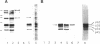Abstract
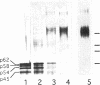
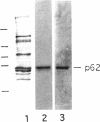
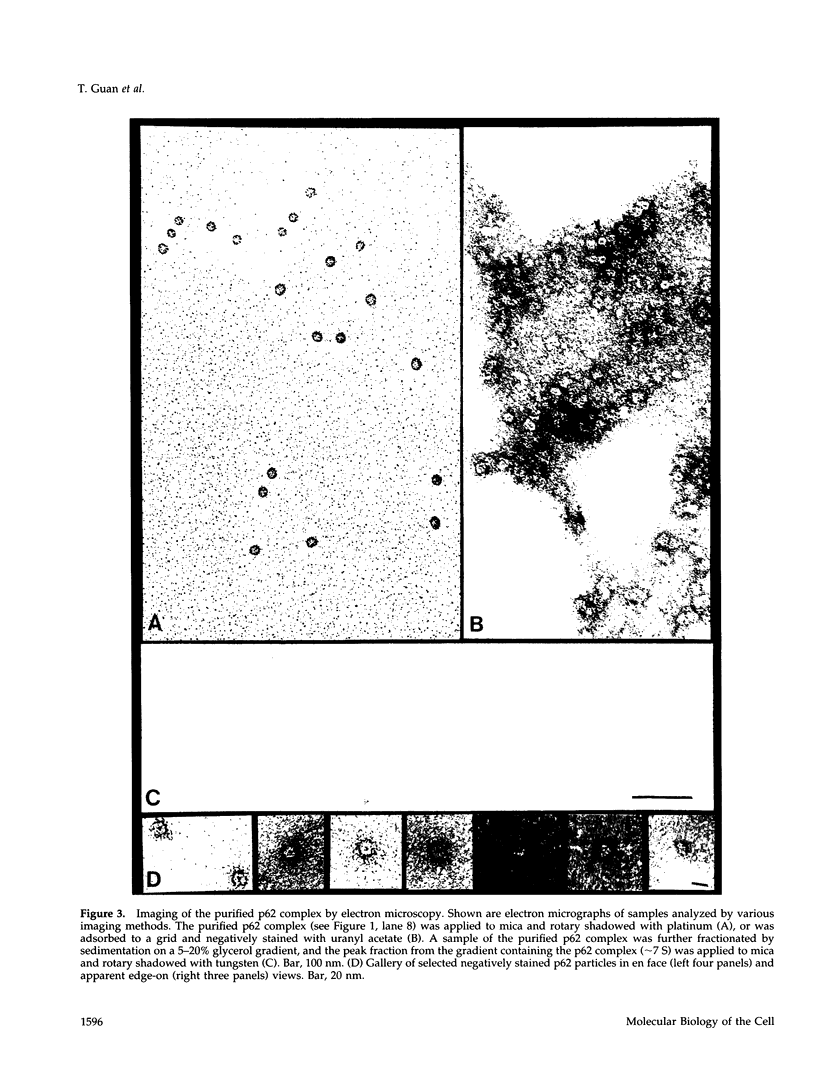
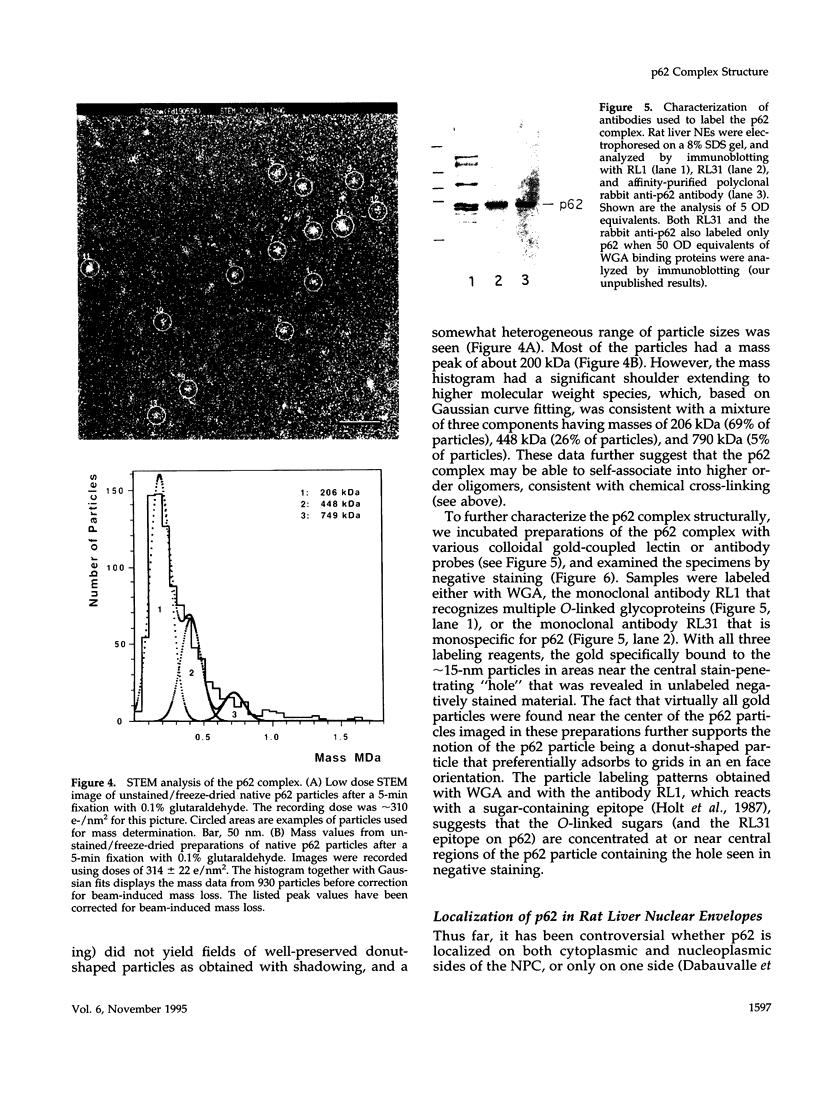
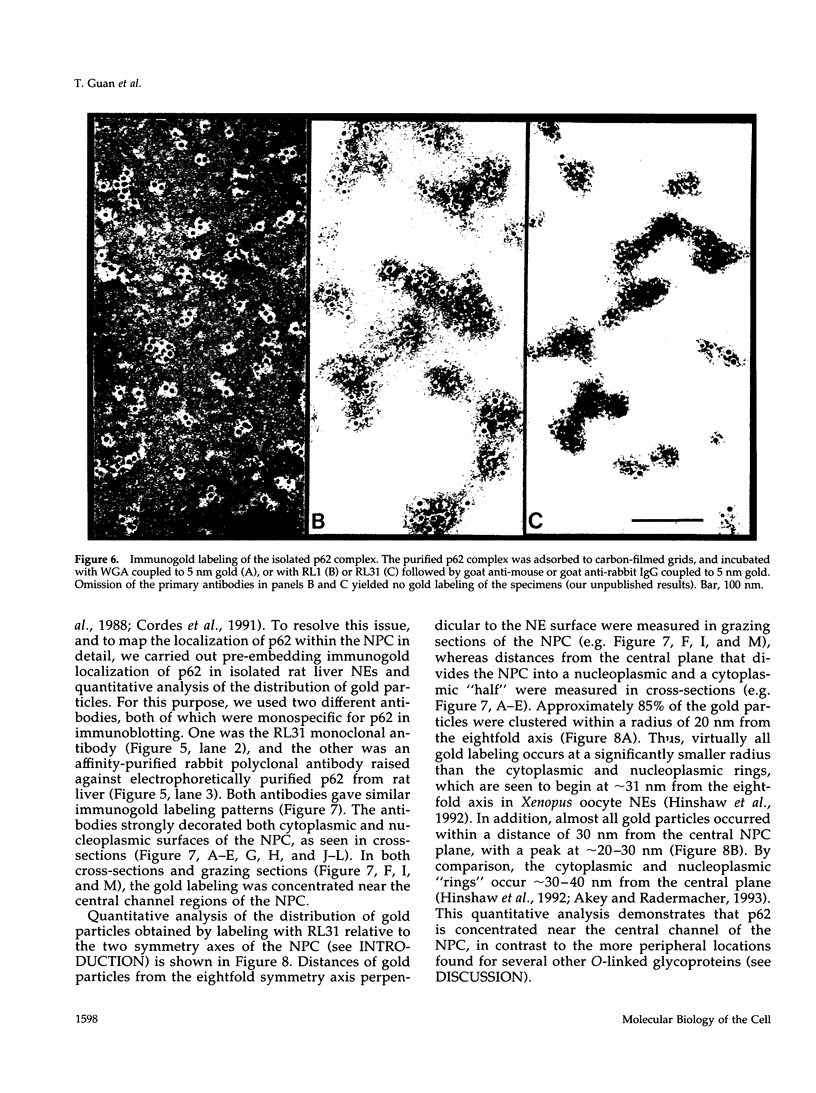
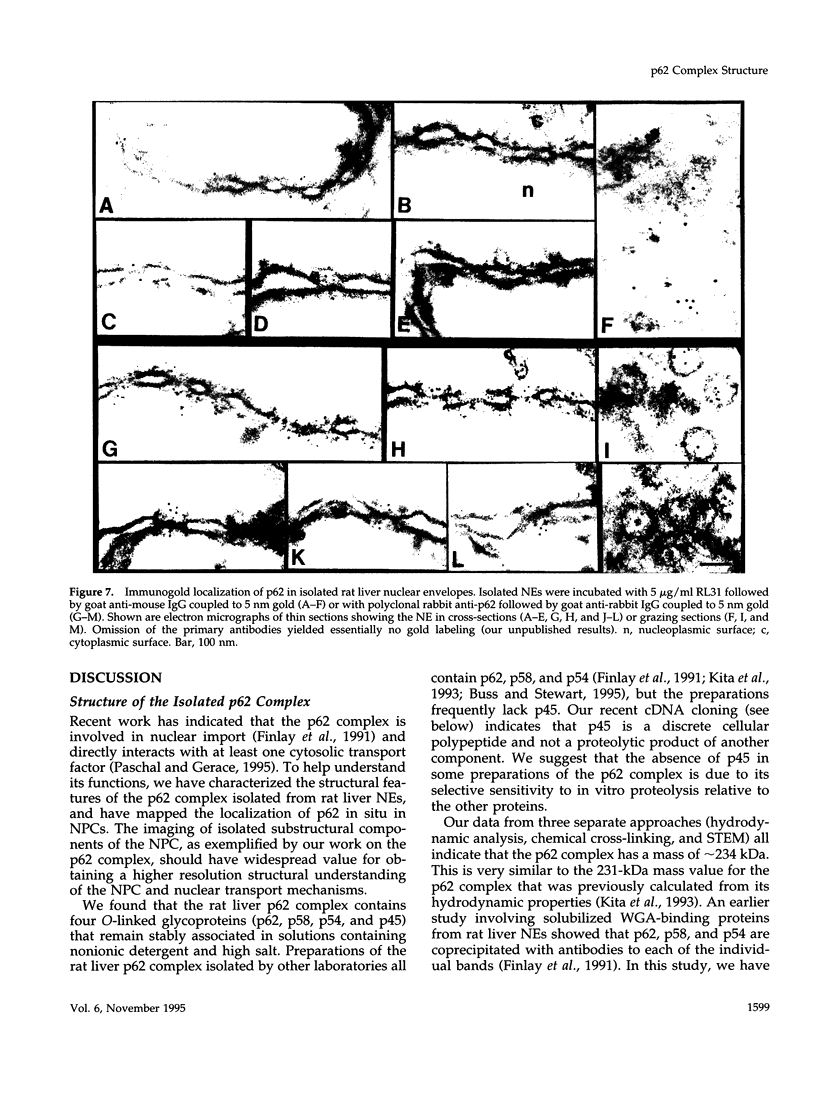
The p62 complex is an oligomeric assembly of O-linked glycoproteins of the nuclear pore complex that interacts with cytosolic transport factors and is part of the machinery for nuclear protein import. In this study we have purified the p62 complex from rat liver nuclear envelopes and analyzed its structure and composition. The p62 complex consists of four distinct polypeptides (p62, p58, p54, and p45) and has a mass of approximately 234 kDa, calculated from its hydrodynamic properties and supported by chemical cross-linking and scanning transmission electron microscopy. These data suggest that the p62 complex contains one copy of each constituent polypeptide. Analysis of preparations of the p62 complex by electron microscopy using rotary metal shadowing and negative staining revealed donut-shaped particles with a diameter of approximately 15 nm. Immunogold electron microscopy of isolated rat liver nuclear envelopes demonstrated that p62 occurs on both the nucleoplasmic and cytoplasmic sides of the pore complex near the central gated channel involved in active transport of proteins and RNAs. The properties and localization of the p62 complex suggest that it may be involved in binding transport ligands near the center of the nuclear pore complex and in subsequently transferring them to the gated transport channel.