Abstract
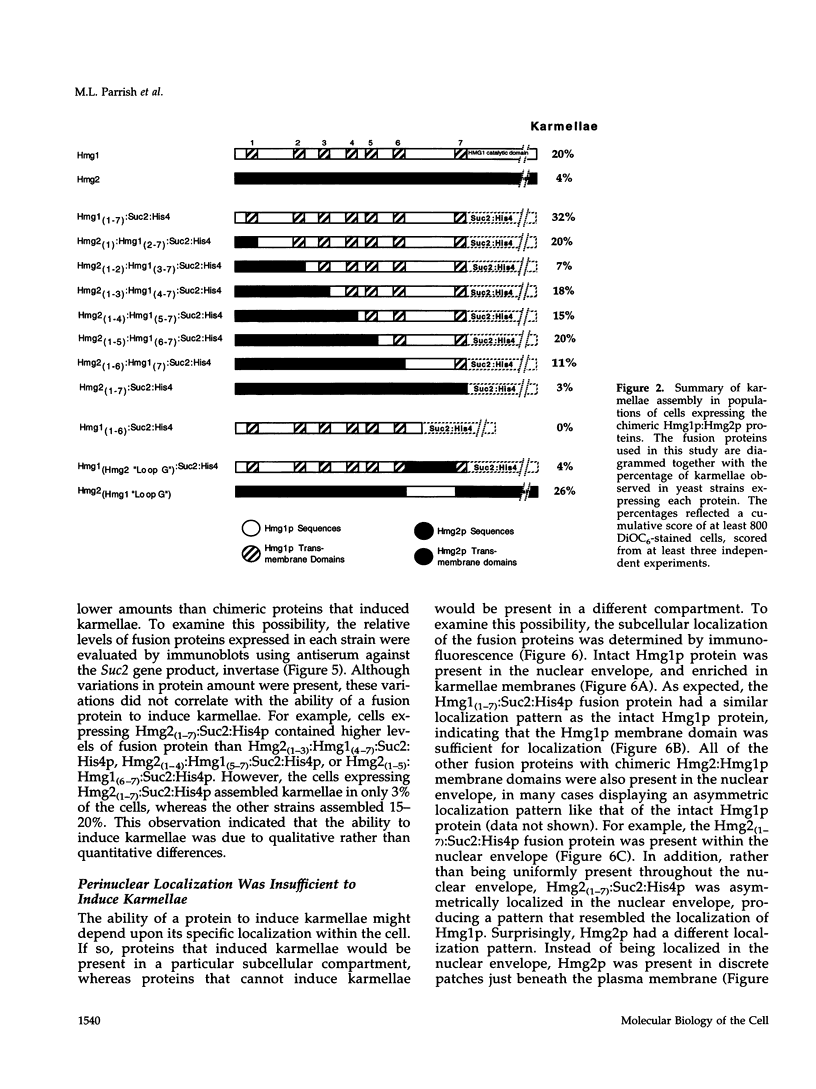
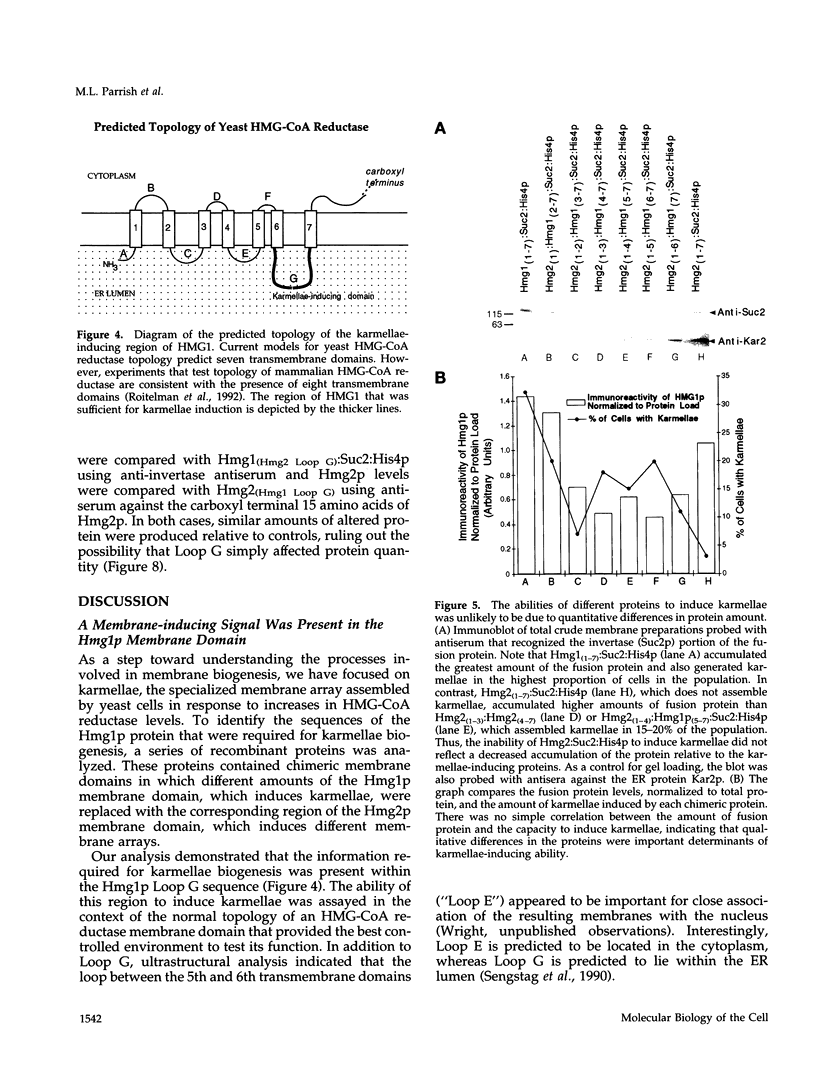
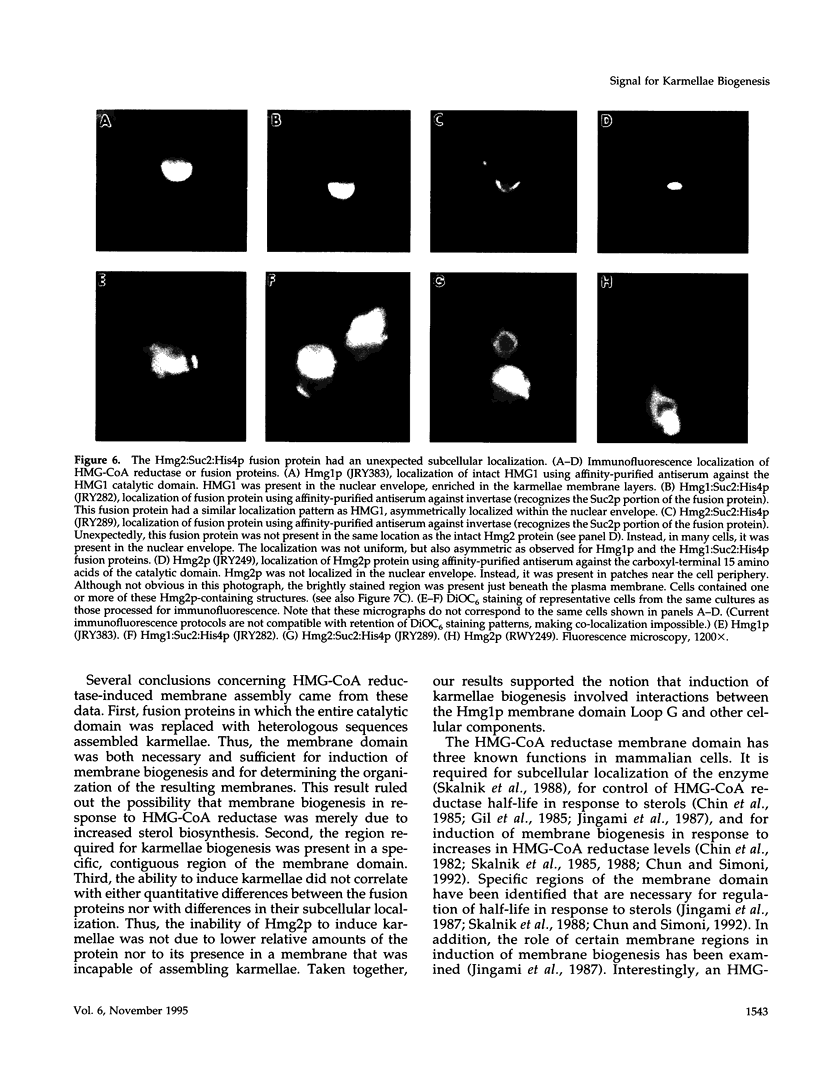
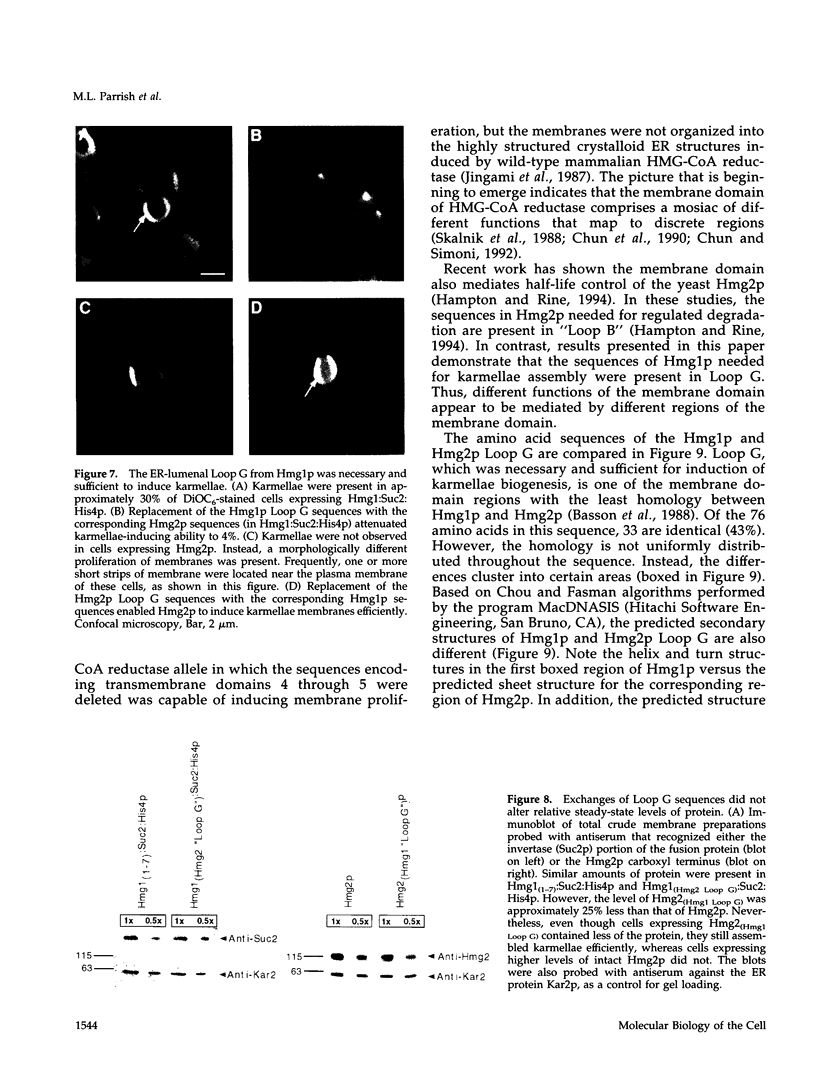
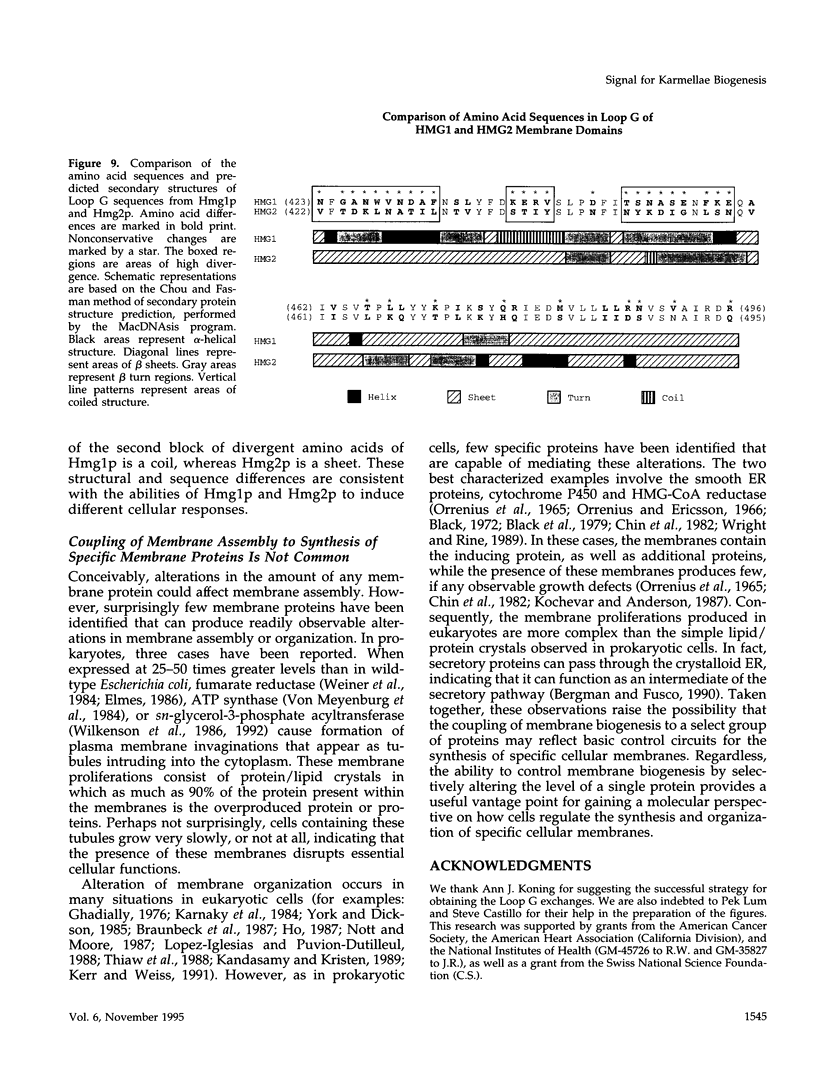
In all eukaryotic cells that have been examined, specific membrane arrays are induced in response to increased levels of the ER membrane protein, HMG-CoA reductase. Analysis of these inducible membranes has the potential to reveal basic insights into general membrane assembly. Yeast express two HMG-CoA reductase isozymes, and each isozyme induces a morphologically distinct proliferation of the endoplasmic reticulum. The isozyme encoded by HMG1 induces karmellae, which are long stacks of membranes that partially enclose the nucleus. In contrast, the isozyme encoded by HMG2 induces short stacks of membrane that may be associated with the nucleus, but are frequently present at the cell periphery. To understand the molecular nature of the different cellular responses to Hmg1p and Hmg2p, we mapped the region of Hmg1p that is needed for karmellae assembly. For this analysis, a series of exchange alleles was examined in which a portion of the Hmg2p membrane domain was replaced with the corresponding Hmg1p sequences. Results of this analysis indicated that the ER lumenal loop between predicted transmembrane domains 6 and 7 was both necessary and sufficient for karmellae assembly, when present in the context of an HMG-CoA reductase membrane domain. Immunoblotting experiments ruled out the simple possibility that differences in the amounts of the various chimeric HMG-CoA reductase proteins was responsible for the altered cellular responses. Our results are consistent with the hypothesis that each yeast isozyme induces or organizes a qualitatively different organization of ER membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Orci L., Brown M. S., Garcia-Segura L. M., Goldstein J. L. Ultrastructural analysis of crystalloid endoplasmic reticulum in UT-1 cells and its disappearance in response to cholesterol. J Cell Sci. 1983 Sep;63:1–20. doi: 10.1242/jcs.63.1.1. [DOI] [PubMed] [Google Scholar]
