosphate composites, with the mineral to organic ratio similar to bone, are 10 times less stiff and have significantly lower strength values than bone 179. It has been also shown that the ultimate strength of the bone tissues does not show such a profound correlation with the mineral content as the Young’s modulus does, although there is a general trend for increase in strength with increase in mineralization 80, 178. Several factors contribute to these unique mechanical properties of bone tissues. In particular, due to extremely small, nanoscopic, size of the basic elements comprising bone nanocomposites there is a high level of integration of the mineral and organic phases at atomic and molecular levels. Other factors, such as structural characteristics of the basic elements and their hierarchical organization at different also contribute to the unique mechanical properties of bone tissues.
Basic elements
Mineral crystallites
As has been mentioned earlier the mineral phase of bone tissues consists of nonstoichiometric carbonated hydroxyapatite (dahlite). Both the total amount of carbonate and the type of substitution affect mechanical properties of the mineral 180, 181
The bone plate-like crystallites with average dimensions of ~50×30×3 nm are probably the smallest biogenic crystals 10, 50, 68. These small dimensions result in the unique mechanical behavior of these crystallites. Specifically, it has been calculated that the apatitic crystals of this size are extremely tolerant to flaws, and have strength of a perfect crystal 182. Furthermore, the extremely high surface to bulk ratio of these crystallites increases their chemical reactivity and the potential for interactions with the organic matrix components at the molecular level 183.
Interestingly the values of the compressive elastic modulus of the crysatallites in bone was reported to be around ~40 GPa 184, which is significantly lower than the modulus of synthetic or geological apatites which varies between 100 and 120 GPa 185, 186. Another important property of the bone crystallites is their mechanical anisotropy reflecting the anisotropic structural properties of the crystals. Specifically it has been reported that the deformation of the crystallites under load in different crystallographic directions is different 184, 187.
Collagen fibrils
Collagen fibrils greatly influence the functional properties of the bone tissues. For example, degradation of the bone collagen matrix by radiation dramatically decreases the strength and work to fracture of the specimens 188, 189. Furthermore in osteogenesis imperfecta, a disease caused by mutations in collagen type I genes which lead to abnormalities in the structure of the triple helices, both structural and mechanical properties of bones are severely compromised 165, 166.
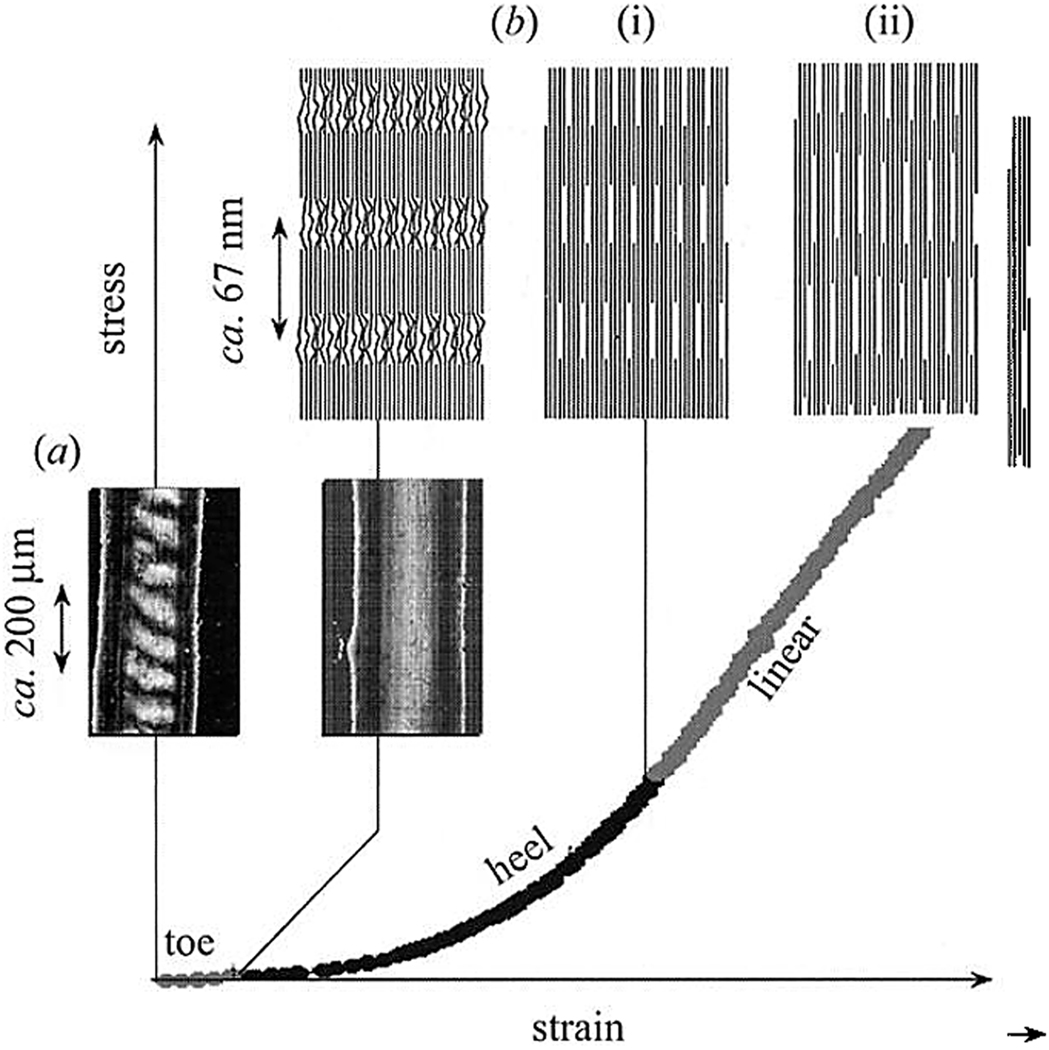
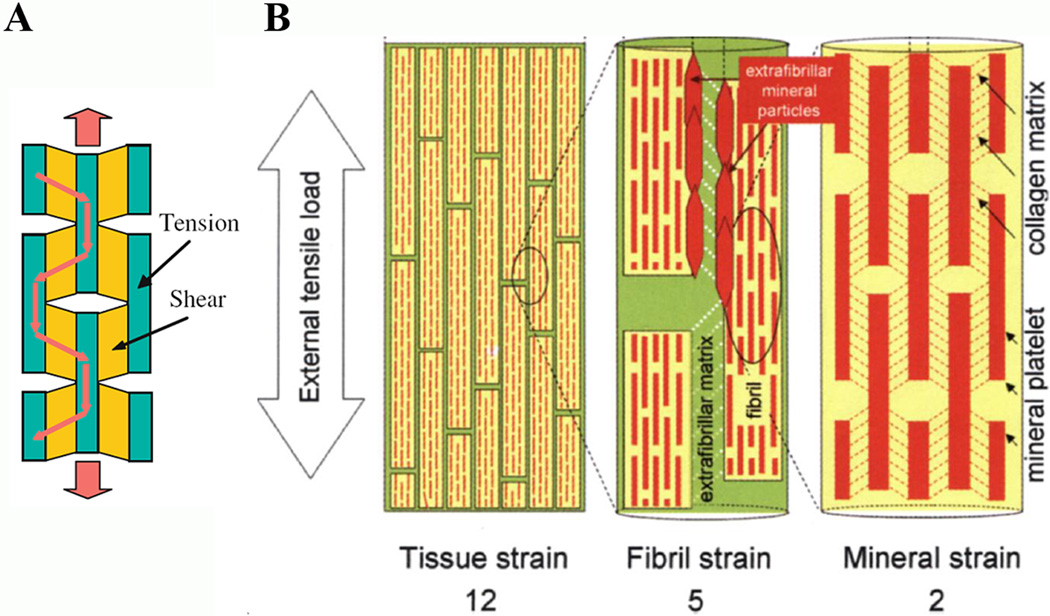
The collagen fibrils are highly anisotropic and mechanical properties of the tissues to a large extent depend on the organization of the collagen fibrils on the meso and microscale. However in some cases where the collagen fibrils are co-aligned, such as in tendons, it is possible to study their mechanical behavior at different scales starting from the nanoscale. In tension, the stress-strain curve of the tendons can be divided into several phases corresponding to different mechanisms of the deformation 190, 191 (Figure 11). Initial phase, so called toe region, is characterized by large tissue strain at very low stresses applied and corresponds to the straightening of the macroscopic crimp in the tissue (Figure 11a). At the next phase, represented by the hill region of the stress-strain curve, the stress needed to induce an equal amount of strain increases exponentially (Figure 11b(i)). This phase involves lateral ordering of the triple helical collagen molecules in the fibrils. Such rearrangement might involve breaking and reestablishing noncovalent hydrophobic and hydrogen bonds between collagen molecules. Finally, the linear region of the stress-strain curve represents sheering or sliding of the collagen molecules inside the fibrils, as represented by the elongation of the D-period (Figure 11b(ii)). Such process would involve breaking of covalent cross-links between the neighboring collagen molecules in addition to the rearrangement of noncovalent hydrogen and hydrophobic bonds, which would require higher forces. This observation is further supported by the fact that in the tissues lacking intermolecular cross-links the shape of the stress-strain curve is different and the maximum stress is an order magnitude less than in the norm 191. Interestingly, the macroscopic strain in the linear region is much greater than the fibril strain, as determined by the increase in the D-spacing, indicating that the fibrils also move along each other in the viscous medium, composed of hydrated charged extrafibrillar macromolecules such as GAGs or glycoproteins 191. This example illustrates that the deformation in the collagenous tissues under load is determined by a number of mechanisms at several hierarchical levels from intermolecular interactions in the collagen fibrils at the nanoscale and interfibrillar mesoscale interactions to the rearrangement of the tissue at the macroscale. It has to be noted however that tendons have a unique structural organization with the majority of the fibrils co-aligned, which is not shared by the majority of collagenous tissue, and bone tissues in particular, which exhibit much more complicated fibrillar arrangements 10. It is also worth mentioning that the experiments described above were performed in tension, which is not the major form of load for the skeletal tissues such as bone. Nevertheless these studies provide a deep insight into the hierarchical mechanical behavior of the collagenous tissues which can be extrapolated to different types of tissues.