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991 Sep 6;66(5):837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- Akey C. W., Goldfarb D. S. Protein import through the nuclear pore complex is a multistep process. J Cell Biol. 1989 Sep;109(3):971–982. doi: 10.1083/jcb.109.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey C. W., Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol. 1993 Jul;122(1):1–19. doi: 10.1083/jcb.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F., Kent H., Stewart M., Bailer S. M., Hanover J. A. Role of different domains in the self-association of rat nucleoporin p62. J Cell Sci. 1994 Feb;107(Pt 2):631–638. doi: 10.1242/jcs.107.2.631. [DOI] [PubMed] [Google Scholar]
- Buss F., Stewart M. Macromolecular interactions in the nucleoporin p62 complex of rat nuclear pores: binding of nucleoporin p54 to the rod domain of p62. J Cell Biol. 1995 Feb;128(3):251–261. doi: 10.1083/jcb.128.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Kern H., Hurt E. C. Human nucleoporin p62 and the essential yeast nuclear pore protein NSP1 show sequence homology and a similar domain organization. Eur J Cell Biol. 1991 Jun;55(1):17–30. [PubMed] [Google Scholar]
- Cordes V. C., Reidenbach S., Köhler A., Stuurman N., van Driel R., Franke W. W. Intranuclear filaments containing a nuclear pore complex protein. J Cell Biol. 1993 Dec;123(6 Pt 1):1333–1344. doi: 10.1083/jcb.123.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes V., Waizenegger I., Krohne G. Nuclear pore complex glycoprotein p62 of Xenopus laevis and mouse: cDNA cloning and identification of its glycosylated region. Eur J Cell Biol. 1991 Jun;55(1):31–47. [PubMed] [Google Scholar]
