Abstract
Human reproductive behaviour is marked by exceptional variation at the population and individual level. Human behavioural ecologists propose adaptive hypotheses to explain this variation as shifting phenotypic optima in relation to local socioecological niches. Here we review evidence that variation in fertility (offspring number), in both traditional and modern industrialized populations, represents optimization of the life-history trade-off between reproductive rate and parental investment. While a reliance on correlational methods suggests the true costs of sibling resource competition are often poorly estimated, a range of anthropological and demographic studies confirm that parents balance family size against offspring success. Evidence of optimization is less forthcoming. Declines in fertility associated with modernization are particularly difficult to reconcile with adaptive models, because fertility limitation fails to enhance offspring reproductive success. Yet, considering alternative measures, we show that modern low fertility confers many advantages on offspring, which are probably transmitted to future generations. Evidence from populations that have undergone or initiated demographic transition indicate that these rewards to fertility limitation fall selectively on relatively wealthy individuals. The adaptive significance of modern reproductive behaviour remains difficult to evaluate, but may be best understood in response to rising investment costs of rearing socially and economically competitive offspring.
Keywords: fertility, parental investment, human behavioural ecology, demographic transition, life history, sibling competition
1. Introduction
Reproduction does not ensure reproductive success. At our best estimate, an average of less than 50 per cent of offspring born over the course of human evolutionary history contributed to future generations through their own survival and reproduction [1]. Parental strategies to enhance the competitive success of offspring will therefore be subject to strong forces of positive selection, even to the potential detriment of other components of fitness [2]. In this sense, fertility limitation, through a reallocation of resources to parental investment, can represent an adaptive strategy to ensure offspring success [3,4]. Demographers and anthropologists have long recognized that all human societies limit birth rates to some extent, ensuring that few women reach the biological maximum, even under the most favourable conditions [5,6]. Human offspring are also born highly vulnerable and slow-maturing, remaining an energetic burden on parents and extended kin often well into the second decade [7], and in many societies later transfers of wealth at marriage and inheritance are substantial [8,9]. Thus, there is good reason to believe that a trade-off between quantity and ‘quality’ of offspring is fundamental to human life history.
Concentrating initially on traditional high-fertility populations, we review evidence for quantity–quality trade-off effects in the human family, and the key factors that may shift the costs and benefits associated with their resolution. Total fertility rates, a population estimate of the number of children expected for a women surviving throughout her reproductive years (i.e. 15–49), average around four to six for contemporary hunter–gatherers [10]. Agriculturalists usually achieve slightly higher fertility rates, but all subsistence types demonstrate a high degree of heterogeneity both between and within groups [11]. We outline the physiological and psychological mechanisms of human fertility regulation and assess evidence that observed patterns represent the local optimization of family size and parental investment to maximize inclusive fitness at the individual level.
We then consider populations that have undergone or initiated the demographic transition to below replacement fertility universally associated with socioeconomic development from a pre-industrial to industrialized economy [12]. It is often suggested that fertility limitation on this scale can only be understood in evolutionary terms as adaptive lag to novel socioecological factors such as contraception [13,14], or as the product of interaction between cultural evolutionary processes and changing social networks [15,16]. In such models, modern reproductive decision-making is seen as both maladaptive and decoupled from the costs and benefits associated with raising children.
Contrary to this perspective, and following the work of Kaplan and colleagues [17,18], we argue that modernization serves to intensify relationships between parental investment and offspring success, triggering evolved mechanisms of fertility regulation to value offspring quality over quantity(see also [9,19]). Since the earliest work on the European demographic transition, historic and economic demographers have theorized on the importance of increasing child rearing costs in initiating fertility decline [20]. However, such costs are more commonly conceptualized at the level of the parent rather than a function of child outcomes, and have largely been examined through crude correlations between population socioeconomic indicators and the timing of transitions, which are often weak [21,22]. Surprisingly few studies have provided direct tests of how modernization influences the returns on parental investment to dimensions of offspring success. Here we show that quantity–quality trade-offs are often substantial in modern populations and highlight new research showing that increases in population or individual wealth can serve to magnify the benefits of low fertility for parents and offspring.