- Andreis P. G., Cavallini L., Mazzocchi G., Nussdorfer G. G. Effects of prolonged administration of lovastatin, an inhibitor of cholesterol synthesis, on the morphology and function of rat Leydig cells. Exp Clin Endocrinol. 1990 Sep;96(1):15–24. doi: 10.1055/s-0029-1210983. [DOI] [PubMed] [Google Scholar]
- Basson M. E., Thorsness M., Finer-Moore J., Stroud R. M., Rine J. Structural and functional conservation between yeast and human 3-hydroxy-3-methylglutaryl coenzyme A reductases, the rate-limiting enzyme of sterol biosynthesis. Mol Cell Biol. 1988 Sep;8(9):3797–3808. doi: 10.1128/mcb.8.9.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson M. E., Thorsness M., Rine J. Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5563–5567. doi: 10.1073/pnas.83.15.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Fusco P. J. The G protein of vesicular stomatitis virus has free access into and egress from the smooth endoplasmic reticulum of UT-1 cells. J Cell Biol. 1990 Mar;110(3):625–635. doi: 10.1083/jcb.110.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black V. H., Robbins D., McNamara N., Huima T. A correlated thin-section and freeze-fracture analysis of guinea pig adrenocortical cells. Am J Anat. 1979 Dec;156(4):453–503. doi: 10.1002/aja.1001560404. [DOI] [PubMed] [Google Scholar]
- Black V. H. The development of smooth-surfaced endoplasmic reticulum in adrenal cortical cells of fetal guinea pigs. Am J Anat. 1972 Nov;135(3):381–417. doi: 10.1002/aja.1001350307. [DOI] [PubMed] [Google Scholar]