Figure 11.
Stress-strain curve of parallel arrays of collagen fibrils as found in tendons can be divided into several regions, corresponding to different strain mechanisms. (a) In the toe region, the strain is due to the straightening of the macroscopic crimp in the tissue. (b(i)) In the hill region the strain is due to the lateral alignment of the collagen molecules inside the fibrils. (b(ii)_) In the linear region the strain is due to the sliding of the collagen molecules along each other, in this region the D-spacing of collagen fibrils increases by up to 10%. The figure is reprinted with permission from reference 191.
Mechanical properties of mineralized collagen fibrils (Nanoscale)
It is a long standing believe that the mechanical behavior of organic phase in the mineralized collagen fibrils differs significantly from their non-mineralized counterparts 192. The mineral crystallites substitute water the spaces between collagen triple helices and establish intermolecular chemical interactions between organic and mineral phase. These interactions can occur between mineral particles and collagen molecules directly. A recent study of the collagen fibril organization suggests that by the juxtaposition of the neighboring molecules, local concentrations of negatively charged residues form along the fibril axis and such clusters can bind the mineral surfaces via ionic interactions 62. Alternatively, the mineral-organic interactions can be mediated by acidic noncollagenous proteins bound to the triple helices. Furthermore, the disordered hydrated surface layer of biogenic apatitic crystals 54, 56 can facilitate protein-mineral interactions. Such anchoring of the collagen molecules to the mineral crystallites should by itself stiffen the organic phase of the composite fibril as is observed in dehydrated collagenous tissues in which the labile hydrogen bonds with water are substituted by intermolecular bonds between neighboring collagen molecules 193. On the other hand the collagen molecules in the mineralized fibrils are physically “straightjacketed” between parallel mineral plates which further limits their flexibility in the lateral direction 192.
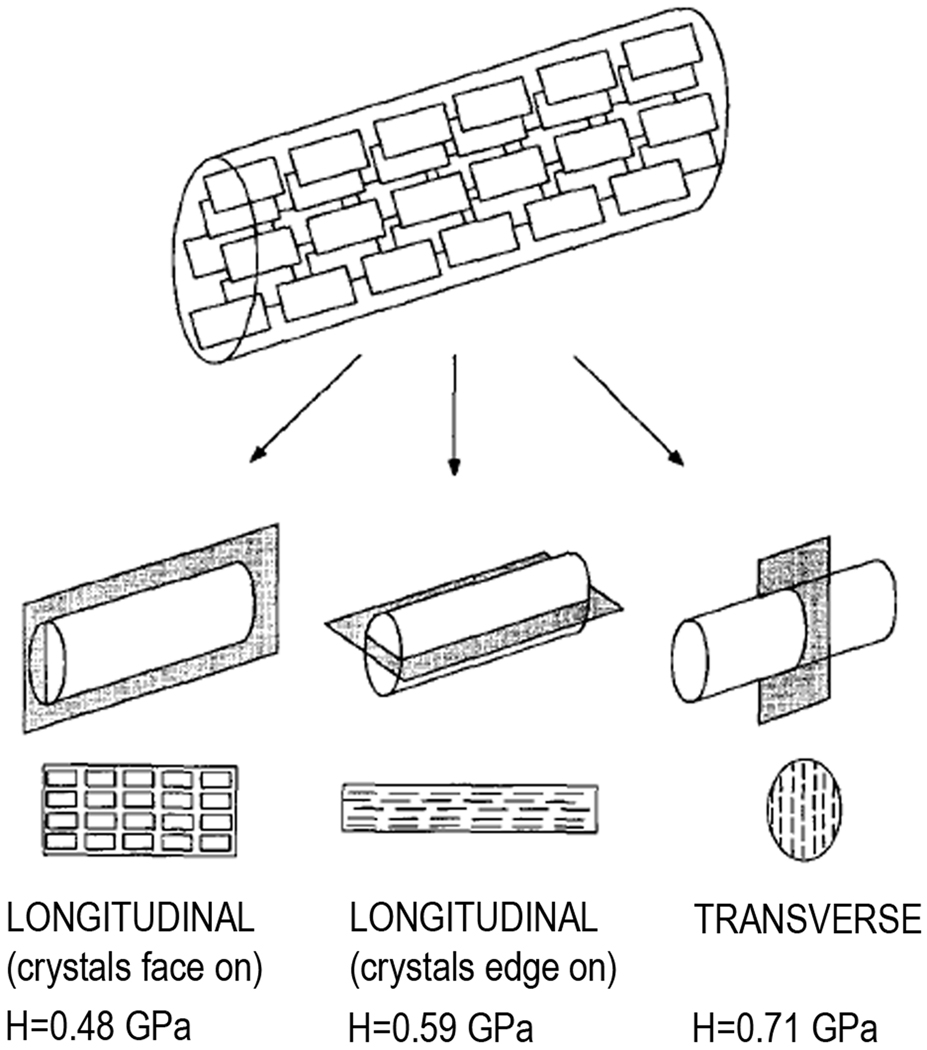
The structural anisotropy of mineralized collagen fibrils translates into the mechanical anisotropy. Although it is hard to measure mechanical properties of individual fibrils, use of bone types, comprised of large parallel fibrillar arrays allows us to assess the mechanical anisotropy of mineralized fibrils. The shaft of bovine femur is comprised of so called parallel-fibred bone, made of parallel arrays of mineralized collagen fibrils running along the bone axis. The microindentation testing of the bovine femur in the directions normal to transverse plane of the mineralized collagen fibrils, longitudinal plane of the fibrils with crystals edge-on and longitudinal plane of the fibrils with the crystals face-on revealed significant differences in the hardness values 76 (Figure 12). Specifically, the hardness values in the transverse plane were 0.706±0.088 GPa, followed by longitudinal, edge-on, 0.598±0.058 GPa with longitudinal, face-on being softest at 0.48±0.54 GPa. Although hardness is a complex property which cannot be directly linked to other mechanical properties via universal mathematical equations, for materials which belong to the same class, there is a direct correlation between hardness and elastic modulus values, hence higher hardness values of a bone sample will correspond to higher stiffness. The differences observed in this study can be explained by the relative contribution of mineral and organic phases to the mechanical properties of the fibrils in different orientations. In the longitudinal, crystals face-on plane, 2–4 nm thin crystallite plates are sandwiched between the layers of collagen molecules of similar thickness, with the organic matrix to a large degree dictating the mechanical properties of the material. In the longitudinal, edge-on and transverse planes the crystals are oriented edge-on, with small gaps between the crystals filled with organic material. Hence in these orientations mineral contributes much more to the mechanical properties of the tissue. Other factors, such as differences in stiffness of the mineral crystals in different crystallographic orientations 184 as well as the differences in Young’s modulus in transverse vs. longitudinal planes of the collagen fibrils 194 can also contribute to the mechanical anisotropy of the mineralized collagen fibrils.
Figure 12.
Mechanical anisotropy of the mineralized collagen fibrils is determined by their structural anisotropy. Reprinted with permission from reference 76.
