Abstract
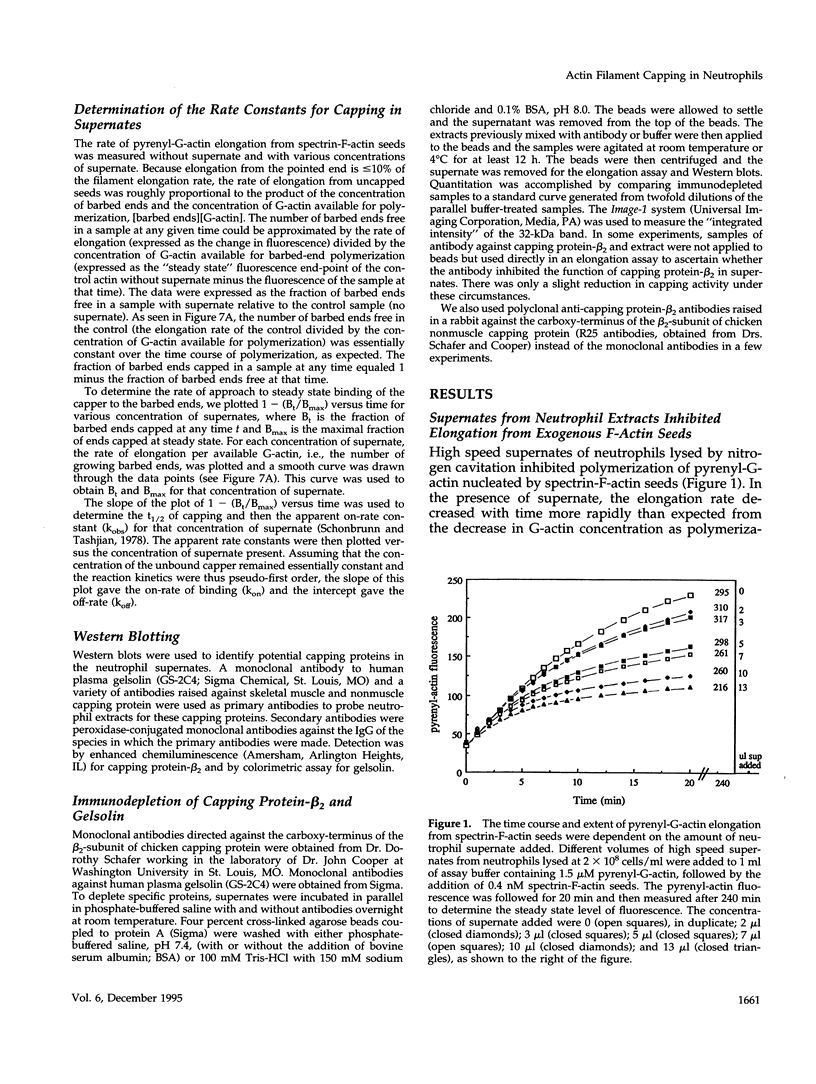
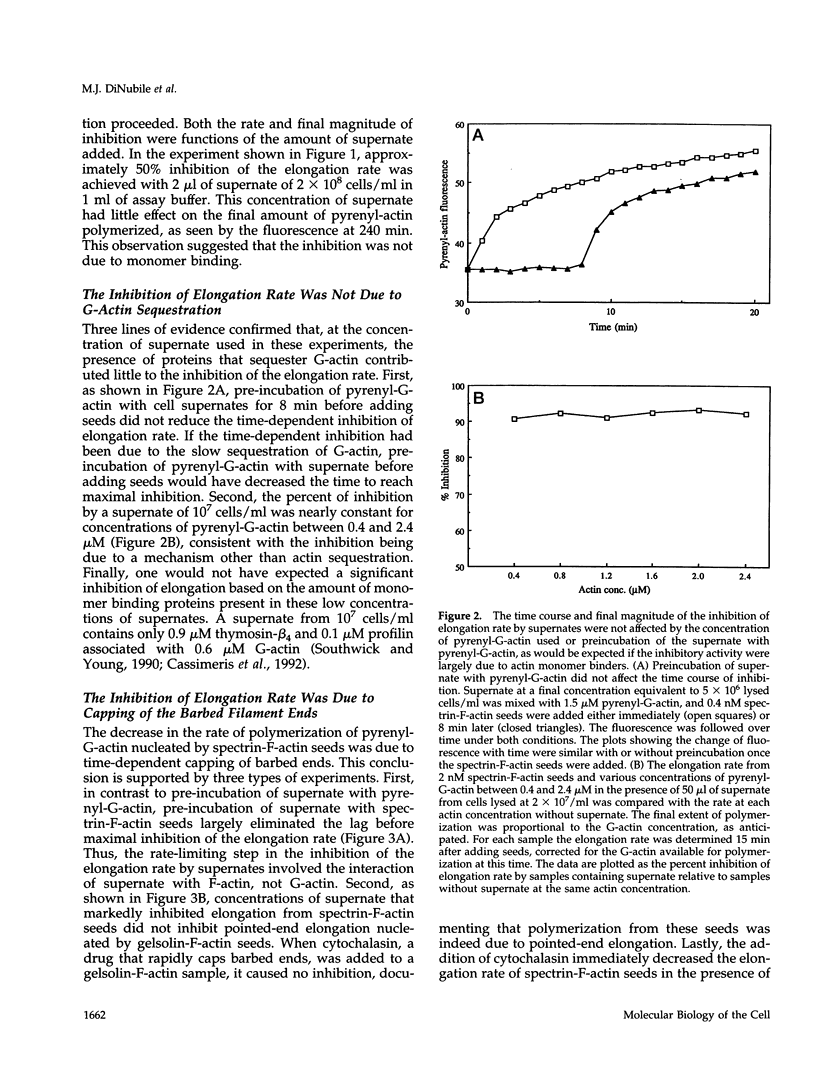
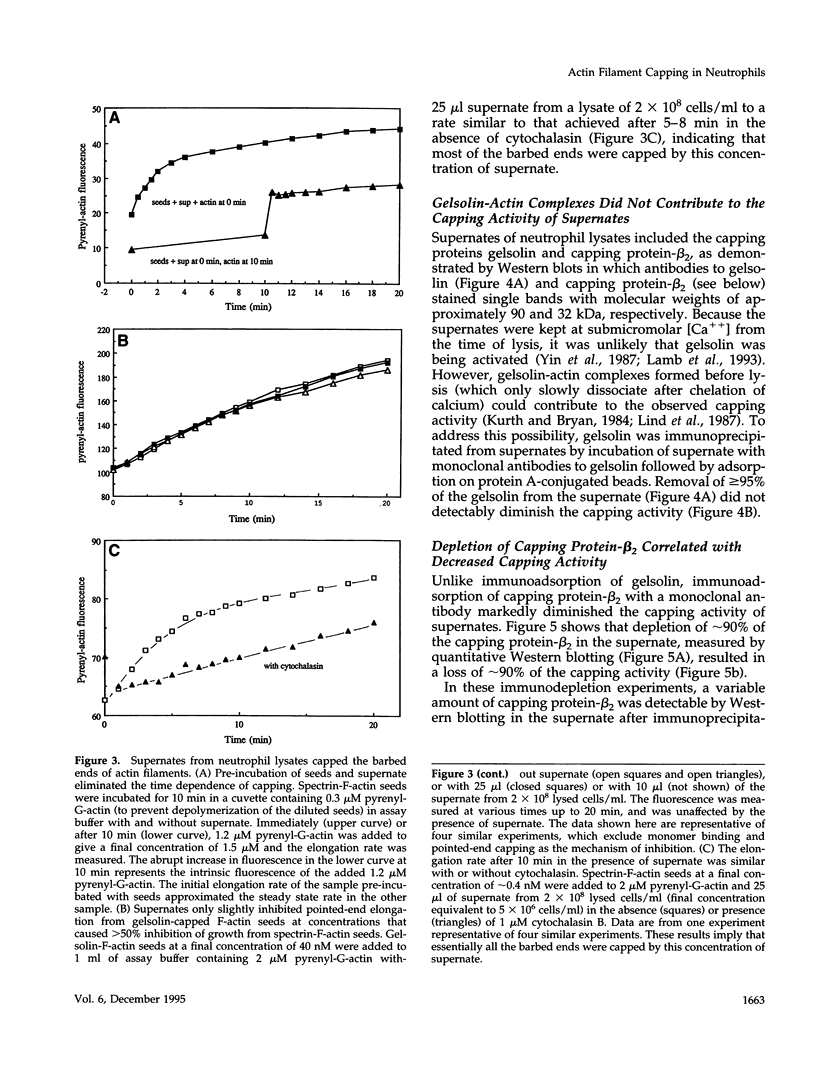
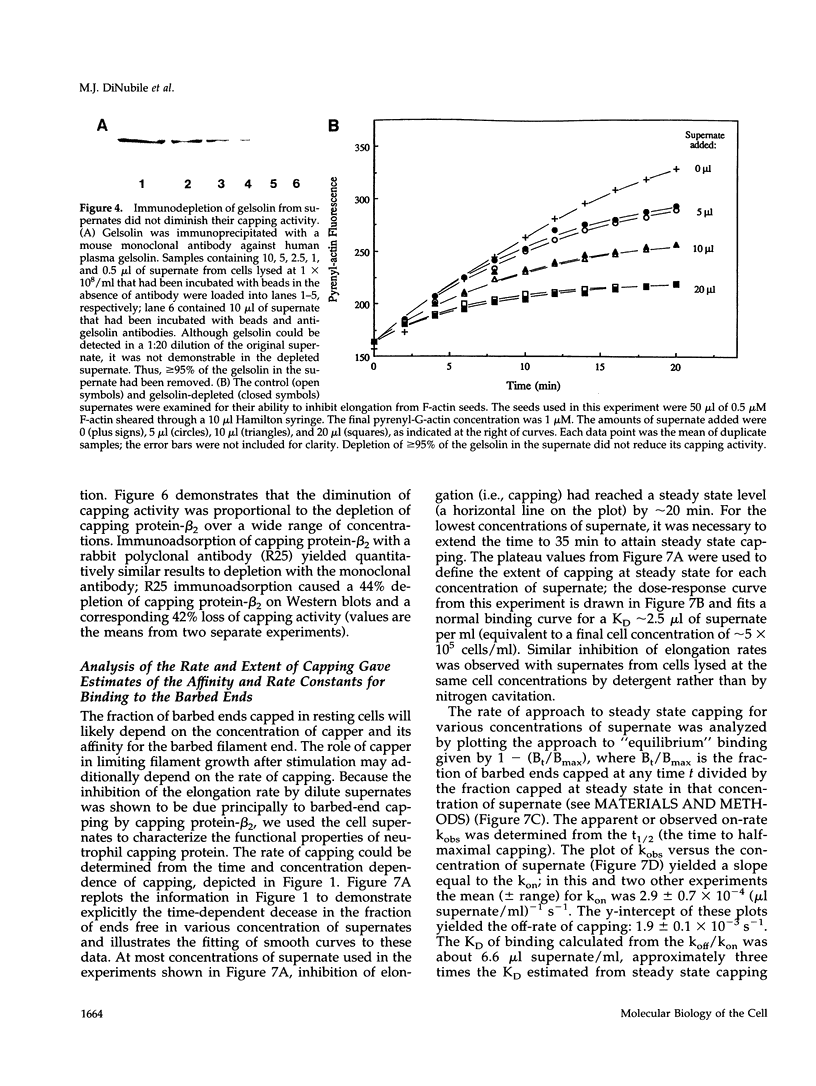
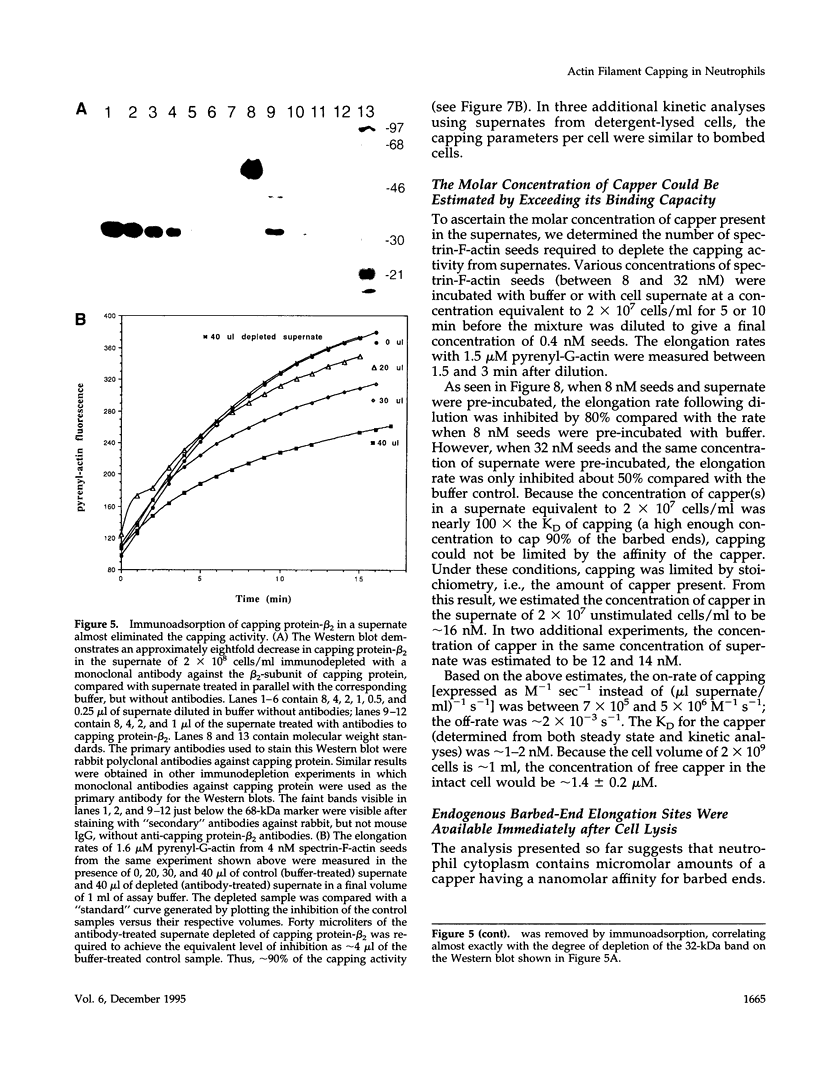
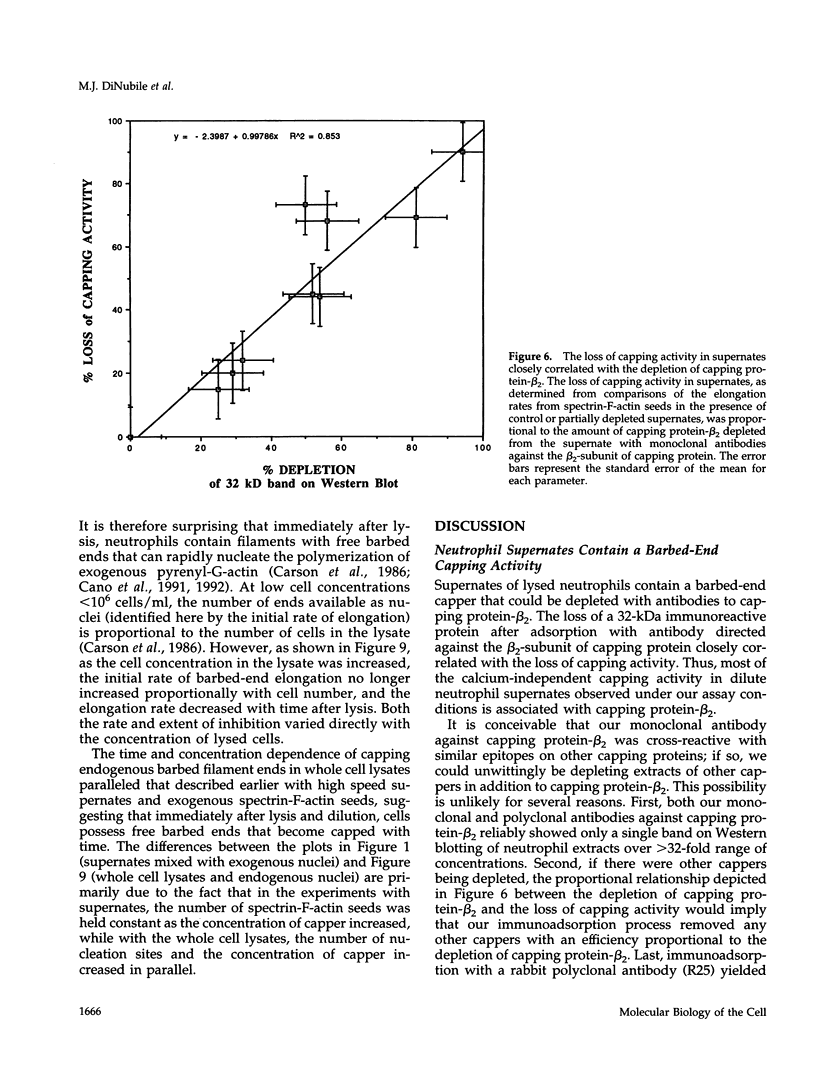
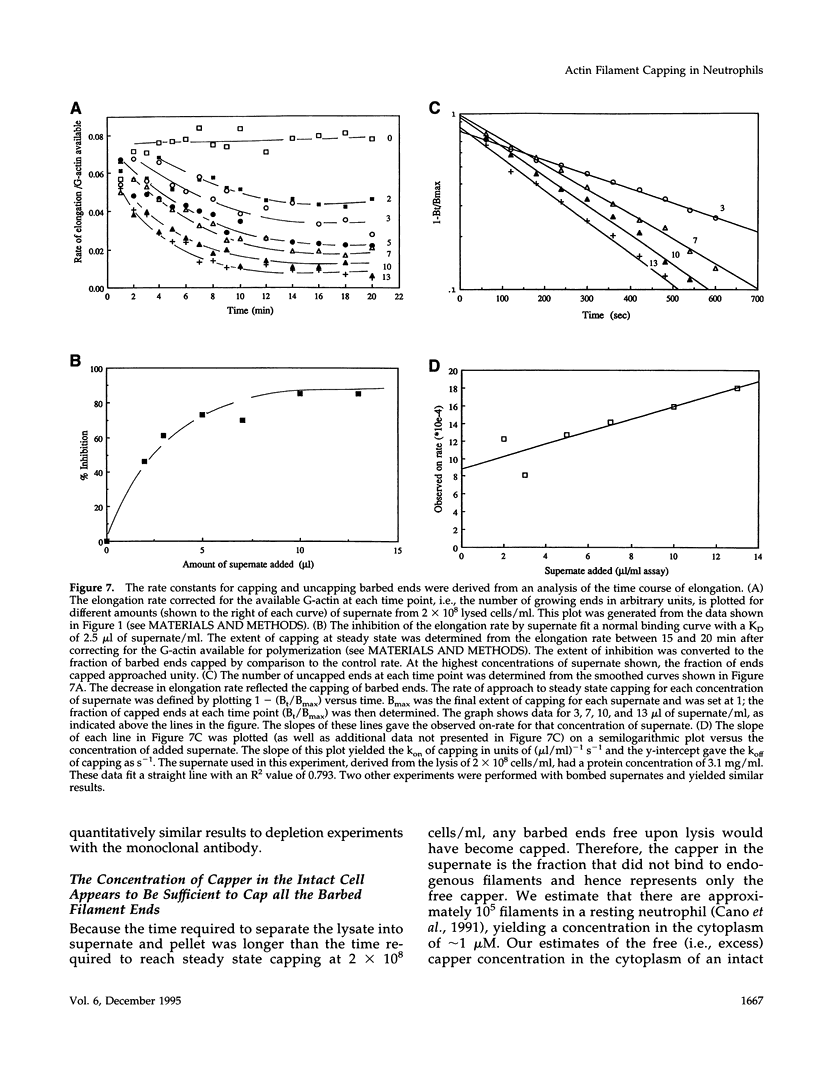
A barbed-end capping activity was found in high speed supernates of neutrophils lysed in submicromolar calcium. In dilute supernate (> or = 100-fold dilution