- Braunbeck T., Gorgas K., Storch V., Völkl A. Ultrastructure of hepatocytes in golden ide (Leuciscus idus melanotus L.; Cyprinidae: Teleostei) during thermal adaptation. Anat Embryol (Berl) 1987;175(3):303–313. doi: 10.1007/BF00309844. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Gil G., Faust J. R., Goldstein J. L., Brown M. S., Luskey K. L. Sterols accelerate degradation of hamster 3-hydroxy-3-methylglutaryl coenzyme A reductase encoded by a constitutively expressed cDNA. Mol Cell Biol. 1985 Apr;5(4):634–641. doi: 10.1128/mcb.5.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Anderson R. G., Faust J. R., Goldstein J. L., Brown M. S. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1185–1189. doi: 10.1073/pnas.79.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Faust J. R., MacDonald R. J., Brown M. S., Goldstein J. L. Molecular cloning of 3-hydroxy-3-methylglutaryl coenzyme a reductase and evidence for regulation of its mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7704–7708. doi: 10.1073/pnas.79.24.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun K. T., Bar-Nun S., Simoni R. D. The regulated degradation of 3-hydroxy-3-methylglutaryl-CoA reductase requires a short-lived protein and occurs in the endoplasmic reticulum. J Biol Chem. 1990 Dec 15;265(35):22004–22010. [PubMed] [Google Scholar]
- Chun K. T., Simoni R. D. The role of the membrane domain in the regulated degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Biol Chem. 1992 Feb 25;267(6):4236–4246. [PubMed] [Google Scholar]
- Deschenes R. J., Broach J. R. Fatty acylation is important but not essential for Saccharomyces cerevisiae RAS function. Mol Cell Biol. 1987 Jul;7(7):2344–2351. doi: 10.1128/mcb.7.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmes M. L., Scraba D. G., Weiner J. H. Isolation and characterization of the tubular organelles induced by fumarate reductase overproduction in Escherichia coli. J Gen Microbiol. 1986 Jun;132(6):1429–1439. doi: 10.1099/00221287-132-6-1429. [DOI] [PubMed] [Google Scholar]