2. Family size and offspring success in humans
(a). The evidence for quantity–quality trade-offs
Studies of child mortality provide strong support for a quantity–quality trade-off model when spacing between births is narrow, with most populations demonstrating elevated mortality in the presence of a closely spaced sibling [23–25]. These costs are probably best explained by poor recovery of maternal somatic resources between births and by dilution of the particularly intense care required in the first years of life. Chances of early survival are also substantially reduced in children from multiple births [26,27].
Considering associations between family size and offspring outcomes across the full range of observed birth intervals presents a more complex picture. Table 1 summarizes studies by evolutionary anthropologists and demographers exploring relationships between sibling number and fitness-related outcomes (i.e. survival, marriage and reproduction). Many of these studies confirm that the human family is characterized by trade-off effects in the quantity and quality of children. However, for each outcome considered, the effects of large family size appear somewhat variable and in a significant number of cases trade-offs are absent or positive effects are reported.
Table 1.
Evidence for quantity–quality trade-offs in high fertility populations. (≠, no relationship; ↓, negative relationship; ↑, positive relationship; /, relationship varies by population subgroup (see reference for details); ♂, in males; ♀, in females. In all other cases both sexes are lumped into the same analysis. ‘Marital success’: a negative relationship indicates lower likelihood of marriage, later age at marriage and/or poor spousal quality. ‘Fertility’: no. of offspring born, except for Borgerhoff Mulder [33,69] and Gibson [41] who measure number of surviving offspring. Note that relationships between sibling number and marital success/fertility are estimated in living adults and consequently do not adjust for the zero success of non-survivors.)
| population | predictor | outcome |
references | ||
|---|---|---|---|---|---|
| survival | marital success | fertility | |||
| contemporary hunter–gatherer | |||||
| !Kung of Botswana | no. of siblings | ≠♂, ≠♀ | — | ↑♂, ≠♀ | [28,29] |
| Aché of Paraguay | no. of siblings | ↓ | — | ↑♂, ≠♀ | [30] |
| contemporary agriculturalist/pastoralist | |||||
| Dogon of Mali | no. of siblings | ↓ | — | — | [32] |
| Bimoba and Kusasi of Ghana | no. of siblings | ↓ | — | — | [31] |
| Gabbra of Kenya | no. of older brothers | — | ↓♂ | ↓♂ | [40] |
| no. of older sisters | — | — | ≠♀ | ||
| no. of sisters | — | — | ↑♂ | ||
| Kipsigis of Kenya | no. of brothers | — | ↓♂ | ↓♂, ≠♀ | [33,69] |
| no. of sisters | — | ↑♂ | ↑♂, ≠♀ | ||
| no. of older siblings | ↑ | ||||
| Arsi Oromo of Ethiopia | no. of older brothers | — | ≠/↓♂ | ≠/↓♂ | [41] |
| historical agriculturalist | |||||
| nineteenth-century N. America | no. of siblings | ↓ | — | — | [35] |
| eighteenth- to nineteenth-century Finland | no. of siblings | ↓ | — | ≠/↑ | [34] |
| nineteenth-century Sweden | no. of siblings | — | — | ↓♂, ↓♀ | [43] |
| eighteenth- to nineteenth-century Germany | no. of same-sex siblings | ≠/↓♂, ≠/↓♀ | ≠/↓♂, ≠/↓♀ | — | [36] |
Studies of hunter–gatherer communities have not found strong evidence of a quantity–quality trade-off. In the !Kung, an African hunter–gatherer group on which the earliest studies of human life history were carried out [23], researchers have failed to demonstrate higher mortality in children with many siblings [28,29]. In the South American Aché, number of siblings depressed likelihood of survival between the ages of 5 and 9. However, mortality below these ages was uninfluenced by parental fertility [30, p. 382]. Furthermore, in both populations, large sibships failed to depress female fertility and were actually associated with higher fertility for males [28,30].