- Dabauvalle M. C., Schulz B., Scheer U., Peters R. Inhibition of nuclear accumulation of karyophilic proteins in living cells by microinjection of the lectin wheat germ agglutinin. Exp Cell Res. 1988 Jan;174(1):291–296. doi: 10.1016/0014-4827(88)90163-2. [DOI] [PubMed] [Google Scholar]
- Dargemont C., Schmidt-Zachmann M. S., Kühn L. C. Direct interaction of nucleoporin p62 with mRNA during its export from the nucleus. J Cell Sci. 1995 Jan;108(Pt 1):257–263. doi: 10.1242/jcs.108.1.257. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7552–7556. doi: 10.1073/pnas.84.21.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Engel A., Meyer J. Preparation of unstained protein structures for mass determination by electron scattering. J Ultrastruct Res. 1980 Aug;72(2):212–222. doi: 10.1016/s0022-5320(80)90059-3. [DOI] [PubMed] [Google Scholar]
- Fabre E., Hurt E. C. Nuclear transport. Curr Opin Cell Biol. 1994 Jun;6(3):335–342. doi: 10.1016/0955-0674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Meier E., Bradley P., Horecka J., Forbes D. J. A complex of nuclear pore proteins required for pore function. J Cell Biol. 1991 Jul;114(1):169–183. doi: 10.1083/jcb.114.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler W. E., Aebi U. Preparation of single molecules and supramolecular complexes for high-resolution metal shadowing. J Ultrastruct Res. 1983 Jun;83(3):319–334. doi: 10.1016/s0022-5320(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Gerace L., Ottaviano Y., Kondor-Koch C. Identification of a major polypeptide of the nuclear pore complex. J Cell Biol. 1982 Dec;95(3):826–837. doi: 10.1083/jcb.95.