- Gil G., Faust J. R., Chin D. J., Goldstein J. L., Brown M. S. Membrane-bound domain of HMG CoA reductase is required for sterol-enhanced degradation of the enzyme. Cell. 1985 May;41(1):249–258. doi: 10.1016/0092-8674(85)90078-9. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Rine J. Regulated degradation of HMG-CoA reductase, an integral membrane protein of the endoplasmic reticulum, in yeast. J Cell Biol. 1994 Apr;125(2):299–312. doi: 10.1083/jcb.125.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. L. Ultrastructure of cerebellar capillary hemangioblastoma. VI. Concentric lamellar bodies of endoplasmic reticulum in stromal cells. Acta Neuropathol. 1987;74(4):345–353. doi: 10.1007/BF00687211. [DOI] [PubMed] [Google Scholar]
- Jingami H., Brown M. S., Goldstein J. L., Anderson R. G., Luskey K. L. Partial deletion of membrane-bound domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase eliminates sterol-enhanced degradation and prevents formation of crystalloid endoplasmic reticulum. J Cell Biol. 1987 Jun;104(6):1693–1704. doi: 10.1083/jcb.104.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnaky K. J., Jr, Lau K. R., Garretson L. T., Schultz S. G. Seasonal variations in the fine structure of the Necturus maculosus urinary bladder epithelium: low transporters and high transporters. Am J Anat. 1984 Oct;171(2):227–242. doi: 10.1002/aja.1001710208. [DOI] [PubMed] [Google Scholar]
- Kerr J. B., Weiss M. Spontaneous or experimentally induced formation of a special zone in the adrenal cortex of the adult brush-tailed possum (Trichosurus vulpecula). Am J Anat. 1991 Feb;190(2):101–117. doi: 10.1002/aja.1001900202. [DOI] [PubMed] [Google Scholar]
- Kochevar D. T., Anderson R. G. Purified crystalloid endoplasmic reticulum from UT-1 cells contains multiple proteins in addition to 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Biol Chem. 1987 Jul 25;262(21):10321–10326. [PubMed] [Google Scholar]