Predicted negative relationships between family size and child survival have been more successfully demonstrated in a number of contemporary African agriculturalist societies ([31,32]; but see [33]) and historical European and American populations [34–36]. It should also be noted that a number of related studies have presented evidence of an association between family size and child anthropometric status which probably predicts future survival. Negative effects of high parental fertility have been suggested in the South American Yanomamö and Shuar ([37,38]; see also [39]).
Studies of marital and reproductive success focusing on the division of inherited capital such as land or cattle, show clear costs of resource division between siblings who survive childhood. As inheritance usually goes to males, these effects are particularly visible in sons. For example, Mace [40] found a negative effect of older brothers on male fertility in the Kenyan Gabbra. This resulted from smaller initial bridewealth herds and later age at marriage for later born sons in comparison with their elder brothers. Number of sisters, however, had a moderately positive effect on male fertility. Similar effects on the number of surviving offspring have been demonstrated on the Kenyan Kipsigis [33] and the Ethiopian Arsi Oromo [41]. Gillespie et al. [34] found that large sibships reduced survival, but not fertility among survivors in eighteenth- to nineteenth-century Finland. However, this analysis did not test for sex-specific effects (see [42]). In an analysis of nineteenth-century Swedish data, Low [43] found that fertility reported in both men and women decreased as the number of siblings increased, but particularly for men, and particularly with respect to number of brothers. Voland & Dunbar [36] show that in eighteenth- to nineteenth-century Germany, the number of same-sex siblings reduced the likelihood of marriage, which is expected to reduce reproductive success for both sexes. Much of the variation between studies may be attributable to socioecological context.
(b). Socioecological context
Quality of offspring is determined by a range of factors in addition to parental investment, and not all contributions of parents are subject to allocation trade-offs (e.g. the quality of genetic inheritance or the social reputation of belonging to a particular family). Variation in local subsistence and inheritance systems, and in the wider environmental determinants of offspring success, will thus be influential to the form and consequence of sibling competition.
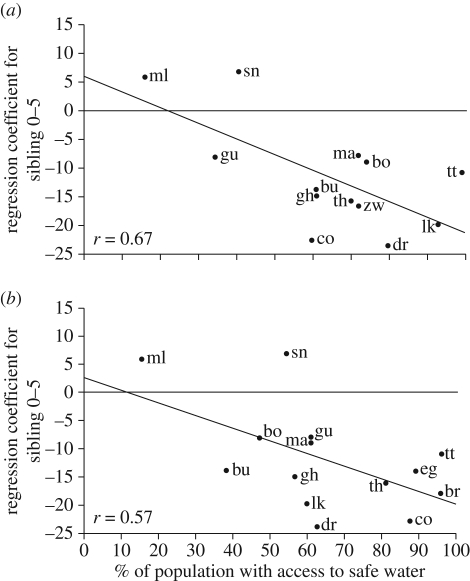
Many researchers have stressed the importance of extrinsic, or ‘care-independent’, environmental risks in establishing the local returns to parental investment [29,44,45]. High pathogen loads, unpredictable and frequent food or water shortages, warfare and intra-group violence all have the potential to introduce negative outcomes largely beyond parental control. This may be particularly true during early childhood, when offspring are vulnerable and mortality high. Desai [39] demonstrates support for the idea that such risks can by extension reduce the impact of sibling competition. In her comparative study of developing populations, using data from the Demographic and Health Surveys, negative effects of large sibship size on child height were weakest in populations with poor access to healthcare facilities or safe drinking water. In the absence of such initiatives, parents may have limited ability to protect children from environmental assaults, such as pathogen stress or crop failure, weakening any advantage to concentrating investment in relatively few offspring (figure 1).