In 2000 Jäger and Fratzl came up with a model of mechanical behavior of a single mineralized collagen fibril suggesting that the load is transferred between stiff mineral particles, which are primarily in tension, and the organic fraction of the fibrils, where the neighboring triple helical molecules slide along each other 195 (Figure 13A). Later experimental studies of bone samples, in tension, using a combination of Small Angle X-ray Scattering (SAXS) (to monitor collagen strain) and Wide Angle X-ray Scattering (WAXS) (to monitor crystal strain) provided further support for this model 184, 196. The results of these studies indicate that the strain of the organic fraction of the mineralized collagen fibril is significantly less than for the nonmineralized fibrils, with the ratio between the strains of collagen and mineral ~5:2 196 (Figure 13B). Considering the fact that the elastic modulus of collagen fibrils is between 1 and 2 GPa and the modulus of geological hydroxyapatite is more than 100 GPa, such ratio should be much greater. Hence, these results suggest a number of possible explanations. For example, the collagen fraction in the mineralized fibrils is much stiffer than in nonmineralized tissues; alternatively, the strain of the biogenic nanocrystals is higher than that of geological apatite. Indeed, the strain of the mineral in this study was shown to be twice of that of fracture strain of bulk apatite 196. Similar data were obtained in a study of dog femur samples in compression using similar approach, namely a combination of SAXS and WAXS techniques to separately measure strains in the collagen fibrils and mineral 184. The Young’s modulus of the collagen fibrils in this experiment was 18.3 ±1.2 GPa, which is 10 times higher, that the modulus of nonmineralized collagenous tissues, while the modulus of mineral was around 40 GPa, roughly 3 times less than bulk hydroxyapatite. The authors suggested that such high modulus of collagen fibrils is due to the fact that the triple helices are physically confined between the mineral platelets. At the same time the chemical interactions between collagen molecules and mineral crystallites might further decrease their ability to move. The low modulus of mineral nanocrystals was attributed to their extremely small size and plate-like morphology, with any atom in the lattice not further that a few dozens angstroms from the surface, leading to a much higher lattice strain and flexibility 184 183.
Figure 13.
Models of mechanical behavior of bone tissues at several hierarchical levels. A. The stress in the mineralized collagen fibrils is transferred from stiff mineral particles in tension to the softer organic matrix in shear mode. Reprinted with permission from reference 208 B. Model of bone deformation in tension at several hierarchical levels. In the fibrils the load is transferred between stiff mineral platelets deforming in tension and shearing collagen molecules. The load between adjacent stiff mineralized fibrils is transferred by shearing in the extrafibrillar matrix, containing noncollagenous macromolecules and extrafibrillar mineral. Reprinted with permission from reference 196.
Mechanical Properties of Fibrillar Arrays (Mesoscale)
At the mesoscale level of organization, presented by fibril arrays and bundles, the interactions between neighboring fibrils play the major role in determining the mechanical response of the tissue. The spaces between the fibrils contain extrafibrillar mineral crystallites and noncollagenous molecules, such as glycoproteins and glycosaminoglycans providing a viscoelastic medium isolating the collagen fibrils 191, 197. Tensile tests of parallel-fibrous bones, which are made of arrays of the mineralized collagen fibrils aligned along the axis of the bone, indicate that the fibrils slide along each other under load. The strain produced by this interfibrillar shear is 6 times greater than the mineral strain and more than 2 times greater than the strain in collagen fibrils 196, 197 (Figure 13B). Hence in the bone tissues there is a hierarchical increase in strain from nano- to meso- scale, where the stiff nanostructures are bound by a softer matrix at the mesocale.
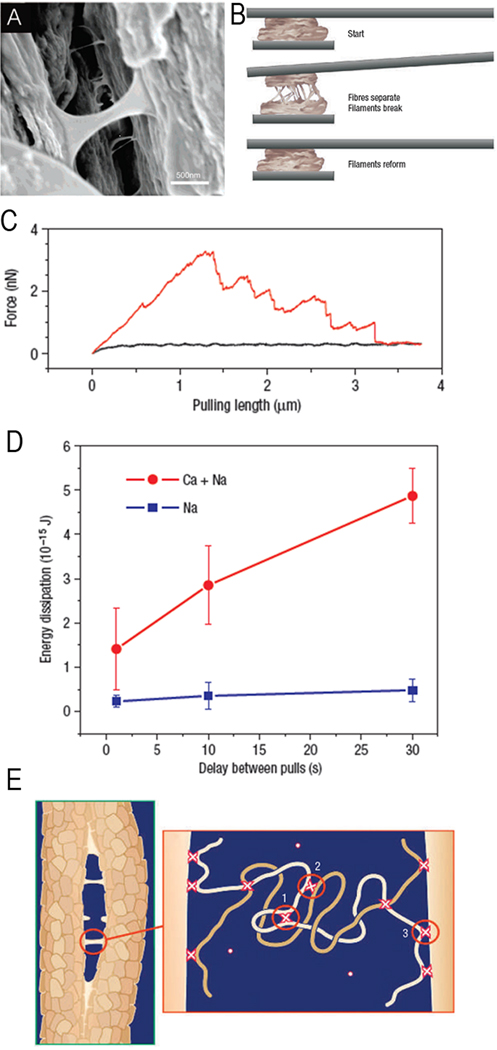
The extrafibrillar component in the bone tissues plays an extremely important mechanical role. The noncollagenous acidic proteins in the interfibrillar space form a supra-molecular network based on the ionic cross-links between their acidic side chains and calcium ions in solution, as well as the interactions between these acidic moieties and calcium ions at the surfaces of mineral crystal coating the fibrils 18, 127, 198, 199 (Figure 14). Such a network held together by reversible, sacrificial ionic bonds can dissipate large amounts of energy under mechanical stress and then reform quickly when the load is off. It is believed that these supramolecular networks can hold the collagen fibrils together and contribute to the fracture resilience of the bone tissues. The arrays of stiff rods, such as the mineralized collagen fibrils, glued together by supramolecular networks leads to the material with the unique combination of stiffness and toughness 3. Futhermore, such design is highly tunable and even small changes in the mineral content inside and outside of the fibrils and in the chemical make up of the interfibrillar supramolecular glue can significantly alter the mechanical properties of the tissue.
Figure 14.
Extrafibrillar organic matrix in bone tissues forms supramolecular networks based on the reversible sacrificial ionic bonds with calcium ions. A. SEM micrograph of a crack in the bone tissue, showing filaments formed by the extrafibrillar macromolecules. B. The experimental set up for measuring the forces in the extrafibrillar matrix. A piece of bone attached to the AFM cantilever is pressed against another piece of bone. When pulled apart the forces holding these two pieces of bone together can be measured. C. The representative pulling curve; large amounts of energy (the area under the curve) required to break two bone pieces apart. D. In the medium containing only monovalent ions (Na+) the amount of energy dissipated is much lower than in the medium containing polyvalent ions (Ca2+) E. The model showing different types of sacrificial bonds in the extrafibrillar matrix: 1) intramolecular bonds; 2) intermolecular bonds 3) protein-mineral bonds. Reprinted with permission from 18.
Arrangements of the fibril arrays (Microscopic level)
There are several types of different arrangements of the fibrillar arrays, uniquely adapted to their function. One is a parallel-lamellar bone in which all the fibrils are co-aligned has been discussed to some extent in the preceding paragraphs. Another one found in the woven bone, consists of randomly oriented mineralized fibrils or small bundles of the fibrils. This type is found primarily during embryonic development and as the initial bone type in the repair sites and is eventually substituted by lamellar bone. Another specialized bone tissue type, dentin is comprised of layers of collagen fibrils randomly oriented in the plane of the layer. Here we will only focus on the most sophisticated and most prominent human bone type- lamellar bone and the osteonal structure as a specialized variation of this bone type.