- Koning A. J., Lum P. Y., Williams J. M., Wright R. DiOC6 staining reveals organelle structure and dynamics in living yeast cells. Cell Motil Cytoskeleton. 1993;25(2):111–128. doi: 10.1002/cm.970250202. [DOI] [PubMed] [Google Scholar]
- Li A. C., Tanaka R. D., Callaway K., Fogelman A. M., Edwards P. A. Localization of 3-hydroxy-3-methylglutaryl CoA reductase and 3-hydroxy-3-methylglutaryl CoA synthase in the rat liver and intestine is affected by cholestyramine and mevinolin. J Lipid Res. 1988 Jun;29(6):781–796. [PubMed] [Google Scholar]
- Liscum L., Finer-Moore J., Stroud R. M., Luskey K. L., Brown M. S., Goldstein J. L. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J Biol Chem. 1985 Jan 10;260(1):522–530. [PubMed] [Google Scholar]
- Lopez-Iglesias C., Puvion-Dutilleul F. Ultrastructural localization of glycoproteins in rabbit fibroblasts altered by Herpes simplex virus type 1 infection. Biol Cell. 1988;62(1):47–56. [PubMed] [Google Scholar]
- Nott J. A., Moore M. N. Effects of polycyclic aromatic hydrocarbons on molluscan lysosomes and endoplasmic reticulum. Histochem J. 1987 Jun-Jul;19(6-7):357–368. doi: 10.1007/BF01680453. [DOI] [PubMed] [Google Scholar]
- Olender E. H., Simon R. D. The intracellular targeting and membrane topology of 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem. 1992 Feb 25;267(6):4223–4235. [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L. Enzyme-membrane relationship in phenobarbital induction of synthesis of drug-metabolizing enzyme system and proliferation of endoplasmic membranes. J Cell Biol. 1966 Feb;28(2):181–198. doi: 10.1083/jcb.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak R. K., Luskey K. L., Anderson R. G. Biogenesis of the crystalloid endoplasmic reticulum in UT-1 cells: evidence that newly formed endoplasmic reticulum emerges from the nuclear envelope. J Cell Biol. 1986 Jun;102(6):2158–2168. doi: 10.1083/jcb.102.6.