of cytoplasm), this activity accounted for most of the inhibition of barbed-end elongation of pyrenyl-G-actin from spectrin-F-actin seeds. Pointed-end elongation from gelsolin-capped F-actin seeds was not inhibited at comparable concentrations of supernate, thus excluding actin monomer sequestration as a cause of the observed inhibition. Most of the capping activity was due to capping protein-beta 2 (a homologue of cap Z). Thus, while immunoadsorption of > or = 95% of the gelsolin in the supernate did not decrease capping activity, immunoadsorption of capping protein-beta 2 reduced capping activity proportionally to the amount of capping protein-beta 2 adsorbed. Depletion of > 90% of capping protein-beta 2 from the supernate removed 90% of its capping activity. The functional properties of the capping activity were defined. The dissociation constant for binding to barbed ends (determined by steady state and kinetic analyses) was approximately 1-2 nM; the on-rate of capping was between 7 x 10(5) and 5 x 10(6) M-1 s-1; and the off-rate was approximately 2 x 10(-3) s-1. The concentration of capper free in the intact cell (determined by adsorption of supernate with spectrin-actin seeds) was estimated to be approximately 1-2 microM. Thus, there appeared to be enough high affinity capper to cap all the barbed ends in vivo. Nevertheless, immediately after lysis with detergent, neutrophils contained sites that nucleate barbed-end elongation of pyrenyl-G-actin. These barbed ends subsequently become capped with a time course and concentration dependence similar to that of spectrin-F-actin seeds in high speed supernates. These observations suggest that, despite the excess of high affinity capper, some ends either are not capped in vivo or are transiently uncapped upon lysis and dilution.