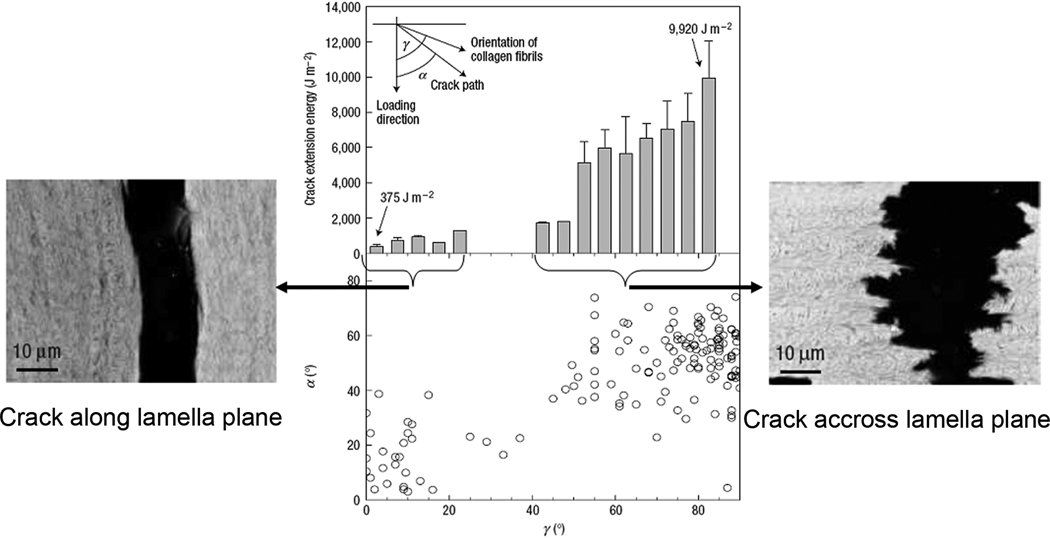
In the lamellar bone, few micron layers of co-aligned fibrils are arranged such that the directions of the fibrils in the neighboring layers are rotated in respect to each other, forming a micro-laminate composite, so called rotated plywood structure 4. Furthermore, it has been reported that the individual lamellae are made of sublayers which are also rotated in respect to each other 78, 80. The resulting complex hierarchical structure is extremely crack resistant in the functionally relevant direction- normal to the lamellae plans, with relatively low resistance along the lamellae 200 (Figure 15). The mechanical properties of bulk lamellar bone (compact circumferential bone) and osteonal bone, made of microscopic cylindrical arrangements of lamellar bone, for the most part are similar 201. The major difference in the mechanical behavior of these two tissues is that after fracture the pieces of osteonal bone remain connected while in the bulk lamellar bone samples the pieces would completely separate. This difference in the facture behavior might be beneficial for the fracture healing process in osteonal bone 201.
Figure 15.
In the lamellar bone the energy required for crack to propagate along the lamellae (left side) is much lower than across the lamellae (right side). Reprinted with permission from 40.
Osteonal bone (Tissue Level)
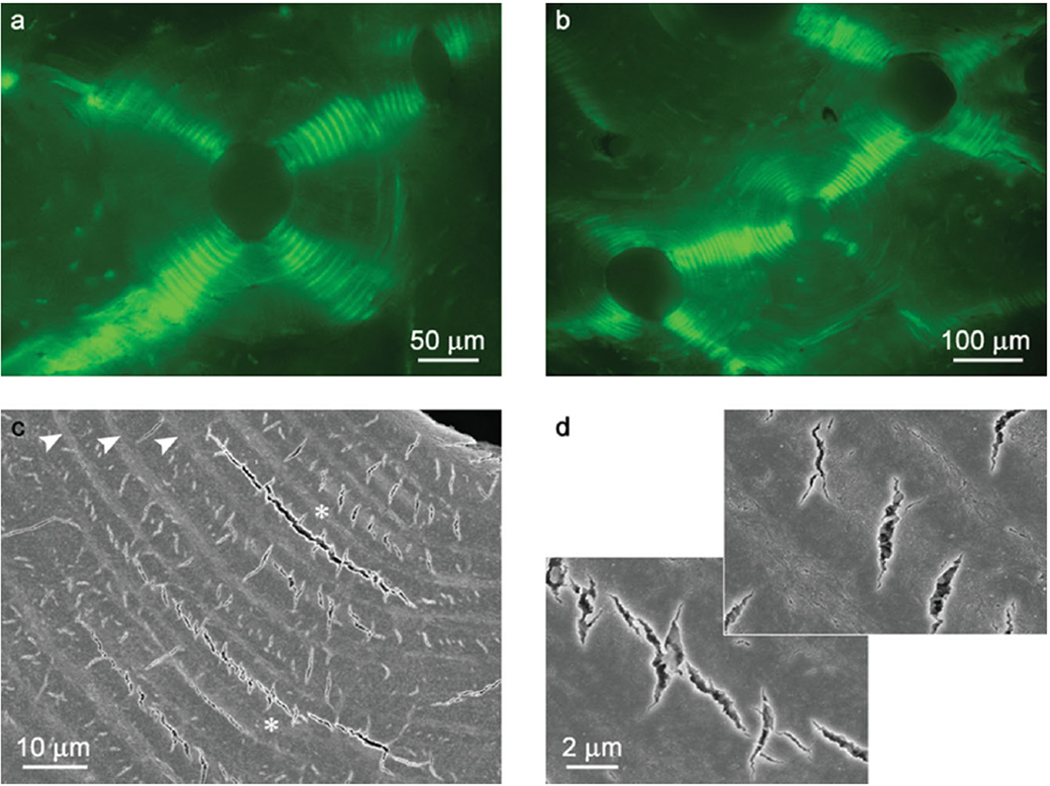
As has been mentioned above osteonal bone is made of cylindrical lamellar motifs, osteons, embedded in circumferential lamellar bone. The number of osteons increases with age, resulting in a tissue completely filled with osteons, which are in direct contact with each other. The studies of osteonal bone in compression, revealed a unique crack pattern, consistent of stacks of ark-shaped circumferential microcracks propagating radially from the centre of each osteon 202. At higher loads these radial crack concentrations in neighboring osteons connect, forming network of cracks throughout the tissue, and redistributing the stress such that its vector on the tissue scale is always normal to the lamellae planes (Figure 16A,B). High magnification observations reveal that the microcracking pattern consists of less then a few micron short radial cracks confined to one or two lamellae and longer circumferential cracks formed by delamination of the neighboring lamellae (Figure 16C,D), suggesting that a combination of ligament bridging and deflection toughening mechanisms are in play here. The combinations of short radial and circumferential microcracks dissipate large amounts of energy and prevent the catastrophic failure. Furthermore, this unique type of microcracking allows the material to maintain its high strength and resilience even in the inelastic regime of deformation. This example illustrates how the hierarchical organization of osteonal bone determines its unique mechanical resilience.
Figure 16.
Crack propagation in the osteonal bone under compression a) Epi-fluorescence image showing four groups of arc-shaped circumferential microcracks (bright green) arranged in the quasiorthogonal pattern; b) Epi-fluorescence image showing the crack propagation across neighboring osteons. The cracking of the central osteon is transfered to the osteons to the lower left and upper right; c) SEM micrograph showing arc-shaped microcracks. d) Closer observations of (c) (asterisks) showing the short micro-radial cracks in the thick lamellae and a circumferential microcrack. The figure is reprinted with permission from reference 202.
Concluding remarks
Although, bone tissues are the primary focus of this review, the principles of structural organization, mechanisms of formation and mechanical behavior described here are not unique to these tissues and are found in many other biomineralization systems. Similarly to bone other biominerals such as dental enamel, mollusk shells or sponge skeletons are hierarchical nanocomposites 1, 5, 203. As in bone, the hierarchical structure of these materials, as well as the nanoscopic size of their basic elements determine to a large extend their mechanical properties 19, 177, 203. Furthermore, a number of features of mineralization processes present in bone tissues are also found in other systems. For example, metastable, often amorphous, mineral phases are utilized in many biomineralization processes 11, 25, 204, 205. Similarly, acidic proteins are found in a broad range of biominerals 112, 145, 206. The fact that many of the features of bone tissues are found in other biomineralization systems throughout the animal kingdom suggest that there are some universal principles of synthesis and structural organization of biominerals. Such strategies of materials design, found in nature, can be utilized for the development of novel bioinspired materials 207 and our continuing quest for the understanding of basic principles guiding formation, structural organization and functional properties of biominerals without any doubt will uncover novel mechanisms, further advancing the field of bioinspired materials design.
Acknowledgements
The author is grateful for funding from NIH/NIDCR (R01 DE016376) which supports his work in this area.