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R., Preston R. A., Adams A. E., Stearns T., Drubin D. G., Haarer B. K., Jones E. W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Roitelman J., Olender E. H., Bar-Nun S., Dunn W. A., Jr, Simoni R. D. Immunological evidence for eight spans in the membrane domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase: implications for enzyme degradation in the endoplasmic reticulum. J Cell Biol. 1992 Jun;117(5):959–973. doi: 10.1083/jcb.117.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengstag C., Stirling C., Schekman R., Rine J. Genetic and biochemical evaluation of eucaryotic membrane protein topology: multiple transmembrane domains of Saccharomyces cerevisiae 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol Cell Biol. 1990 Feb;10(2):672–680. doi: 10.1128/mcb.10.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kazazis D. M., Huff J. W. Lovastatin, an inhibitor of cholesterol synthesis, induces hydroxymethylglutaryl-coenzyme A reductase directly on membranes of expanded smooth endoplasmic reticulum in rat hepatocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5264–5268. doi: 10.1073/pnas.85.14.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalnik D. G., Brown D. A., Brown P. C., Friedman R. L., Hardeman E. C., Schimke R. T., Simoni R. D. Mechanisms of 3-hydroxy-3-methylglutaryl coenzyme A reductase overaccumulation in three compactin-resistant cell lines. J Biol Chem. 1985 Feb 25;260(4):1991–1994. [PubMed] [Google Scholar]
- Skalnik D. G., Narita H., Kent C., Simoni R. D. The membrane domain of 3-hydroxy-3-methylglutaryl-coenzyme A reductase confers endoplasmic reticulum localization and sterol-regulated degradation onto beta-galactosidase. J Biol Chem. 1988 May 15;263(14):6836–6841. [PubMed] [Google Scholar]