Figure 1.
Siblings and childhood height in populations at varying levels of economic development. This graph plots the regression coefficient for associations between each additional sibling aged 0–5 years and child's height-for-age in each population against population access to (a) health services; (b) safe water supply (adapted from [39]). Access to health services and safe water supply is associated with relatively larger costs of large family size on childhood height. All data are from the Demographic and Health Surveys (bo, Bolivia; br, Brazil; bu, Burundi; co, Colombia; dr, Dominican Republic; eg, Egypt; gh, Ghana; gu, Guatemala; lk, Sri Lanka; ma, Morocco; ml, Mali; sn, Senegal; th, Thailand; tt, Trinidad and Tobago; zw, Zimbabwe). (Reprinted with permission from Desai S. 1995. Copyright © [Taylor & Francis Ltd.], http://www.informaworld.com).
Children may also relax their early dependence on parents by varying degrees through contribution to subsistence activities, including the direct care of younger siblings (see [46] for discussion of how subsistence type influences children's ability to help). However, in no population has it been demonstrated that children entirely offset their own energetic costs, implying that additional siblings will always dictate some division of family resources [7]. Furthermore, while it is possible that caring for younger siblings may provide some benefit to later-borns, offspring engaged in helping activities may consequently take a longer time to reach independence, limiting the overall benefit to parents. Wider patterns of cooperative breeding, whereby extended kin share the burden of child-rearing, may be more effective at relieving competition between siblings [39,47]. This will particularly be the case when kin support responds to demand (i.e. parents with more children receive more help).
The extent to which resources are transferred across generations will influence competition between siblings in adulthood, when resource transfers determine chances of marriage and future means of production [33,40]. This may explain why trade-off effects have proved easier to demonstrate in agriculturalist populations rather than hunter–gatherers, where resource inequality and intergenerational resource transmission are both relatively low [8]. Supporting this explanation, Voland & Dunbar [36] found that negative effects of large family size were unique to landowning families in the Krummhörn, while for peasants, offspring success was determined by other means. Gibson and Gurmu [41] also shows that number of older brothers holds negative associations with marital and reproductive success (number of surviving offspring) in rural Ethiopian farmers only when land quality had been determined by inheritance from parents. In otherwise comparable men who received land allocations from a government redistribution scheme, the existence of older brothers had no negative consequences.
Finally, it is worth noting that the local opportunity for sibling aggression can exaggerate the negative consequences of resource competition when the stakes are especially high (for animal examples: [48,49]). Human history provides many examples of intra-family conflict in the succession to inheritance. For example, during the fifteenth to seventeenth century, it was law that all surviving brothers be murdered at the appointment of a new Sultan of the Ottoman Empire—in the most famous case Mehmet III ordered the execution of 19 brothers [50]. This practice was explicitly intended to minimize disputes to the throne and associated political instability. Alternatively, when other factors ensure little resource competition, siblings may boost each other's success through cooperative activities by, for example, gaining political advantage in community disputes [28].
(c). Methodological issues
It is essential to recognize that all of the studies reviewed here are correlational in design and so potentially subject to confounding associations which may mask true relationships between reproductive behaviour and offspring success. This issue is well recognized in life-history research, but often poorly addressed because relevant heterogeneity is difficult to measure effectively [30,51,52]. Failure to adequately account for differences in resource access is especially important as ‘wealthier’ individuals may invest relatively more in all traits, creating the illusion of a positive association between competing functions [53]. As such, it is likely that much research underestimates the true costs of sibling competition.