References
- 1.Aizenberg J, Weaver JC, Thanawala MS, Sundar VC, Morse DE, Fratzl P. Science. 2005;309(5732):275–278. doi: 10.1126/science.1112255. [DOI] [PubMed] [Google Scholar]
- 2.Fratzl P, Gupta HS, Paschalis EP, Roschger P. J. Mater. Chem. 2004;14(14):2115–2123. [Google Scholar]
- 3.Fratzl P, Weinkamer R. Prog. Mater. Sci. 2007;52(8):1263–1334. [Google Scholar]
- 4.Weiner S, Wagner HD. Annual Review of Materials Science. 1998;28:271–298. [Google Scholar]
- 5.Margolis HC, Beniash E, Fowler CE. J. Dent. Res. 2006;85(9):775–793. doi: 10.1177/154405910608500902. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert P, Abrecht M, Frazer BH. Molecular Geomicrobiology. Vol. 59. Chantilly: Mineralogical Soc America; 2005. The organic-mineral interface in biominerals; pp. 157–185. [Google Scholar]
- 7.Dove PM. Elements. 6(1):37–42. [Google Scholar]
- 8.Weiner S, Dove PM. An overview of biomineralization processes and the problem of the vital effect. In: Dove PM, DeYoreo JJ, Weiner S, editors. Biomineralization. Vol. 54. 2003. pp. 1–29. [Google Scholar]
- 9.Lowenstam HA. Science. 1981;211(4487):1126–1131. doi: 10.1126/science.7008198. [DOI] [PubMed] [Google Scholar]
- 10.Weiner S, Addadi L. J. Mater. Chem. 1997;7(5):689–702. [Google Scholar]
- 11.Aizenberg J, Weiner S, Addadi L. Connect. Tissue Res. 2003;44:20–25. [PubMed] [Google Scholar]
- 12.Belcher AM, Wu XH, Christensen RJ, Hansma PK, Stucky GD, Morse DE. Nature. 1996;381(6577):56–58. [Google Scholar]
- 13.Falini G, Albeck S, Weiner S, Addadi L. Science. 1996;271(5245):67–69. [Google Scholar]
- 14.Kwak SY, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Litman A, Margolis HC. J. Biol. Chem. 2009;284(28):18972–18979. doi: 10.1074/jbc.M109.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albeck S, Addadi L, Weiner S. Connect. Tissue Res. 1996;35(1–4):365–370. doi: 10.3109/03008209609029213. [DOI] [PubMed] [Google Scholar]
- 16.Moradian-Oldak J, Tan J, Fincham AG. Biopolymers. 1998;46(4):225–238. doi: 10.1002/(SICI)1097-0282(19981005)46:4<225::AID-BIP4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Beniash E, Simmer JP, Margolis HC. J. Struct. Biol. 2005;149(2):182–190. doi: 10.1016/j.jsb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GAG, Stucky GD, Morse DE, Hansma PK. Nat Mater. 2005;4(8):612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj D, Arola DD. Biomaterials. 2009;30(23–24):4037–4046. doi: 10.1016/j.biomaterials.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldassarri M, Margolis HC, Beniash E. J. Dent. Res. 2008;87(7):645–649. doi: 10.1177/154405910808700711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He G, Dahl T, Veis A, George A. Nat. Mater. 2003;2(8):552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 22.Weiner S, Addadi L. Trends in Biochemical Sciences. 1991;16:252–256. doi: 10.1016/0968-0004(91)90098-g. [DOI] [PubMed] [Google Scholar]
- 23.Colfen H, Mann S. Angew. Chem.-Int. Edit. 2003;42(21):2350–2365. doi: 10.1002/anie.200200562. [DOI] [PubMed] [Google Scholar]
- 24.Beniash E, Aizenberg J, Addadi L, Weiner S. Proc. R. Soc. B. 1997;264(1380):461–465. [Google Scholar]
- 25.Beniash E, Metzler RA, Lam RSK, Gilbert P. J. Struct. Biol. 2009;166(2):133–143. doi: 10.1016/j.jsb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahamid J, Sharir A, Addadi L, Weiner S. Proc. Natl. Acad. Sci. U. S. A. 2008;105(35):12748–12753. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner S. J. Struct. Biol. 2008;163(3):229–234. doi: 10.1016/j.jsb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Weiner S, Sagi I, Addadi L. Science. 2005;309(5737):1027–1028. doi: 10.1126/science.1114920. [DOI] [PubMed] [Google Scholar]
- 29.Antonietti M, Breulmann M, Goltner CG, Colfen H, Wong KKW, Walsh D, Mann S. Chem. Eur. J. 1998;4(12):2493–2500. [Google Scholar]
- 30.Green D, Walsh D, Mann S, Oreffo ROC. Bone. 2002;30(6):810–815. doi: 10.1016/s8756-3282(02)00727-5. [DOI] [PubMed] [Google Scholar]
- 31.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 32.Heuer AH, Fink DJ, Laraia VJ, Arias JL, Calvert PD, Kendall K, Messing GL, Blackwell J, Rieke PC, Thompson DH, Wheeler AP, Veis A, Caplan AI. Science. 1992;255(5048):1098–1105. doi: 10.1126/science.1546311. [DOI] [PubMed] [Google Scholar]
- 33.Mann S, Ozin GA. Nature. 1996;382(6589):313–318. [Google Scholar]
- 34.Huo QS, Margolese DI, Ciesla U, Feng PY, Gier TE, Sieger P, Leon R, Petroff PM, Schuth F, Stucky GD. Nature. 1994;368(6469):317–321. [Google Scholar]
- 35.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Chem. Rev. 2008;108(11):4754–4783. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espinosa HD, Rim JE, Barthelat F, Buehler MJ. Prog. Mater. Sci. 2009;54(8):1059–1100. [Google Scholar]
- 37.Liao SS, Cui FZ, Zhang W, Feng QL. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2004;69B(2):158–165. doi: 10.1002/jbm.b.20035. [DOI] [PubMed] [Google Scholar]
- 38.Tan J, Saltzman WM. Biomaterials. 2004;25(17):3593–3601. doi: 10.1016/j.biomaterials.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 39.Veis A. Reviews in Mineralogy and Geochemistry. 2003;54(1):249–289. [Google Scholar]
- 40.Fratzl P. Nat Mater. 2008;7(8):610–612. doi: 10.1038/nmat2240. [DOI] [PubMed] [Google Scholar]
- 41.Prockop DJ, Fertala A. J. Struct. Biol. 1998;122(1–2):111–118. doi: 10.1006/jsbi.1998.3976. [DOI] [PubMed] [Google Scholar]
- 42.Brodsky B, Ramshaw JAM. Matrix Biol. 1997;15(8–9):545–554. doi: 10.1016/s0945-053x(97)90030-5. [DOI] [PubMed] [Google Scholar]
- 43.Brodsky B, Shah NK. Faseb Journal. 1995;9(15):1537–1546. doi: 10.1096/fasebj.9.15.8529832. [DOI] [PubMed] [Google Scholar]
- 44.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Biochem. J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuznetsova N, Leikin S. J. Biol. Chem. 1999;274(51):36083–36088. doi: 10.1074/jbc.274.51.36083. [DOI] [PubMed] [Google Scholar]
- 46.Hulmes DJS. J. Struct. Biol. 2002;137(1–2):2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 47.Chapman JA. Connect. Tissue Res. 1974;2(2):137–150. doi: 10.3109/03008207409152099. [DOI] [PubMed] [Google Scholar]
- 48.Ou-Yang H, Paschalis EP, Mayo WE, Boskey AL, Mendelsohn R. J. Bone Miner. Res. 2001;16(5):893–900. doi: 10.1359/jbmr.2001.16.5.893. [DOI] [PubMed] [Google Scholar]
- 49.Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. Microsc. Res. Tech. 1996;33(2):192–202. doi: 10.1002/(SICI)1097-0029(19960201)33:2<192::AID-JEMT9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 50.Ziv V, Weiner S. Connect. Tissue Res. 1994;30(3):165–175. doi: 10.3109/03008209409061969. [DOI] [PubMed] [Google Scholar]
- 51.Grabner B, Landis WJ, Roschger P, Rinnerthaler S, Peterlik H, Klaushofer K, Fratzl P. Bone. 2001;29(5):453–457. doi: 10.1016/s8756-3282(01)00594-4. [DOI] [PubMed] [Google Scholar]
- 52.Tesch W, Eidelman N, Roschger P, Goldenberg F, Klaushofer K, Fratzl P. Calcif Tissue Int. 2001;69(3):147–157. doi: 10.1007/s00223-001-2012-z. [DOI] [PubMed] [Google Scholar]
- 53.Camacho NP, Rinnerthaler S, Paschalis EP, Mendelsohn R, Boskey AL, Fratzl P. Bone. 1999;25(3):287–293. doi: 10.1016/s8756-3282(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 54.Huang S-J, Tsai Y-L, Lee Y-L, Lin C-P, Chan JCC. Chemistry of Materials. 2009;21(13):2583–2585. [Google Scholar]
- 55.Christian J, Thea W, Wolfgang M-Z, Matthias E. Magnetic Resonance in Chemistry. 2006;44(6):573–580. doi: 10.1002/mrc.1774. [DOI] [PubMed] [Google Scholar]