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P., Schlaich N., Tekotte H., Hurt E. C. Functional interaction of Nic96p with a core nucleoporin complex consisting of Nsp1p, Nup49p and a novel protein Nup57p. EMBO J. 1995 Jan 3;14(1):76–87. doi: 10.1002/j.1460-2075.1995.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw J. E., Carragher B. O., Milligan R. A. Architecture and design of the nuclear pore complex. Cell. 1992 Jun 26;69(7):1133–1141. doi: 10.1016/0092-8674(92)90635-p. [DOI] [PubMed] [Google Scholar]
- Holt G. D., Snow C. M., Senior A., Haltiwanger R. S., Gerace L., Hart G. W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987 May;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnik M., Aebi U. Toward a more complete 3-D structure of the nuclear pore complex. J Struct Biol. 1991 Dec;107(3):291–308. doi: 10.1016/1047-8477(91)90054-z. [DOI] [PubMed] [Google Scholar]
- Kita K., Omata S., Horigome T. Purification and characterization of a nuclear pore glycoprotein complex containing p62. J Biochem. 1993 Mar;113(3):377–382. doi: 10.1093/oxfordjournals.jbchem.a124054. [DOI] [PubMed] [Google Scholar]
- Kraemer D., Wozniak R. W., Blobel G., Radu A. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1519–1523. doi: 10.1073/pnas.91.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave R., Fürst D. O., Weber K. Visualization of the polarity of isolated titin molecules: a single globular head on a long thin rod as the M band anchoring domain? J Cell Biol. 1989 Nov;109(5):2177–2187. doi: 10.1083/jcb.109.5.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Panté N., Aebi U. The nuclear pore complex. J Cell Biol. 1993 Sep;122(5):977–984. doi: 10.1083/jcb.122.