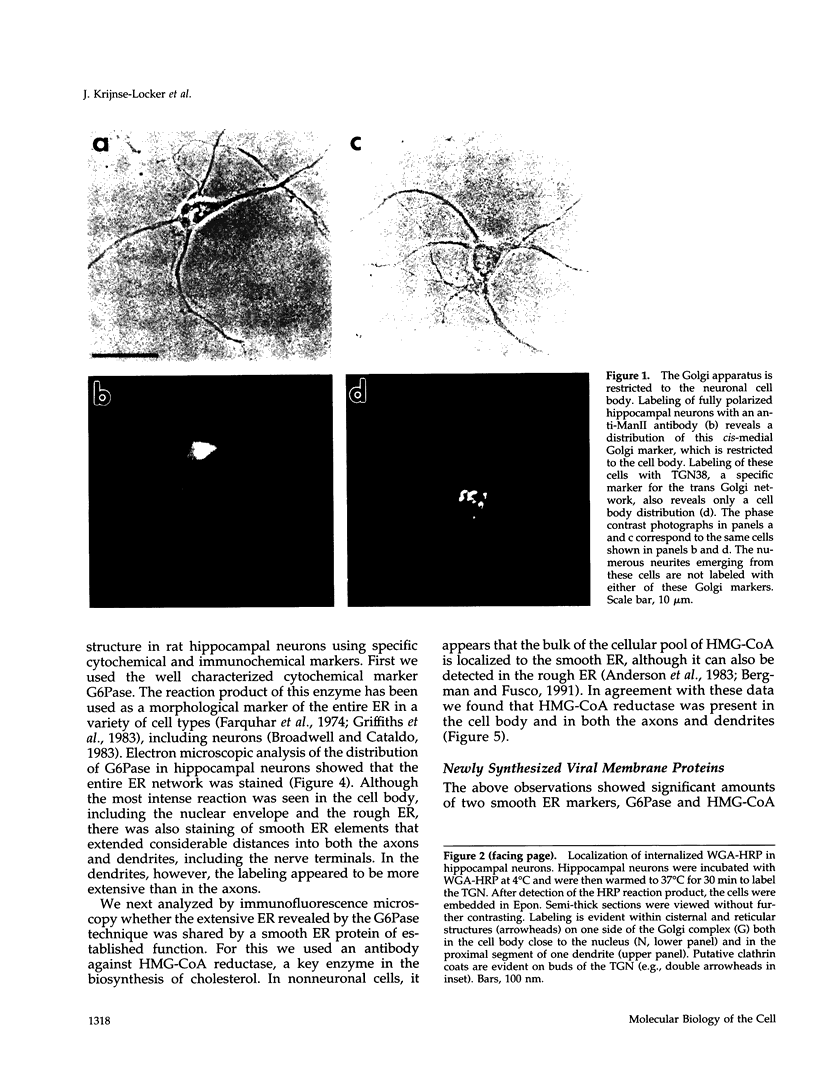
Abstract
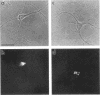
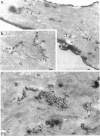
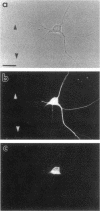
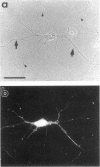
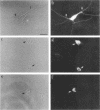
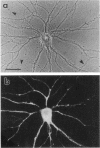
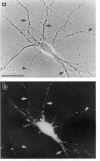
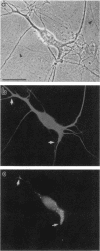
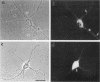
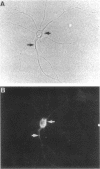
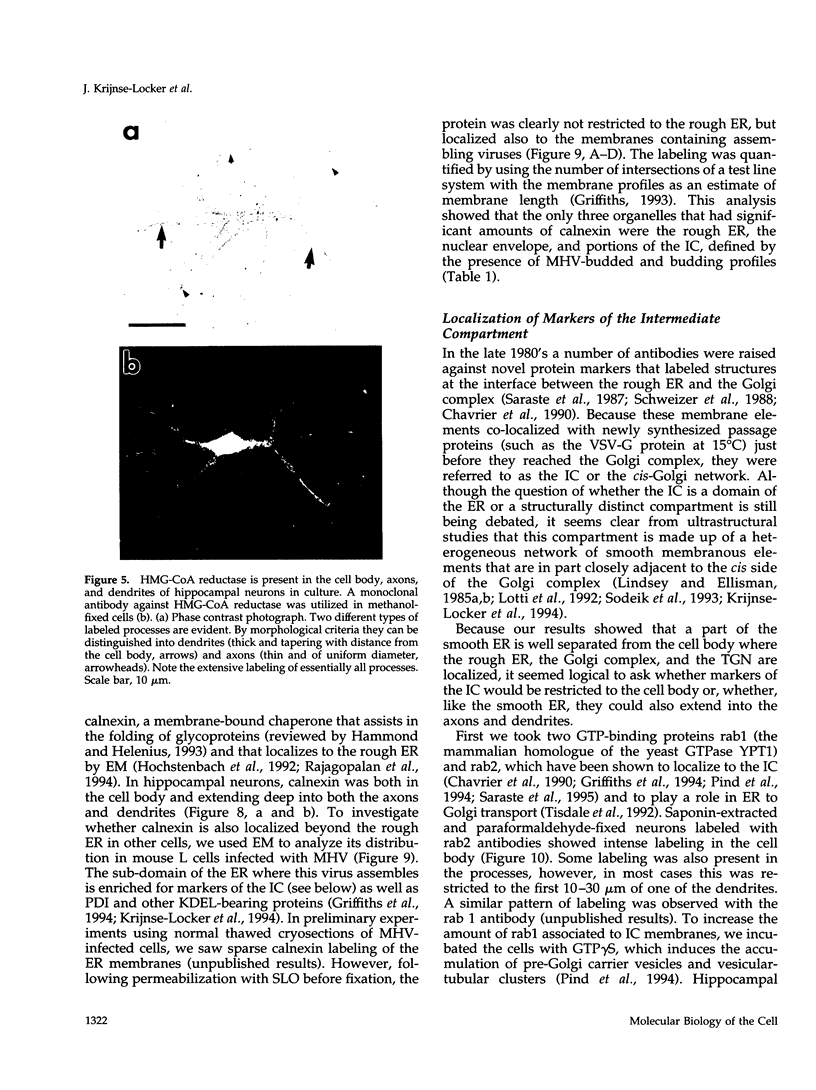
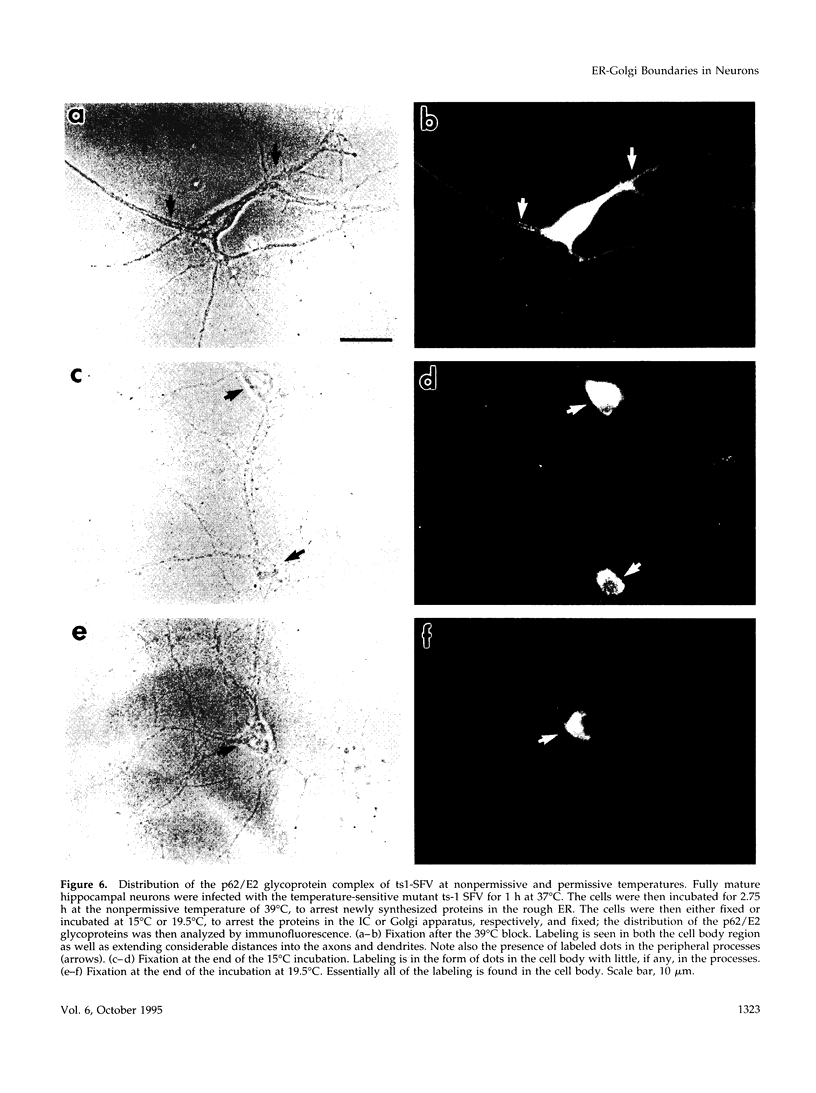