Draper & Hames (2000) for example, who found positive relationships between family size and offspring success in the !Kung, made no statistical adjustment for between-family differences, arguing that confounding effects of resource variation are unlikely because hunter–gatherer groups are ‘egalitarian’. This is a weak argument, as even in the absence of significant material wealth, other dimensions of parental condition may be important. For instance, in the foraging-farmer Tsimane of Bolivia, von Rueden et al. [54] document variation in males, by physical condition, skill in resource accumulation, social support and level of acculturation. All of these factors could allow some parents to invest more in both reproductive rate and parental investment, while nevertheless remaining subject to allocation trade-offs. Female physical condition is also clearly important; it is now well established that in many populations children of tall mothers experience significantly lower mortality [55,56].
In agricultural or wage-labour communities, indicators such as land size or quality, amount of livestock and occupation are easy to measure and probably encapsulate related aspects of resource variation such as physical health reasonably well. This may account for the relative success in documenting predicted trade-offs in these populations. However, even here it is not easy to ensure that all relevant differences between small and large families have been taken into account. For example, public health research in western populations demonstrates that socioeconomic status is multi-dimensional and dynamic across the life course, so that choice of measure can influence results considerably [57]. Furthermore, practically all studies conceptualize and measure resource access at the individual level, but this is unlikely to provide an accurate representation when in reality resources occupy ‘pooled energy budgets’ shared between kin and non-kin [58].
In light of these methodological problems, caution must be exercised in directly comparing the studies summarized in table 1. More comparative studies, which seek to replicate research designs across environments are needed. Desai's [39] cross-national study of demographic surveys (figure 1) illustrates that this approach may be fruitful in isolating variation in sibling costs associated with socioecology rather than methodological differences between studies. However, it should be noted that this particular analysis also has strong limitations. The use of country as the unit of analysis in the face of considerable regional social and environmental variation is questionable. It is also true that a multi-level modelling approach is required for a considered and statistically appropriate analysis of such hierarchical data (e.g. [55,59]).
Compared with experimental designs, correlational studies also suffer from weak inference of causality. This may be a particular problem when considering associations between family size and offspring mortality owing to possible ‘replacement’ or ‘insurance’ effects, whereby a mother has additional births to make up for early deaths, or to compensate for predicted high extrinsic child mortality. Some studies have made attempts to adjust models for this possibility. For example, Strassmann & Gillespie [32] exclude cases of very early death from their model of family size and child mortality in the Dogon (see also [31]). Concerns about causality need to be more explicitly addressed in future research by testing whether a division of parental investment truly drives quantity–quality trade-off effects.
3. Mechanisms of fertility regulation
The behavioural ecology approach predicts that observed life histories represent ecologically dependent optima of inclusive fitness maximization (sometimes referred to as the ‘individual optimization hypothesis’: [60]). Pathways to inheritance (genetic or cultural) and the physiological and cognitive mechanisms that lie behind reaction norms are not seen to seriously limit adaptive responses to the environment. As our species has successfully colonized a diverse range of environments, which could only be possible with inherently high levels of adaptive phenotypic plasticity [1], this assumption may not be unreasonable, even for industrialized populations [61]. Nevertheless, a complete understanding of human reproductive behaviour requires consideration of the evolved proximate mechanisms that regulate responses to the environment.
For many demographers, physiological mechanisms are seen as the principal regulators of reproduction in high fertility populations. For example, levels of physical stress, nutrition and energy availability are important determinants of age at menarche and the probability of conception [62,63]. Ovulation is also hormonally suppressed when nursing a young infant, preventing subsequent dilution of parental investment at a time when current offspring are highly vulnerable.
We can also expect reproductive decision-making to be regulated by cognitive mechanisms using environmental information on observed or expected relationships between parental investment and offspring development [17,64]. For example, Mathews & Sear [65] used an experimental design to consider the effects of mortality risks on ideal family size in a British university sample. In the treatment group, primed by questions on local mortality risks, men stated a preference for relatively high fertility. Newson et al. [15] have also argued that as successful reproduction is often dependent on alloparental help [47], our fertility decisions have evolved to be particularly sensitive to the local composition and influence of social networks.