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panté N., Bastos R., McMorrow I., Burke B., Aebi U. Interactions and three-dimensional localization of a group of nuclear pore complex proteins. J Cell Biol. 1994 Aug;126(3):603–617. doi: 10.1083/jcb.126.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Gerace L. Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore complex protein p62. J Cell Biol. 1995 May;129(4):925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim Biophys Acta. 1986 Dec 22;864(3-4):305–359. doi: 10.1016/0304-4157(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Rabilloud T., Carpentier G., Tarroux P. Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis. 1988 Jun;9(6):288–291. doi: 10.1002/elps.1150090608. [DOI] [PubMed] [Google Scholar]
- Reichelt R., Holzenburg A., Buhle E. L., Jr, Jarnik M., Engel A., Aebi U. Correlation between structure and mass distribution of the nuclear pore complex and of distinct pore complex components. J Cell Biol. 1990 Apr;110(4):883–894. doi: 10.1083/jcb.110.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Snow C. M., Senior A., Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987 May;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr C. M., D'Onofrio M., Park M. K., Hanover J. A. Primary sequence and heterologous expression of nuclear pore glycoprotein p62. J Cell Biol. 1990 Jun;110(6):1861–1871. doi: 10.1083/jcb.110.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T., Nimmesgern E., Nitsch M., Peters J., Pfeifer G., Müller S., Kellermann J., Engel A., Hartl F. U., Baumeister W. The thermosome of Thermoplasma acidophilum and its relationship to the eukaryotic chaperonin TRiC. Eur J Biochem. 1995 Feb 1;227(3):848–856. doi: 10.1111/j.1432-1033.1995.tb20210.x. [DOI] [PubMed] [Google Scholar]