Full text
PDF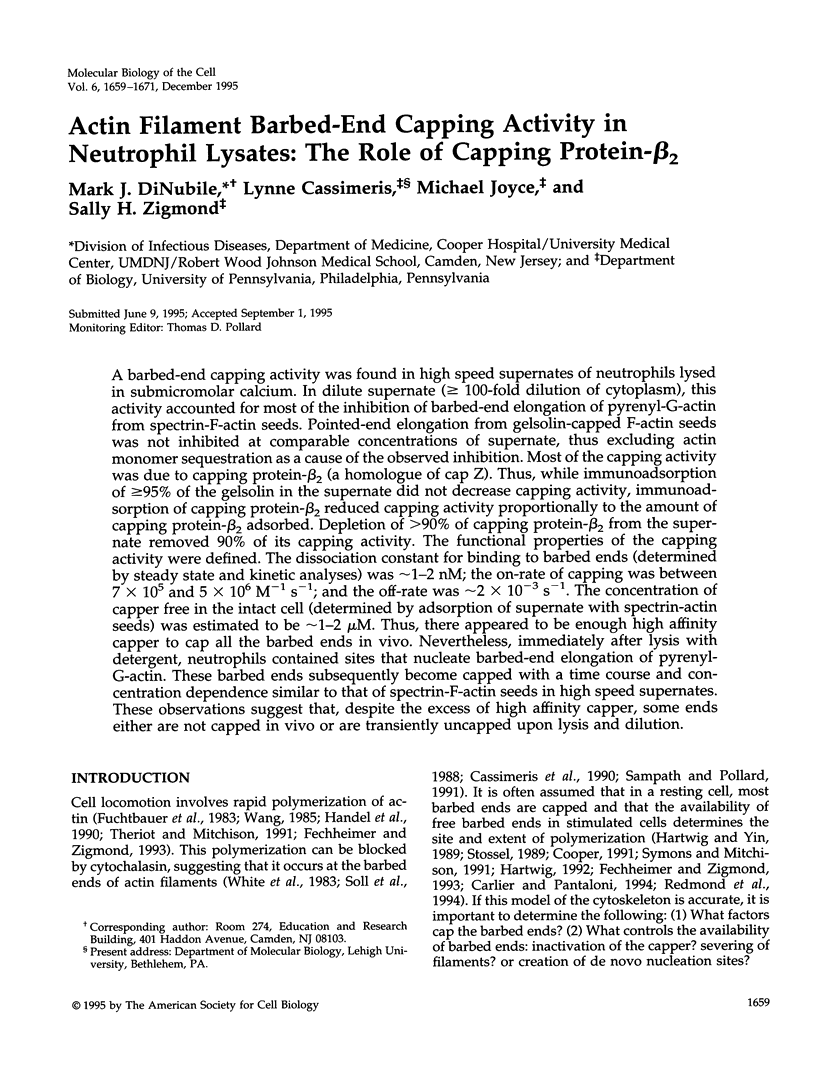




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. F., Gattermeir D. J., Karpova T. S., Cooper J. A. Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J Cell Biol. 1992 Dec;119(5):1151–1162. doi: 10.1083/jcb.119.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E., Brink M., Gerisch G., Isenberg G., Noegel A., Schleicher M., Segall J. E., Wallraff E. A Dictyostelium mutant deficient in severin, an F-actin fragmenting protein, shows normal motility and chemotaxis. J Cell Biol. 1989 Mar;108(3):985–995. doi: 10.1083/jcb.108.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. E., Heiss S. G., Mermall V., Cooper J. A. Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry. 1989 Oct 17;28(21):8506–8514. doi: 10.1021/bi00447a036. [DOI] [PubMed] [Google Scholar]
- Cano M. L., Cassimeris L., Fechheimer M., Zigmond S. H. Mechanisms responsible for F-actin stabilization after lysis of polymorphonuclear leukocytes. J Cell Biol. 1992 Mar;116(5):1123–1134. doi: 10.1083/jcb.116.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M. L., Lauffenburger D. A., Zigmond S. H. Kinetic analysis of F-actin depolymerization in polymorphonuclear leukocyte lysates indicates that chemoattractant stimulation increases actin filament number without altering the filament length distribution. J Cell Biol. 1991 Nov;115(3):677–687. doi: 10.1083/jcb.115.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Actin assembly in response to extracellular signals: role of capping proteins, thymosin beta 4 and profilin. Semin Cell Biol. 1994 Jun;5(3):183–191. doi: 10.1006/scel.1994.1023. [DOI] [PubMed] [Google Scholar]
- Carson M., Weber A., Zigmond S. H. An actin-nucleating activity in polymorphonuclear leukocytes is modulated by chemotactic peptides. J Cell Biol. 1986 Dec;103(6 Pt 2):2707–2714. doi: 10.1083/jcb.103.6.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella J. F., Craig S. W., Maack D. J., Brown A. E. Cap Z(36/32), a barbed end actin-capping protein, is a component of the Z-line of skeletal muscle. J Cell Biol. 1987 Jul;105(1):371–379. doi: 10.1083/jcb.105.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella J. F., Maack D. J., Lin S. Purification and initial characterization of a protein from skeletal muscle that caps the barbed ends of actin filaments. J Biol Chem. 1986 Aug 15;261(23):10915–10921. [PubMed] [Google Scholar]
- Cassimeris L., McNeill H., Zigmond S. H. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J Cell Biol. 1990 Apr;110(4):1067–1075. doi: 10.1083/jcb.110.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L., Safer D., Nachmias V. T., Zigmond S. H. Thymosin beta 4 sequesters the majority of G-actin in resting human polymorphonuclear leukocytes. J Cell Biol. 1992 Dec;119(5):1261–1270. doi: 10.1083/jcb.119.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Blum J. D., Pollard T. D. Acanthamoeba castellanii capping protein: properties, mechanism of action, immunologic cross-reactivity, and localization. J Cell Biol. 1984 Jul;99(1 Pt 1):217–225. doi: 10.1083/jcb.99.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Eddy R. J., Sauterer R. A., Condeelis J. S. Aginactin, an agonist-regulated F-actin capping activity is associated with an Hsc70 in Dictyostelium. J Biol Chem. 1993 Nov 5;268(31):23267–23274. [PubMed] [Google Scholar]