Finally, behavioural pathways of fertility regulation may often be institutionalized in social institutions and cultural practices, such as rules regulating marriage, inheritance, celibacy, contraception, infanticide and abandonment [17,66]. In modern societies it has also been argued that we can add use of reproductive technologies including abortion and artificial fertility treatments to the list of strategic tools available to optimize reproductive timing and achieved family size [67].
4. The optimization of family size in traditional populations
(a). Predicted optima and observed fertility
Animal behavioural ecologists have tested whether or not observed clutch sizes are optimal with the prediction that neither the experimental addition nor removal of young will result in increased parental fitness relative to control broods (e.g. [60,68]). Anthropologists have to make do with alternative methods. One approach has been to first determine the fertility level that leads to the highest fitness returns in some measurable currency (while controlling for heterogeneity in parental resources) and then to compare this with the population mode. If fertility is optimized, then optimal and modal fertility should converge.
Studies of the !Kung [28,29] and Aché [30] indicate strong positive linear relationships between fertility and number of grandchildren. This implies that both groups of hunter–gatherers failed to optimize family size, as higher fitness could have been achieved by increasing fertility beyond observed levels. Borgerhoff Mulder's [69] study of the Kipsigis found that intermediate numbers of children maximized grandchildren for women, but not for men. For women, the calculated optima corresponded with the population mode. In the Dogon, Strassmann & Gillespie [32] found that family size had a negative effect on child survival rates, so that an intermediate level of fertility (eight offspring) optimized this measure of reproductive success. A majority of women had a completed fertility within the confidence limits of this estimate, leading the authors to conclude that observed family size optimized parental fitness. However, more recent studies of child survival attempting to replicate the results of Strassmann & Gillespie [32] have found no evidence that intermediate levels of fertility maximize the number of surviving children (e.g. [31]).
The mixed success of these studies may largely rest in the difficulty involved in calculating precise fertility optima with available data. Lifetime reproductive success, as measured by the number of surviving children or grandchildren, is probably an effective proxy for fitness in many ecologies, provided mortality rates are relatively high. However, studies focusing on child survival alone will not detect negative effects of large family size, which become apparent in later life, such as through early death of the mother [31] or in future generations caused by the division of inherited resources [9,69]. Hence, such studies are likely to systematically overestimate the optimum family size. This line of reasoning is consistent with the fact that all studies that have failed to demonstrate a convergence between modal and optimal fertility have suggested that observed levels lie below the optimum.
(b). Resources and reproductive success
A more generalized approach, that does not require the calculation of precise optima, is to consider covariation in the strength of trade-off effects and observed fertility patterns. Life-history models have emphasized that negative effects of resource competition between offspring will be most severe when resources are relatively scarce, as this reinforces the assumption of finite parental resources [28,30,31,34,38,69]. This position is also empirically supported by a number of studies [31,34,69]. Consistent with widespread optimization of fertility, wealthier individuals have been shown to raise larger families in practically all traditional societies where such relationships have been considered [70].
5. Parental investment and fertility decline
(a). Modern low fertility and adaptive lag
Around half of the world's population now lives in countries where total fertility rates have fallen below replacement level [12,71]. This dramatic shift immediately appears at odds with adaptive models of fertility optimization [14]. Firstly, the substantial increases in personal and societal wealth associated with fertility decline eliminate any fitness cost of large family size on offspring survival or reproduction [72,73]. Secondly, fertility decline within societies is generally characterized by markedly larger reductions of fertility in wealthy families compared with the rest of the population [74,75]. As a consequence, modern fertility is not only dramatically reduced in comparison with traditional populations but is also typified by relative socioeconomic levelling [70]. Thus, contrary to adaptive predictions, relationships between wealth and fertility are typically recorded as null or negative in demographic surveys [18,76]. Some studies have suggested that when education is held constant, positive correlations between income and fertility persist, at least for males [70,77]. However, these associations remain considerably weaker than comparable relationships in traditional populations [70].