- 56.Cazalbou S, Combes C, Eichert D, Rey C, Glimcher MJ. J. Bone Miner. Metab. 2004;22(4):310–317. doi: 10.1007/s00774-004-0488-0. [DOI] [PubMed] [Google Scholar]
- 57.Tang R, Wang LAOC, Bonstein T, Bush Pj, Nancollas GH. Angewandte Chemie International Edition. 2004;43(20):2697–2701. doi: 10.1002/anie.200353652. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Nancollas GH, Henneman ZJ, Klein E, Weiner S. Biointerphases. 2006;1(3):106–111. doi: 10.1116/1.2354575. [DOI] [PubMed] [Google Scholar]
- 59.Traub W, Arad T, Weiner S. Proc. Natl. Acad. Sci. U. S. A. 1989;86(24):9822–9826. doi: 10.1073/pnas.86.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiner S, Traub W. Faseb Journal. 1992;6(3):879–885. [PubMed] [Google Scholar]
- 61.Su X, Sun K, Cui FZ, Landis WJ. Bone. 2003;32(2):150–162. doi: 10.1016/s8756-3282(02)00945-6. [DOI] [PubMed] [Google Scholar]
- 62.Landis WJ, Silver FH. Cells Tissues Organs. 2009;189(1–4):20–24. doi: 10.1159/000151454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sang-Hoon R, Jae Do L, Junzo T. Journal of the American Ceramic Society. 2000;83(11):2890–2892. [Google Scholar]
- 64.Elangovan S, Margolis HC, Oppenheim FG, Beniash E. Langmuir. 2007;23(22):11200–11205. doi: 10.1021/la7013978. [DOI] [PubMed] [Google Scholar]
- 65.Johnston N, Krimm S. Biopolymers. 1971;10(12):2597–2605. doi: 10.1002/bip.360101219. [DOI] [PubMed] [Google Scholar]
- 66.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, Douglas EP, Gower LB. Materials Science and Engineering: R: Reports. 2007;58(3–5):77–116. [Google Scholar]
- 67.Bonar LC, Lees S, Mook HA. J. Mol. Biol. 1985;181(2):265–270. doi: 10.1016/0022-2836(85)90090-7. [DOI] [PubMed] [Google Scholar]
- 68.Landis WJ, Hodgens KJ, Song MJ, Arena J, Kiyonaga S, Marko M, Owen C, McEwen BF. J. Struct. Biol. 1996;117(1):24–35. doi: 10.1006/jsbi.1996.0066. [DOI] [PubMed] [Google Scholar]
- 69.Prostak KS, Lees S. Calcif. Tissue Int. 1996;59(6):474–479. doi: 10.1007/BF00369213. [DOI] [PubMed] [Google Scholar]
- 70.Lees S, Prostak KS, Ingle VK, Kjoller K. Calcif. Tissue Int. 1994;55(3):180–189. doi: 10.1007/BF00425873. [DOI] [PubMed] [Google Scholar]
- 71.Kinney JH, Pople JA, Driessen CH, Breunig TM, Marshall GW, Marshall SJ. J. Dent. Res. 2001;80(6):1555–1559. doi: 10.1177/00220345010800061501. [DOI] [PubMed] [Google Scholar]
- 72.Tong W, Glimcher MJ, Katz JL, Kuhn L, Eppell SJ. Calcif. Tissue Int. 2003;72(5):592–598. doi: 10.1007/s00223-002-1077-7. [DOI] [PubMed] [Google Scholar]
- 73.Su XW, Feng QL, Cui FZ, Zhu XD. Connect. Tissue Res. 1997;36(3):271–286. doi: 10.3109/03008209709160227. [DOI] [PubMed] [Google Scholar]
- 74.Berglundh T, Abrahamsson I, Lang NP, Lindhe J. Clin. Oral Implants Res. 2003;14(3):251–262. doi: 10.1034/j.1600-0501.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 75.Lacroix D, Prendergast PJ. J. Biomech. 2002;35(9):1163–1171. doi: 10.1016/s0021-9290(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 76.Ziv V, Wagner HD, Weiner S. Bone. 1996;18(5):417–428. doi: 10.1016/8756-3282(96)00049-x. [DOI] [PubMed] [Google Scholar]
- 77.Landis WJ, Silver FH. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology. 2002;133(4):1135–1157. doi: 10.1016/s1095-6433(02)00248-9. [DOI] [PubMed] [Google Scholar]
- 78.Weiner S, Traub W, Wagner HD. J. Struct. Biol. 1999;126(3):241–255. doi: 10.1006/jsbi.1999.4107. [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto T, Domon T, Takahashi S, Islam N, Suzuki R. Anat. Embryol. (Berl) 2000;202(1):25–30. doi: 10.1007/pl00008241. [DOI] [PubMed] [Google Scholar]
- 80.Weiner S, Arad T, Sabanay I, Traub W. Bone. 1997;20(6):509–514. doi: 10.1016/s8756-3282(97)00053-7. [DOI] [PubMed] [Google Scholar]
- 81.Qin C, Baba O, Butler WT. Critical Reviews in Oral Biology & Medicine. 2004;15(3):126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 82.Mackie EJ. Int. J. Biochem. Cell Biol. 2003;35(9):1301–1305. doi: 10.1016/s1357-2725(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 83.Nanci A. Ten Cate's Oral Histology: Development, Structure, and function. 7th ed. St. Luis: Mosby; 2008. p. 411. [Google Scholar]
- 84.Dimuzio MT, Veis A. J. Biol. Chem. 1978;253(19):6845–6852. [PubMed] [Google Scholar]
- 85.Beniash E, Traub W, Veis A, Weiner S. J. Struct. Biol. 2000;132(3):212–225. doi: 10.1006/jsbi.2000.4320. [DOI] [PubMed] [Google Scholar]
- 86.Goldberg M, Rapoport O, Septier D, Palmier K, Hall R, Embery G, Young M, Ameye L. Connect. Tissue Res. 2003;44:184–188. [PubMed] [Google Scholar]
- 87.Septier D, Hall RC, Lloyd D, Embery G, Goldberg M. Histochem. J. 1998;30(4):275–284. doi: 10.1023/a:1003216024158. [DOI] [PubMed] [Google Scholar]
- 88.Weinstock M, Leblond CP. J. Cell Biol. 1974;60(1):92–127. doi: 10.1083/jcb.60.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ameye L, Aria D, Jepsen K, Oldberg AKE, Xu T, Young MF. FASEB J. 2002;16(7):673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 90.Ameye L, Young MF. Glycobiology. 2002;12(9):107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 91.Embery G, Hall R, Waddington R, Septier D, Goldberg M. Critical Reviews in Oral Biology & Medicine. 2001;12(4):331–349. doi: 10.1177/10454411010120040401. [DOI] [PubMed] [Google Scholar]
- 92.Haruyama N, Sreenath TL, Suzuki S, Yao X, Wang Z, Wang Y, Honeycutt C, Iozzo RV, Young MF, Kulkarni AB. Matrix Biol. 2009;28(3):129–136. doi: 10.1016/j.matbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro S, Iozzo RV. J. Biol. Chem. 2000;275(29):21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 94.Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Cell. Mol. Life Sci. 2000;57(5):859–863. doi: 10.1007/s000180050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weber IT, Harrison RW, Iozzo RV. J. Biol. Chem. 1996;271(50):31767–31770. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 96.Milan AM, Sugars RV, Embery G, Waddington RJ. Calcif. Tissue Int. 2005;76(2):127–135. doi: 10.1007/s00223-004-0033-0. [DOI] [PubMed] [Google Scholar]
- 97.Plate U, Hohling HJ, Reimer L, Barckhaus RH, Wienecke R, Wiesmann HP, Boyde A. J. Microsc. 1992;166:329–341. doi: 10.1111/j.1365-2818.1992.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 98.Dallas SL, Veno PA, Rosser JL, Barragan-Adjemian C, Rowe DW, Kalajzic I, Bonewald LF. Cells Tissues Organs. 2009;189(1–4):6–11. doi: 10.1159/000151745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irie K, Ejiri S, Sakakura Y, Shibui T, Yajima T. J. Histochem. Cytochem. 2008;56(6):561–567. doi: 10.1369/jhc.2008.950527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li C, Siegel S, Paris O, Fratzl P, Weiner S, Addadi L. Proceedings of the National Academy of Sciences. 2010;107(14):6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Landis WJ, Glimcher MJ. J. Ultrastruct. Res. 1982;78(3):227–268. doi: 10.1016/s0022-5320(82)80001-4. [DOI] [PubMed] [Google Scholar]
- 102.Wu LNY, Genge BR, Dunkelberger DG, LeGeros RZ, Concannon B, Wuthier RE. J. Biol. Chem. 1997;272(7):4404–4411. doi: 10.1074/jbc.272.7.4404. [DOI] [PubMed] [Google Scholar]