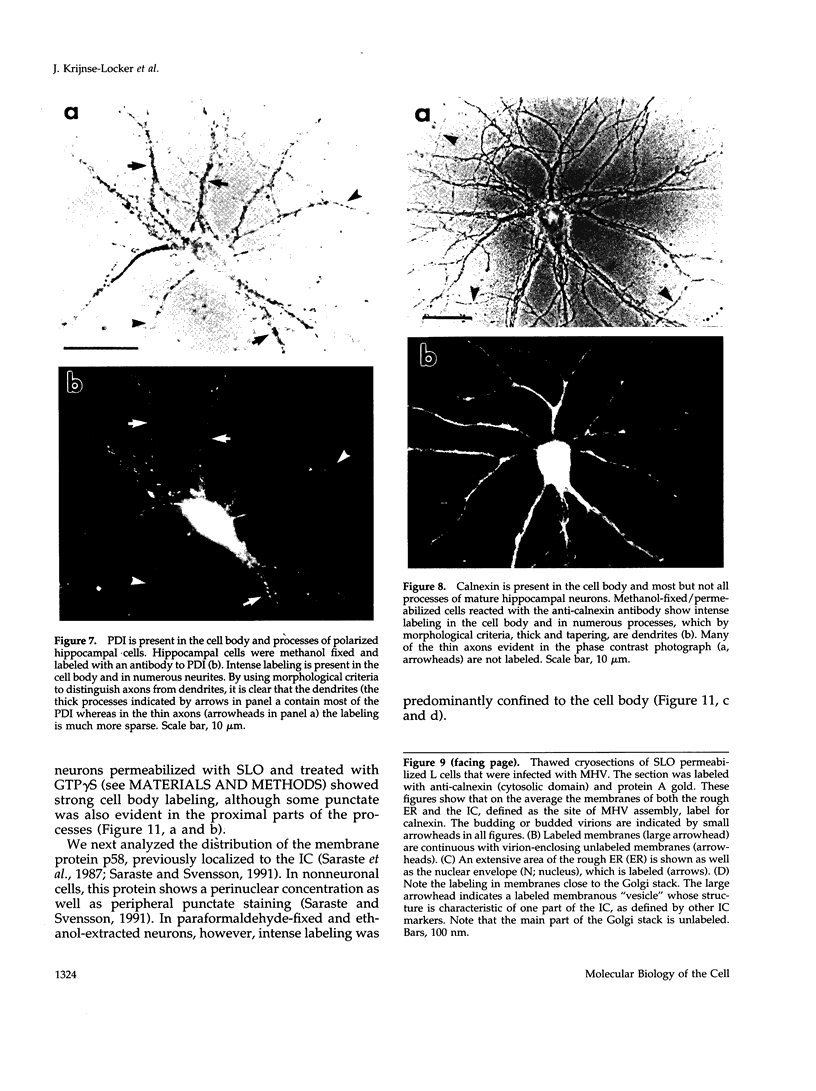
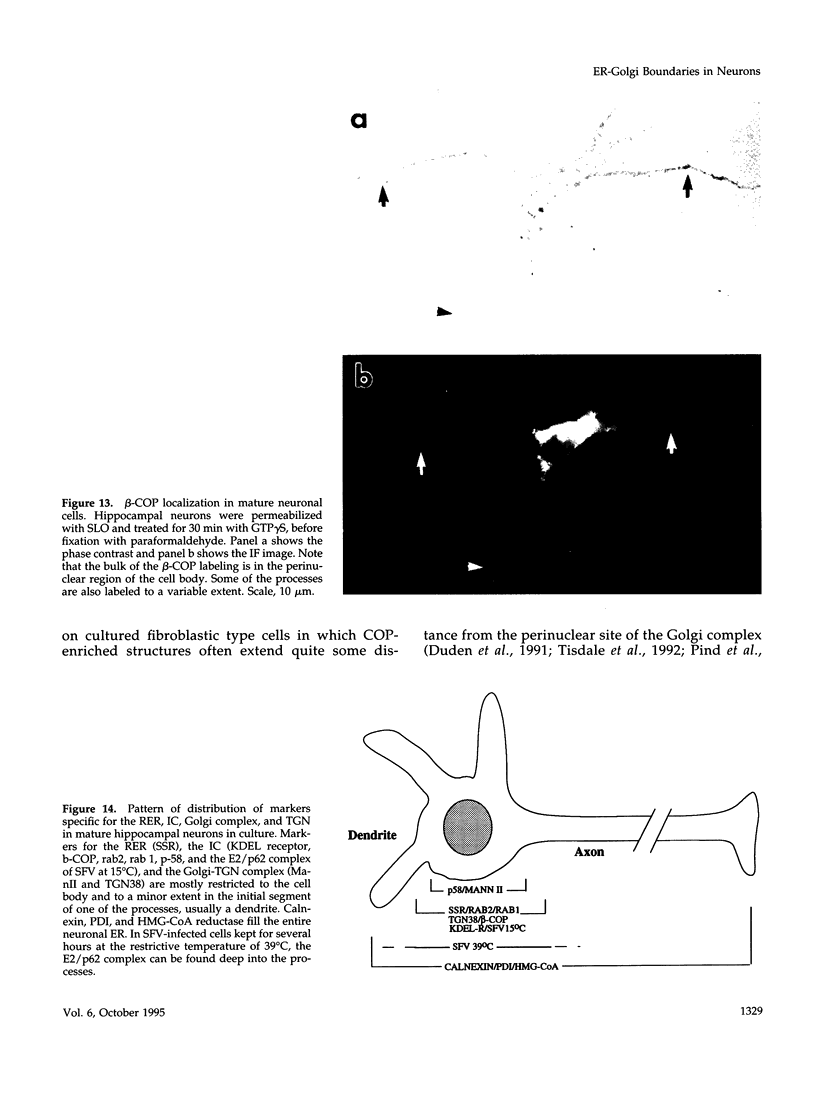
The boundaries of the organelles of the biosynthetic endomembrane system are still controversial. In this paper we take advantage of the unique architectural organization of neurons to investigate the localization of a spectrum of compartment-specific markers with the goal of defining the location of the rough endoplasmic reticulum (ER), smooth ER, intermediate compartment, and the Golgi complex. Markers of the rough ER (signal sequence receptor), Golgi complex (mannosidase II), and the trans Golgi network (TGN38) were essentially restricted to the cell body and the initial segment of one of the cell's dendrites. In contrast the cytochemical reaction product for glucose 6 phosphate, a classical ER marker, in addition to staining ER structures in the cell body also reacted with smooth ER elements that extended into both axons and dendrites. These peripheral smooth ER elements also reacted at the immunofluorescence level for ER marker 3-hydroxy-3-methylglutaryl-coenzyme A reductase, as well as for calnexin and protein disulfide isomerase. We also analyzed the location of rab1, rab2, p58, the KDEL receptor, and beta-subunit of coatomer. These intermediate compartment markers were found predominantly in the cell body but also extended to the proximal parts of the dendrites. Collectively, our data argue that the ER of hippocampal neurons consists of functionally and spatially distinct and separated domains, and they stress the power of the hippocampal neuron system for investigations of the organization of the ER by light microscopy.