For many researchers, fertility limitation on this scale can be understood only in evolutionary terms as the result of adaptive lag to novel cultural change. Maladaptation to new contraceptive technologies [13,78], social changes associated with the fragmentation of extended kin networks [15] and new emerging roles of social prestige in the labour market [79] have all been promoted as explanations for why low fertility persists in the absence of obvious fitness benefits (for parallel frameworks of cultural diffusion and social influence in mainstream demography, see [21,80]). All of these models reject the notion that the costs and benefits of rearing children continue to play a dominant role in reproductive decision-making.
(b). Quantity–quality trade-offs in modern populations
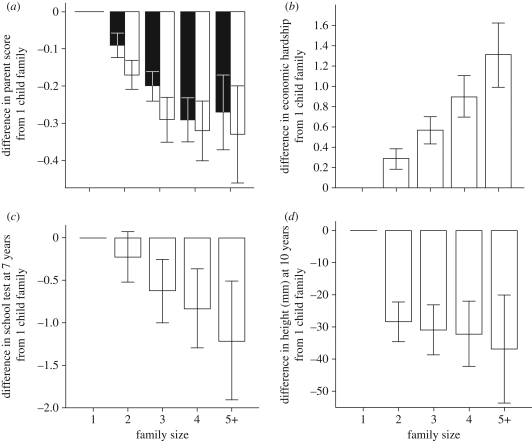
We recently considered relationships between family size, parental investment and a range of child development measures in contemporary British families with detailed longitudinal data (figure 2). While it may be evident that fertility limitation provides little advantage to offspring survival or reproduction, consideration of a broader range of proximate outcomes reveals that high fertility carries important costs to both offspring and parents.
Figure 2.
Family size, parental investment and child development in contemporary British families. The relationship between family size and (a) maternal and paternal allocations of care time (standardized ‘parent scores’) between 1 and 9 years (filled bars, mother score; unfilled bars, partner score; adapted from [51]); (b) maternal perception of economic hardship from 0 to 7 years (adapted from [76]); (c) school test results at 7 years (adapted from [83]); (d) height at age 10 years (from [83,85]). Children with more siblings receive less time from parents, grow up in more economically stressed households and exhibit relatively poor physical and cognitive/educational development. Data are from the Avon Longitudinal Study of Parents and Children, a large cohort study (n = 14 000 and above) of children born in 1991–1992. Confidence intervals are set at 95%.
Lawson & Mace [51] demonstrate that family size has a negative influence on both mother's and father's time spent engaged in childcare over the first decade of life (including activities such as helping with homework, reading to or physical play). Number of siblings had a larger influence on this measure of parental investment than any other covariate considered, including socioeconomic indicators and parental age (see also [81,82]). Lawson & Mace [76] also found that in the struggle to feed, clothe and house more children, parents from large family households reported increased financial difficulty even after adjustment for a range of factors including differences in income, education and ethnicity. Children with many siblings performed significantly worse on formal educational assessments and IQ tests throughout childhood [83], a pattern now well-established across many developed populations [81,84]. Finally, we also find evidence that the presence of siblings is associated with deficits in childhood growth, which may stem from reduced parental attention to healthcare or nutrition in early life [85].
Available evidence from related studies further demonstrates that the costs of large family size to offspring persist into adulthood. Cooney & Uhlenberg [86] for example, have reported that number of siblings is negatively related to a range of later investments including the direct receipt of money or gifts, giving advice in difficult decisions and direct assistance with childcare (see also [87]). Keister [88] has also demonstrated that number of siblings has a strong negative influence on the likelihood of receiving a trust fund or an inheritance. In modern populations, childhood height is closely associated with adult height [89]. On average, taller adults have improved health status and live longer [90]. Poor performance on cognitive tests in childhood is also predictive of poor adult educational qualifications and social mobility [91]. Finally, and of particular relevance to evolutionary theories of modern low fertility, Keister [88,92] shows that the combined effects of large family size on inheritance sums and potential for income generation are responsible for strong negative relationships between family size and adult wealth ownership (see also [72]). This implies that high-fertility strategies in modern populations will have important negative consequences for the wealth of future generations.