- Fechheimer M., Zigmond S. H. Focusing on unpolymerized actin. J Cell Biol. 1993 Oct;123(1):1–5. doi: 10.1083/jcb.123.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füchtbauer A., Jockusch B. M., Maruta H., Kilimann M. W., Isenberg G. Disruption of microfilament organization after injection of F-actin capping proteins into living tissue culture cells. 1983 Jul 28-Aug 3Nature. 304(5924):361–364. doi: 10.1038/304361a0. [DOI] [PubMed] [Google Scholar]
- Hall A. L., Warren V., Dharmawardhane S., Condeelis J. Identification of actin nucleation activity and polymerization inhibitor in ameboid cells: their regulation by chemotactic stimulation. J Cell Biol. 1989 Nov;109(5):2207–2213. doi: 10.1083/jcb.109.5.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel S. E., Hendry K. A., Sheterline P. Microinjection of covalently cross-linked actin oligomers causes disruption of existing actin filament architecture in PtK2 cells. J Cell Sci. 1990 Oct;97(Pt 2):325–333. doi: 10.1242/jcs.97.2.325. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Noegel A. A., Eckerskorn C., Rapp S., Schleicher M. Ca2+-independent F-actin capping proteins. Cap 32/34, a capping protein from Dictyostelium discoideum, does not share sequence homologies with known actin-binding proteins. J Biol Chem. 1989 Jul 25;264(21):12639–12647. [PubMed] [Google Scholar]
- Hartmann H., Schleicher M., Noegel A. A. Heterodimeric capping proteins constitute a highly conserved group of actin-binding proteins. Dev Genet. 1990;11(5-6):369–376. doi: 10.1002/dvg.1020110508. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992 Sep;118(6):1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Yin H. L. The organization and regulation of the macrophage actin skeleton. Cell Motil Cytoskeleton. 1988;10(1-2):117–125. doi: 10.1002/cm.970100116. [DOI] [PubMed] [Google Scholar]
- Howard T., Chaponnier C., Yin H., Stossel T. Gelsolin-actin interaction and actin polymerization in human neutrophils. J Cell Biol. 1990 Jun;110(6):1983–1991. doi: 10.1083/jcb.110.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug C., Jay P. Y., Reddy I., McNally J. G., Bridgman P. C., Elson E. L., Cooper J. A. Capping protein levels influence actin assembly and cell motility in dictyostelium. Cell. 1995 May 19;81(4):591–600. doi: 10.1016/0092-8674(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Aebi U., Pollard T. D. An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature. 1980 Dec 4;288(5790):455–459. doi: 10.1038/288455a0. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth M. C., Bryan J. Platelet activation induces the formation of a stable gelsolin-actin complex from monomeric gelsolin. J Biol Chem. 1984 Jun 25;259(12):7473–7479. [PubMed] [Google Scholar]
- Lamb J. A., Allen P. G., Tuan B. Y., Janmey P. A. Modulation of gelsolin function. Activation at low pH overrides Ca2+ requirement. J Biol Chem. 1993 Apr 25;268(12):8999–9004. [PubMed] [Google Scholar]
- Lind S. E., Janmey P. A., Chaponnier C., Herbert T. J., Stossel T. P. Reversible binding of actin to gelsolin and profilin in human platelet extracts. J Cell Biol. 1987 Aug;105(2):833–842. doi: 10.1083/jcb.105.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. M., Weber A., Knox M. K. Myosin subfragment 1 binding to relaxed actin filaments and steric model of relaxation. Biochemistry. 1981 Feb 3;20(3):641–649. doi: 10.1021/bi00506a030. [DOI] [PubMed] [Google Scholar]
- Redmond T., Tardif M., Zigmond S. H. Induction of actin polymerization in permeabilized neutrophils. Role of ATP. J Biol Chem. 1994 Aug 26;269(34):21657–21663. [PubMed] [Google Scholar]