Full text
PDF


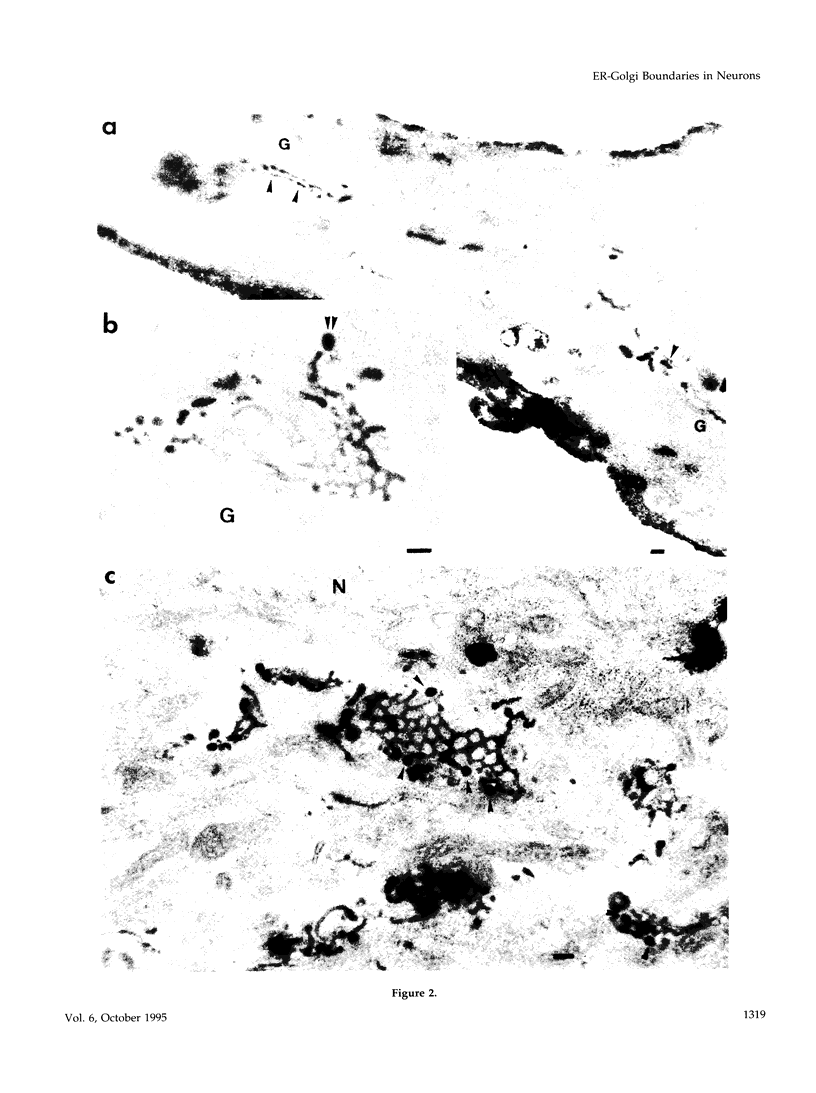
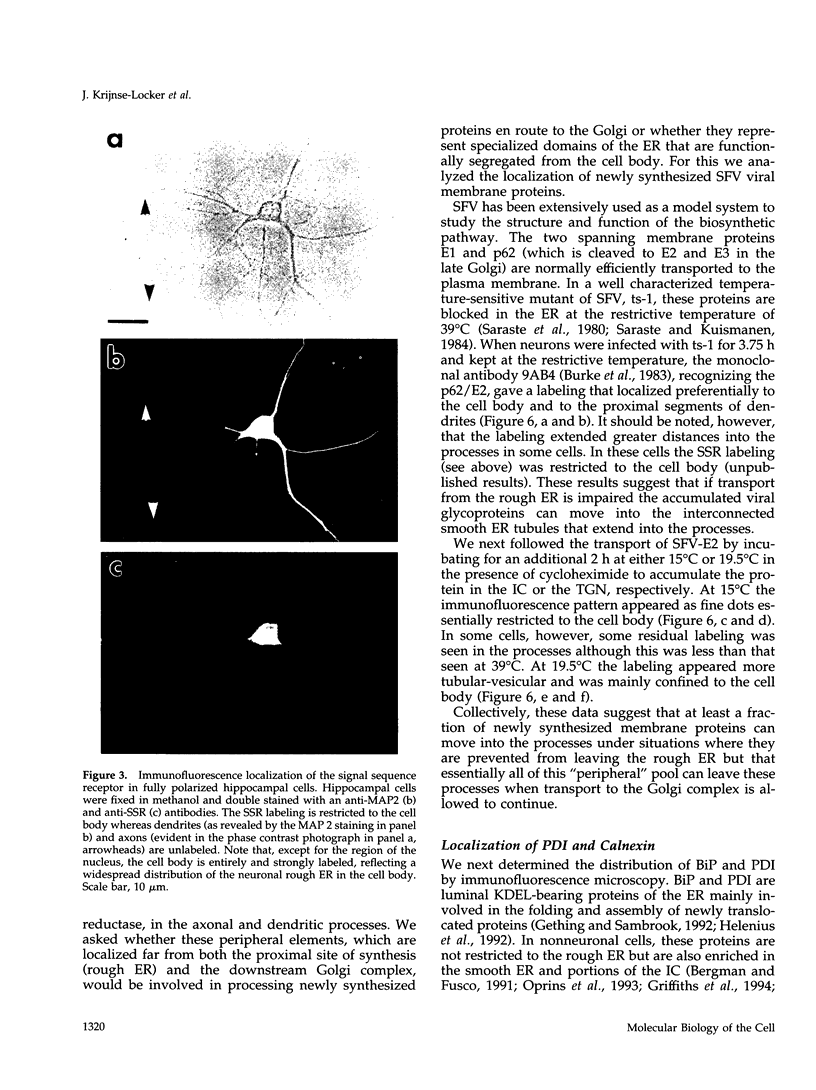








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Orci L., Brown M. S., Garcia-Segura L. M., Goldstein J. L. Ultrastructural analysis of crystalloid endoplasmic reticulum in UT-1 cells and its disappearance in response to cholesterol. J Cell Sci. 1983 Sep;63:1–20. doi: 10.1242/jcs.63.1.1. [DOI] [PubMed] [Google Scholar]
- Banfield D. K., Lewis M. J., Rabouille C., Warren G., Pelham H. R. Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J Cell Biol. 1994 Oct;127(2):357–371. doi: 10.1083/jcb.127.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Fusco P. J. The G protein of vesicular stomatitis virus has free access into and egress from the smooth endoplasmic reticulum of UT-1 cells. J Cell Biol. 1990 Mar;110(3):625–635. doi: 10.1083/jcb.110.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell R. D., Cataldo A. M. The neuronal endoplasmic reticulum: its cytochemistry and contribution to the endomembrane system. I. Cell bodies and dendrites. J Histochem Cytochem. 1983 Sep;31(9):1077–1088. doi: 10.1177/31.9.6309951. [DOI] [PubMed] [Google Scholar]
- Broadwell R. D., Cataldo A. M. The neuronal endoplasmic reticulum: its cytochemistry and contribution to the endomembrane system. II. Axons and terminals. J Comp Neurol. 1984 Dec 1;230(2):231–248. doi: 10.1002/cne.902300208. [DOI] [PubMed] [Google Scholar]
- Burke B., Walter C., Griffiths G., Warren G. Viral glycoproteins at different stages of intracellular transport can be distinguished using monoclonal antibodies. Eur J Cell Biol. 1983 Sep;31(2):315–324. [PubMed] [Google Scholar]
- Cala S. E., Scott B. T., Jones L. R. Intralumenal sarcoplasmic reticulum Ca(2+)-binding proteins. Semin Cell Biol. 1990 Aug;1(4):265–275. [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Moretti M., Donini S. D., Walter U., Lohmann S. M. Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: evidence for its intracellular accumulation on microtubules, microtubule-organizing centers, and in the area of the Golgi complex. J Cell Biol. 1986 Jul;103(1):189–203. doi: 10.1083/jcb.103.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Lamb R. A., Rose J. K., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993 Apr;193(2):545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990 Jul 13;62(1):63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991 Feb 8;64(3):649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Bergeron J. J., Palade G. E. Cytochemistry of Golgi fractions prepared from rat liver. J Cell Biol. 1974 Jan;60(1):8–25. doi: 10.1083/jcb.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Kim S. U., Stieber A., Avrameas S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977 Apr;73(1):1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Ericsson M., Krijnse-Locker J., Nilsson T., Goud B., Söling H. D., Tang B. L., Wong S. H., Hong W. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J Cell Biol. 1994 Dec;127(6 Pt 1):1557–1574. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Helenius A. A chaperone with a sweet tooth. Curr Biol. 1993 Dec 1;3(12):884–886. doi: 10.1016/0960-9822(93)90226-e. [DOI] [PubMed] [Google Scholar]