- 103.Saito T, Arsenault AL, Yamauchi M, Kuboki Y, Crenshaw MA. Bone. 1997;21(4):305–311. doi: 10.1016/s8756-3282(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 104.Hunter GK, Poitras MS, Underhill TM, Grynpas MD, Goldberg HA. J. Biomed. Mater. Res. 2001;55(4):496–502. doi: 10.1002/1097-4636(20010615)55:4<496::aid-jbm1042>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 105.Deshpande AS, Beniash E. Cryst. Growth Des. 2008;8(8):3084–3090. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boskey AL. J. Cell. Biochem. 1998;72(S30–31):83–91. doi: 10.1002/(SICI)1097-4644(1998)72:30/31+<83::AID-JCB12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 107.Gorski J. Calcif. Tissue Int. 1992;50(5):391–396. doi: 10.1007/BF00296767. [DOI] [PubMed] [Google Scholar]
- 108.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Biochem. Biophys. Res. Commun. 2001;280(2):460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 109.George A, Sabsay B, Simonian PA, Veis A. J. Biol. Chem. 1993;268(17):12624–12630. [PubMed] [Google Scholar]
- 110.Stetler-Stevenson WG, Veis A. Biochemistry. 1983;22(18):4326–4335. doi: 10.1021/bi00287a025. [DOI] [PubMed] [Google Scholar]
- 111.George A, Veis A. Chem. Rev. 2008;108(11):4670–4693. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gotliv B-A, Kessler N, Sumerel JL, Morse DE, Tuross N, Addadi L, Weiner S. ChemBioChem. 2005;6(2):304–314. doi: 10.1002/cbic.200400221. [DOI] [PubMed] [Google Scholar]
- 113.Stetler-Stevenson W, Veis A. Calcif. Tissue Int. 1987;40(2):97–102. doi: 10.1007/BF02555712. [DOI] [PubMed] [Google Scholar]
- 114.Goldberg HA, Warner KJ, Li MC, Hunter GK. Connect. Tissue Res. 2001;42(1):25–37. doi: 10.3109/03008200109014246. [DOI] [PubMed] [Google Scholar]
- 115.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Biochem. J. 1996;317(1):59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Atkinson RA, Evans JS, Hauschka PV, Levine BA, Meats R, Triffitt JT, Virdi AS, Williams RJP. Eur. J. Biochem. 1995;232(2):515–521. doi: 10.1111/j.1432-1033.1995.tb20838.x. [DOI] [PubMed] [Google Scholar]
- 117.Evans JS, Chan SI. Biopolymers. 1994;34(4):507–527. doi: 10.1002/bip.360340407. [DOI] [PubMed] [Google Scholar]
- 118.Evans JS. Curr. Opin. in Colloid & Interface Sci. 2003;8(1):48–54. [Google Scholar]
- 119.Delak K, Collino S, Evans JS. Biochemistry. 2009;48(16):3669–3677. doi: 10.1021/bi900113v. [DOI] [PubMed] [Google Scholar]
- 120.Delak K, Harcup C, Lakshminarayanan R, Sun Z, Fan YW, Moradian-Oldak J, Evans JS. Biochemistry. 2009;48(10):2272–2281. doi: 10.1021/bi802175a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He G, Gajjeraman S, Schultz D, Cookson D, Qin CL, Butler WT, Hao JJ, George A. Biochemistry. 2005;44(49):16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li JH, Olton D, Lee D, Kumta PN, Sfeir C. Cells Tissues Organs. 2009;189(1–4):252–255. doi: 10.1159/000158571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aggeli A, Nyrkova IA, Bell M, Harding R, Carrick L, McLeish TCB, Semenov AN, Boden N. Proc. Natl. Acad. Sci. U. S. A. 2001;98(21):11857–11862. doi: 10.1073/pnas.191250198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Nature. 2005;435(7043):773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Serpell LC. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2000;1502(1):16–30. doi: 10.1016/s0925-4439(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 126.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Acta Biomaterialia. 2005;1(4):387–397. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 127.Adams J, Fantner GE, Fisher LW, Hansma PK. Nanotechnology. 2008;19(38) doi: 10.1088/0957-4484/19/38/384008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fujisawa R, Kuboki Y. Calcif. Tissue Int. 1992;51(6):438–442. doi: 10.1007/BF00296677. [DOI] [PubMed] [Google Scholar]
- 129.Stetler-Stevenson W, Veis A. Calcif. Tissue Int. 1986;38(3):135–141. doi: 10.1007/BF02556873. [DOI] [PubMed] [Google Scholar]
- 130.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. J. Biol. Chem. 2002;277(6):4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 131.He G, George A. J. Biol. Chem. 2004;279(12):11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 132.Dahl T, Sabsay B, Veis A. J. Struct. Biol. 1998;123(2):162–168. doi: 10.1006/jsbi.1998.4025. [DOI] [PubMed] [Google Scholar]
- 133.Tye CE, Hunter GK, Goldberg HA. J. Biol. Chem. 2005;280(14):13487–13492. doi: 10.1074/jbc.M408923200. [DOI] [PubMed] [Google Scholar]
- 134.Silverman L, Boskey AL. Calcif. Tissue Int. 2004;75(6):494–501. doi: 10.1007/s00223-004-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang L, Nancollas GH. Chem. Rev. 2008;108(11):4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He G, Ramachandran A, Dahl T, George S, Schultz D, Cookson D, Veis A, George A. J. Biol. Chem. 2005;280(39):33109–33114. doi: 10.1074/jbc.M500159200. [DOI] [PubMed] [Google Scholar]
- 137.Burke EM, Guo Y, Colon L, Rahima M, Veis A, Nancollas GH. Colloid Surf. B-Biointerfaces. 2000;17(1):49–57. [Google Scholar]
- 138.Grohe B, O'Young J, Ionescu DA, Lajoie G, Rogers KA, Karttunen M, Goldberg HA, Hunter GK. J. Am. Chem. Soc. 2007;129(48):14946–14951. doi: 10.1021/ja0745613. [DOI] [PubMed] [Google Scholar]
- 139.Chien YC, Masica DL, Gray JJ, Nguyen S, Vali H, McKee MD. J. Biol. Chem. 2009;284(35):23491–23501. doi: 10.1074/jbc.M109.021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heywood BR, Mann S. Adv. Mater. 1994;6(1):9–20. [Google Scholar]
- 141.Aizenberg J, Black AJ, Whitesides GM. Nature. 1999;398(6727):495–498. [Google Scholar]
- 142.Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Bone and Mineral. 1993;22(2):147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 143.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. J. Biol. Chem. 2004;279(18):18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 144.Tsuji T, Onuma K, Yamamoto A, Iijima M, Shiba K. Proc. Natl. Acad. Sci. U. S. A. 2008;105(44):16866–16870. doi: 10.1073/pnas.0804277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aizenberg J, Lambert G, Addadi L, Weiner S. Adv. Mater. 1996;8(3):222. [Google Scholar]
- 146.Crane NJ, Popescu V, Morris MD, Steenhuis P, Ignelzi MA. Bone. 2006;39(3):434–442. doi: 10.1016/j.bone.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 147.Cross K, Huq N, O’Brien-Simpson N, Perich J, Attard T, Reynolds E. International Journal of Peptide Research and Therapeutics. 2007;13(4):479–495. [Google Scholar]
- 148.Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds EC. J. Biol. Chem. 2005;280(15):15362–15369. doi: 10.1074/jbc.M413504200. [DOI] [PubMed] [Google Scholar]
- 149.Shechter A, Glazer L, Cheled S, Mor E, Weil S, Berman A, Bentov S, Aflalo ED, Khalaila I, Sagi A. Proc. Natl. Acad. Sci. U. S. A. 2008;105(20):7129–7134. doi: 10.1073/pnas.0800193105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aizenberg J, Lambert G, Weiner S, Addadi L. J. Am. Chem. Soc. 2002;124(1):32–39. doi: 10.1021/ja016990l. [DOI] [PubMed] [Google Scholar]
- 151.Bradt J-H, Mertig M, Teresiak A, Pompe W. Chemistry of Materials. 1999;11(10):2694–2701. [Google Scholar]
- 152.Ling YF, Rios HF, Myers ER, Lu YB, Feng JQ, Boskey AL. J. Bone Miner. Res. 2005;20(12):2169–2177. doi: 10.1359/JBMR.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Nature. 1996;382(6590):448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 154.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Bone. 1998;23(3):187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 155.Boskey AL, Young MF, Kilts T, Verdelis K. Cells Tissues Organs. 2005;181(3/4):144–153. doi: 10.1159/000091376. [DOI] [PubMed] [Google Scholar]