- Sampath P., Pollard T. D. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991 Feb 19;30(7):1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- Sauterer R. A., Eddy R. J., Hall A. L., Condeelis J. S. Purification and characterization of aginactin, a newly identified agonist-regulated actin-capping protein from Dictyostelium amoebae. J Biol Chem. 1991 Dec 25;266(36):24533–24539. [PubMed] [Google Scholar]
- Schafer D. A., Korshunova Y. O., Schroer T. A., Cooper J. A. Differential localization and sequence analysis of capping protein beta-subunit isoforms of vertebrates. J Cell Biol. 1994 Oct;127(2):453–465. doi: 10.1083/jcb.127.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher M., Gerisch G., Isenberg G. New actin-binding proteins from Dictyostelium discoideum. EMBO J. 1984 Sep;3(9):2095–2100. doi: 10.1002/j.1460-2075.1984.tb02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbrunn A., Tashjian H., Jr Characterization of functional receptors for somatostatin in rat pituitary cells in culture. J Biol Chem. 1978 Sep 25;253(18):6473–6483. [PubMed] [Google Scholar]
- Soll D. R., Voss E., Varnum-Finney B., Wessels D. "Dynamic Morphology System": a method for quantitating changes in shape, pseudopod formation, and motion in normal and mutant amoebae of Dictyostelium discoideum. J Cell Biochem. 1988 Jun;37(2):177–192. doi: 10.1002/jcb.240370205. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., DiNubile M. J. Rabbit alveolar macrophages contain a Ca2+-sensitive, 41,000-dalton protein which reversibly blocks the "barbed" ends of actin filaments but does not sever them. J Biol Chem. 1986 Oct 25;261(30):14191–14195. [PubMed] [Google Scholar]
- Southwick F. S., Young C. L. The actin released from profilin--actin complexes is insufficient to account for the increase in F-actin in chemoattractant-stimulated polymorphonuclear leukocytes. J Cell Biol. 1990 Jun;110(6):1965–1973. doi: 10.1083/jcb.110.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Symons M. H., Mitchison T. J. Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol. 1991 Aug;114(3):503–513. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. Actin microfilament dynamics in locomoting cells. Nature. 1991 Jul 11;352(6331):126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A., Maciver S. F-actin capping proteins. Curr Opin Cell Biol. 1993 Feb;5(1):63–69. doi: 10.1016/s0955-0674(05)80009-2. [DOI] [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Sha'afi R. I. Stimulation by chemotactic factor of actin association with the cytoskeleton in rabbit neutrophils. Effects of calcium and cytochalasin B. J Biol Chem. 1983 Nov 25;258(22):14041–14047. [PubMed] [Google Scholar]
- Witke W., Sharpe A. H., Hartwig J. H., Azuma T., Stossel T. P., Kwiatkowski D. J. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995 Apr 7;81(1):41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- Yin H. L. Gelsolin: calcium- and polyphosphoinositide-regulated actin-modulating protein. Bioessays. 1987 Oct;7(4):176–179. doi: 10.1002/bies.950070409. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Young C. L., Southwick F. S., Weber A. Kinetics of the interaction of a 41-kilodalton macrophage capping protein with actin: promotion of nucleation during prolongation of the lag period. Biochemistry. 1990 Mar 6;29(9):2232–2240. doi: 10.1021/bi00461a005. [DOI] [PubMed] [Google Scholar]
- Yu F. X., Johnston P. A., Südhof T. C., Yin H. L. gCap39, a calcium ion- and polyphosphoinositide-regulated actin capping protein. Science. 1990 Dec 7;250(4986):1413–1415. doi: 10.1126/science.2255912. [DOI] [PubMed] [Google Scholar]