- Hammond C., Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994 Jul;126(1):41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Schweizer A. The endoplasmic reticulum-Golgi intermediate compartment. Curr Opin Cell Biol. 1992 Aug;4(4):600–608. doi: 10.1016/0955-0674(92)90078-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Hendricks L. C., McCaffery M., Palade G. E., Farquhar M. G. Disruption of endoplasmic reticulum to Golgi transport leads to the accumulation of large aggregates containing beta-COP in pancreatic acinar cells. Mol Biol Cell. 1993 Apr;4(4):413–424. doi: 10.1091/mbc.4.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Görlich D., Wiedmann M., Rapoport T. A., Dobberstein B. The identification of proteins in the proximity of signal-anchor sequences during their targeting to and insertion into the membrane of the ER. J Cell Biol. 1991 Apr;113(1):35–44. doi: 10.1083/jcb.113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach F., David V., Watkins S., Brenner M. B. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila A. P., Eder A. M., Fuller S. D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J Cell Biol. 1992 Sep;118(6):1305–1320. doi: 10.1083/jcb.118.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J., Ericsson M., Rottier P. J., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994 Jan;124(1-2):55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. D., Ellisman M. H. The neuronal endomembrane system. I. Direct links between rough endoplasmic reticulum and the cis element of the Golgi apparatus. J Neurosci. 1985 Dec;5(12):3111–3123. doi: 10.1523/JNEUROSCI.05-12-03111.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. D., Ellisman M. H. The neuronal endomembrane system. II. The multiple forms of the Golgi apparatus cis element. J Neurosci. 1985 Dec;5(12):3124–3134. doi: 10.1523/JNEUROSCI.05-12-03124.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. D., Ellisman M. H. The neuronal endomembrane system. III. The origins of the axoplasmic reticulum and discrete axonal cisternae at the axon hillock. J Neurosci. 1985 Dec;5(12):3135–3144. doi: 10.1523/JNEUROSCI.05-12-03135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lotti L. V., Torrisi M. R., Pascale M. C., Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J Cell Biol. 1992 Jul;118(1):43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein P. R., Morrison E. E., Bain D., Shering A. F., Banting G., Douglas P., Castro M. G. Polarized distribution of the trans-Golgi network marker TGN38 during the in vitro development of neocortical neurons: effects of nocodazole and brefeldin A. Eur J Neurosci. 1994 Sep 1;6(9):1453–1465. doi: 10.1111/j.1460-9568.1994.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Brake B., Banting G., Howell K. E., Braghetta P., Stanley K. K. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem J. 1990 Aug 15;270(1):97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complex: in vitro veritas? Cell. 1992 Mar 6;68(5):829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K. W., Robbins P. W. Isolation, characterization, and expression of cDNAs encoding murine alpha-mannosidase II, a Golgi enzyme that controls conversion of high mannose to complex N-glycans. J Cell Biol. 1991 Dec;115(6):1521–1534. doi: 10.1083/jcb.115.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K. W., Touster O. Biosynthesis and modification of Golgi mannosidase II in HeLa and 3T3 cells. J Biol Chem. 1985 Jun 10;260(11):6654–6662. [PubMed] [Google Scholar]
- Oprins A., Duden R., Kreis T. E., Geuze H. J., Slot J. W. Beta-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993 Apr;121(1):49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Malhotra V., Amherdt M., Serafini T., Rothman J. E. Dissection of a single round of vesicular transport: sequential intermediates for intercisternal movement in the Golgi stack. Cell. 1989 Feb 10;56(3):357–368. doi: 10.1016/0092-8674(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Simons K., Dotti C. G. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992 Oct;119(1):123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak R. K., Luskey K. L., Anderson R. G. Biogenesis of the crystalloid endoplasmic reticulum in UT-1 cells: evidence that newly formed endoplasmic reticulum emerges from the nuclear envelope. J Cell Biol. 1986 Jun;102(6):2158–2168. doi: 10.1083/jcb.102.6.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Scheel J., Horstmann H., Hauri H. P., Griffiths G., Kreis T. E. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993 Jul 16;74(1):71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H., Johnson M. P. Membrane biogenesis in the sprouting neuron. I. Selective transfer of newly synthesized phospholipid into the growing neurite. J Cell Biol. 1983 Oct;97(4):1038–1042. doi: 10.1083/jcb.97.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pind S. N., Nuoffer C., McCaffery J. M., Plutner H., Davidson H. W., Farquhar M. G., Balch W. E. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994 Apr;125(2):239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Xu Y., Brenner M. B. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994 Jan 21;263(5145):387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Putney J. W., Jr The identity of the calcium-storing, inositol 1,4,5-trisphosphate-sensitive organelle in non-muscle cells: calciosome, endoplasmic reticulum ... or both? Trends Neurosci. 1991 Jul;14(7):310–314. doi: 10.1016/0166-2236(91)90143-i. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Saraste J., Lahtinen U., Goud B. Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway. J Cell Sci. 1995 Apr;108(Pt 4):1541–1552. doi: 10.1242/jcs.108.4.1541. [DOI] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J Cell Biol. 1987 Nov;105(5):2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991 Nov;100(Pt 3):415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Käriäinen L., Keränen S. Semliki forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology. 