- 156.Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM. J. Bone Miner. Res. 2003;18(6):1005–1011. doi: 10.1359/jbmr.2003.18.6.1005. [DOI] [PubMed] [Google Scholar]
- 157.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Calcif. Tissue Int. 2002;71(2):145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 158.Lu YB, Ye L, Yu SB, Zhang SB, Xie YX, McKee MD, Li YC, Kong J, Eick JD, Dallas SL, Feng JQ. Dev. Biol. 2007;303(1):191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, Kohler T, Müller R, Goldberg M, Kulkarni AB. Matrix Biol. 2009;28(4):221–229. doi: 10.1016/j.matbio.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. J. Biol. Chem. 2003;278(27):24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 161.Malaval L, Wade-Gueye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, Laroche N, Roux J-P, Burt-Pichat B, Duboeuf F, Boivin G, Jurdic P, Lafage-Proust M-H, Amedee J, Vico L, Rossant J, Aubin JE. J. Exp. Med. 2008;205(5):1145–1153. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Feng JQ, Ward LM, Liu SG, Lu YB, Xie YX, Yuan BZ, Yu XJ, Rauch F, Davis SI, Zhang SB, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Nat. Genet. 2006;38(11):1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Cell. 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang W, Liao SS, Cui FZ. Chemistry of Materials. 2003;15(16):3221–3226. [Google Scholar]
- 165.Fratzl P, Paris O, Klaushofer K, Landis WJ. J. Clin. Invest. 1996;97(2):396–402. doi: 10.1172/JCI118428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Landis WJ. Bone. 1995;16(5):533–544. doi: 10.1016/8756-3282(95)00076-p. [DOI] [PubMed] [Google Scholar]
- 167.Traub W, Arad T, Vetter U, Weiner S. Matrix Biol. 1994;14(4):337–345. doi: 10.1016/0945-053x(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 168.Bella J, Eaton M, Brodsky B, Berman HM. Science. 1994;266(5182):75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 169.McBride DJ, Choe V, Shapiro JR, Brodsky B. J. Mol. Biol. 1997;270(2):275–284. doi: 10.1006/jmbi.1997.1106. [DOI] [PubMed] [Google Scholar]
- 170.Arnold, Plate, Wiesmann, Stratmann, Kohl, Höhling J. Microsc. 1999;195(1):58–63. doi: 10.1046/j.1365-2818.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 171.Plate U, Arnold S, Reimer L, Hohling HJ, Boyde A. Cell Tissue Res. 1994;278(3):543–547. doi: 10.1007/BF00331372. [DOI] [PubMed] [Google Scholar]
- 172.Toroian D, Lim JE, Price PA. J. Biol. Chem. 2007;282(31):22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 173.Gower LB. Chem. Rev. 2008;108(11):4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF. J. Struct. Biol. 1993;110(1):39–54. doi: 10.1006/jsbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- 175.Ofir PBY, Govrin-Lippman R, Garti N, Furedi-Milhofer H. Crystal Growth & Design. 2004;4(1):177–183. [Google Scholar]
- 176.Price PA, Toroian D, Lim JE. J. Biol. Chem. 2009;284(25):17092–17101. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ji B, Gao H. Journal of the Mechanics and Physics of Solids. 2004;52(9):1963–1990. [Google Scholar]
- 178.Currey JD. Bones: Structure and Mechanics. Princeton, NJ: Princeton: Princeton University Press; 2006. p. 436. [Google Scholar]
- 179.Du C, Cui FZ, Zhang W, Feng QL, Zhu XD, Groot Kd. J. Biomed. Mater. Res. 2000;50(4):518–527. doi: 10.1002/(sici)1097-4636(20000615)50:4<518::aid-jbm7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 180.Kavukcuoglu NB, Patterson-Buckendahl P, Mann AB. Journal of the Mechanical Behavior of Biomedical Materials. 2009;2(4):348–354. doi: 10.1016/j.jmbbm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 181.Akkus O, Adar F, Schaffler MB. Bone. 2004;34(3):443–453. doi: 10.1016/j.bone.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 182.Gao H, Ji B, Jäger IL, Arzt E, Fratzl P. Proc. Natl. Acad. Sci. U. S. A. 2003;100(10):5597–5600. doi: 10.1073/pnas.0631609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wopenka B, Pasteris JD. Materials Science and Engineering: C. 2005;25(2):131–143. [Google Scholar]
- 184.Almer JD, Stock SR. J. Struct. Biol. 2007;157(2):365–370. doi: 10.1016/j.jsb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 185.White SN, Luo W, Paine ML, Fong H, Sarikaya M, Snead ML. J. Dent. Res. 2001;80(1):321–326. doi: 10.1177/00220345010800010501. [DOI] [PubMed] [Google Scholar]
- 186.Ruys AJ, Wei M, Sorrell CC, Dickson MR, Brandwood A, Milthorpe BK. Biomaterials. 1995;16(5):409–415. doi: 10.1016/0142-9612(95)98859-c. [DOI] [PubMed] [Google Scholar]
- 187.Giri B, Tadano S, Fujisaki K, Sasaki N. Bone. 2009;44(6):1111–1120. doi: 10.1016/j.bone.2009.01.394. [DOI] [PubMed] [Google Scholar]
- 188.Currey JD, Foreman J, Laketi I, cacute; Mitchell J, Pegg DE, Reilly GC. J. Orthop. Res. 1997;15(1):111–117. doi: 10.1002/jor.1100150116. [DOI] [PubMed] [Google Scholar]
- 189.Currey JD. Osteoporos. Int. 2003;14(0):29–36. [Google Scholar]
- 190.Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. J. Struct. Biol. 1998;122(1–2):119–122. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- 191.Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P, Fratzl P. Philosophical Transactions: Biological Sciences. 2002;357(1418):191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McCutchen CW. J. Theor. Biol. 1975;51(1):51–58. doi: 10.1016/0022-5193(75)90138-1. [DOI] [PubMed] [Google Scholar]
- 193.Maciel KT, Carvalho RM, Ringle RD, Preston CD, Russell CM, Pashley DH. J. Dent. Res. 1996;75(11):1851–1858. doi: 10.1177/00220345960750110601. [DOI] [PubMed] [Google Scholar]
- 194.Lees S, Tao NJ, Lindsay SM. Connect. Tissue Res. 1990;24(3):187–205. doi: 10.3109/03008209009152148. [DOI] [PubMed] [Google Scholar]
- 195.Jäger I, Fratzl P. Biophys. J. 2000;79(4):1737–1746. doi: 10.1016/S0006-3495(00)76426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P. Proc. Natl. Acad. Sci. U. S. A. 2006;103(47):17741–17746. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gupta HS, Wagermaier W, Zickler GA, Raz-Ben Aroush D, Funari SS, Roschger P, Wagner HD, Fratzl P. Nano Letters. 2005;5(10):2108–2111. doi: 10.1021/nl051584b. [DOI] [PubMed] [Google Scholar]
- 198.Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. Nature. 2001;414(6865):773–776. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 199.Fantner GE, Adams J, Turner P, Thurner PJ, Fisher LW, Hansma PK. Nano Letters. 2007;7(8):2491–2498. doi: 10.1021/nl0712769. [DOI] [PubMed] [Google Scholar]
- 200.Peterlik H, Roschger P, Klaushofer K, Fratzl P. Nat Mater. 2006;5(1):52–55. doi: 10.1038/nmat1545. [DOI] [PubMed] [Google Scholar]
- 201.Liu D, Wagner HD, Weiner S. J. Mater. Sci.-Mater. Med. 2000;11(1):49–60. doi: 10.1023/a:1008989719560. [DOI] [PubMed] [Google Scholar]
- 202.Ebacher V, Wang R. Advanced Functional Materials. 2009;19(1):57–66. [Google Scholar]
- 203.Kamat S, Su X, Ballarini R, Heuer AH. Nature. 2000;405(6790):1036–1040. doi: 10.1038/35016535. [DOI] [PubMed] [Google Scholar]
- 204.Politi Y, Arad T, Klein E, Weiner S, Addadi L. Science. 2004;306(5699):1161–1164. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- 205.Weiss IM, Tuross N, Addadi L, Weiner S. J. Exp. Zool. 2002;293(5):478–491. doi: 10.1002/jez.90004. [DOI] [PubMed] [Google Scholar]
- 206.Killian CE, Wilt FH. J. Biol. Chem. 1996;271(15):9150–9159. doi: 10.1074/jbc.271.15.9150. [DOI] [PubMed] [Google Scholar]
- 207.Aizenberg J, Fratzl P. Adv. Mater. 2009;21(4):387–388. [Google Scholar]
- 208.Gao H. International Journal of Fracture. 2006;138(1):101–137. [Google Scholar]