- Weiner J. H., Lemire B. D., Elmes M. L., Bradley R. D., Scraba D. G. Overproduction of fumarate reductase in Escherichia coli induces a novel intracellular lipid-protein organelle. J Bacteriol. 1984 May;158(2):590–596. doi: 10.1128/jb.158.2.590-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkison W. O., Bell R. M., Taylor K. A., Costello M. J. Structural characterization of ordered arrays of sn-glycerol-3-phosphate acyltransferase from Escherichia coli. J Bacteriol. 1992 Oct;174(20):6608–6616. doi: 10.1128/jb.174.20.6608-6616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkison W. O., Walsh J. P., Corless J. M., Bell R. M. Crystalline arrays of the Escherichia coli sn-glycerol-3-phosphate acyltransferase, an integral membrane protein. J Biol Chem. 1986 Jul 25;261(21):9951–9958. [PubMed] [Google Scholar]
- Wright R., Basson M., D'Ari L., Rine J. Increased amounts of HMG-CoA reductase induce "karmellae": a proliferation of stacked membrane pairs surrounding the yeast nucleus. J Cell Biol. 1988 Jul;107(1):101–114. doi: 10.1083/jcb.107.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R., Keller G., Gould S. J., Subramani S., Rine J. Cell-type control of membrane biogenesis induced by HMG-CoA reductase. New Biol. 1990 Oct;2(10):915–921. [PubMed] [Google Scholar]
- Wright R., Rine J. Transmission electron microscopy and immunocytochemical studies of yeast: analysis of HMG-CoA reductase overproduction by electron microscopy. Methods Cell Biol. 1989;31:473–512. doi: 10.1016/s0091-679x(08)61624-6. [DOI] [PubMed] [Google Scholar]
- Yorke M. A., Dickson D. H. Lamellar to tubular conformational changes in the endoplasmic reticulum of the retinal pigment epithelium of the newt, Notophthalmus viridescens. Cell Tissue Res. 1985;241(3):629–637. doi: 10.1007/BF00214585. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]