1980 Jan 30;100(2):229–245. doi: 10.1016/0042-6822(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Fransen J. A., Bächi T., Ginsel L., Hauri H. P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988 Nov;107(5):1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992 Oct;3(10):1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B., Doms R. W., Ericsson M., Hiller G., Machamer C. E., van 't Hof W., van Meer G., Moss B., Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993 May;121(3):521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. L., Wong S. H., Qi X. L., Low S. H., Hong W. Molecular cloning, characterization, subcellular localization and dynamics of p23, the mammalian KDEL receptor. J Cell Biol. 1993 Jan;120(2):325–338. doi: 10.1083/jcb.120.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg S., Holtzman E. Axonal agranular reticulum and synaptic vesicles in cultured embryonic chick sympathetic neurons. J Cell Biol. 1973 Apr;57(1):88–108. doi: 10.1083/jcb.57.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale E. J., Bourne J. R., Khosravi-Far R., Der C. J., Balch W. E. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992 Nov;119(4):749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Van P. N., Peter F., Söling H. D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J Biol Chem. 1989 Oct 15;264(29):17494–17501. [PubMed] [Google Scholar]
- Vance J. E., Pan D., Vance D. E., Campenot R. B. Biosynthesis of membrane lipids in rat axons. J Cell Biol. 1991 Nov;115(4):1061–1068. doi: 10.1083/jcb.115.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Villa A., Podini P., Clegg D. O., Pozzan T., Meldolesi J. Intracellular Ca2+ stores in chicken Purkinje neurons: differential distribution of the low affinity-high capacity Ca2+ binding protein, calsequestrin, of Ca2+ ATPase and of the ER lumenal protein, Bip. J Cell Biol. 1991 May;113(4):779–791. doi: 10.1083/jcb.113.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel F., Hartmann E., Görlich D., Rapoport T. A. Segregation of the signal sequence receptor protein in the rough endoplasmic reticulum membrane. Eur J Cell Biol. 1990 Dec;53(2):197–202. [PubMed] [Google Scholar]
- Warren G. Protein transport. Signals and salvage sequences. Nature. 1987 May 7;327(6117):17–18. doi: 10.1038/327017a0. [DOI] [PubMed] [Google Scholar]
- Weis K., Griffiths G., Lamond A. I. The endoplasmic reticulum calcium-binding protein of 55 kDa is a novel EF-hand protein retained in the endoplasmic reticulum by a carboxyl-terminal His-Asp-Glu-Leu motif. J Biol Chem. 1994 Jul 22;269(29):19142–19150. [PubMed] [Google Scholar]
- Wiedmann M., Goerlich D., Hartmann E., Kurzchalia T. V., Rapoport T. A. Photocrosslinking demonstrates proximity of a 34 kDa membrane protein to different portions of preprolactin during translocation through the endoplasmic reticulum. FEBS Lett. 1989 Nov 6;257(2):263–268. doi: 10.1016/0014-5793(89)81549-2. [DOI] [PubMed] [Google Scholar]