In the presence of these quantity–quality trade-off effects, it must be recognized that modern low fertility may maximize long-term fitness if immediate deficits in reproductive success are eventually offset by acquired benefits to wealth inheritance or other predictors of lineage survival. This scenario is supported by a number of mathematical models([9,93,94]; see also [95]), but remains difficult to evaluate in the absence of sufficient multi-generational data. Alternatively, as argued by Kaplan, low fertility is maladaptive, but nevertheless best understood as the product of an evolved psychology that regulates reproduction in balance with the local effects of parental investment on offspring status. This psychology may fail to function adaptively in modern contexts because novel factors, in particular the establishment of skill-based wage economies, offer radically extended scope for status competition between individuals at levels which now fail to translate into significant survival or reproductive benefits [17,18].
(c). The influence of modernization on sibling competition
Consistent with Kaplan's [17] theoretical model, a number of studies now indicate that, even while overall mortality is in decline, the early stages of demographic transition establish increasing pay-offs to fertility limitation on alternative measures of offspring quality. This reverses the traditional life-history perspective that resource scarcity drives quantity–quality trade-offs.
As we have already discussed, a reduction in extrinsic environmental risks, such as vulnerability to infectious disease, environmental catastrophes or political instability, is one factor that can increase parental returns to concentrating investment in fewer offspring. Desai [39] used a measure of child physical development (early growth) to demonstrate this effect in her cross-national study, indicating that sibling competition for nutritional status can increase with a population's socioeconomic development (figure 1). Child education appears to follow the same pattern. Quantity–quality trade-off effects on educational success, considered as one of the most robust relationships in sociology, are often absent in developing countries [33,39,84]. Yet, recent studies suggest that negative effects of large family size are becoming increasingly evident over time and as communities urbanize ([96], see also [97]). Gibson & Lawson [98] compare parental investments in schooling in rural Ethiopian villages with and without installed tap stands, which have dramatically reduced childhood mortality. In villages where tap stands have been installed, parents were more likely to invest in educating children and more likely to focus this investment on early born children. Parental perceptions of increased reliability of long-term benefits to investment may lie behind this shift.
Once skill-based wage economies become fully established, there is also evidence that the benefits to fertility limitation fall selectively on the wealthy. This may be because investments in skill-acquisition or direct transfers of wealth now dramatically increase an offspring's ability to generate new wealth over the life course and further invest in their own status [17,95]. The presence of a functioning welfare state may also reduce investment competition in the poorest families through guaranteed provisioning of basic schooling, healthcare and social opportunity; consequently families with potential to invest above this ‘base’ level (e.g. in private schooling, healthcare, etc.) may experience more substantial costs to investment division [76].
Keister [92] and Grawe [99] both demonstrate that large family size is associated with negative consequences for the income generation and wealth ownership of offspring in middle and high socioeconomic families in the US, but of relatively little consequence to children from impoverished backgrounds. Lawson & Mace [76] have also reported that relatively well-educated British mothers record larger increases in perceived economic hardship associated with reproducing above the two-child norm, suggesting that the perceived costs of high fertility are magnified in high socioeconomic strata.
(d). Mate choice when parental investment is critical
The findings reviewed above suggest that modern low-fertility patterns emerge in response to new payoffs to high parental investment. With parental investment fundamental to modern reproductive strategies, we can also expect the ability of parents to pass on the skills required for children to compete to become key criteria in mating markets [19]. Lawson & Mace [51] show that in contemporary British families some children have all the luck: those children with a high investing mother tend to also have a high investing father; they tend to be the wealthier and better educated parents; and the results of this investment are apparent in a range of measures of child health and educational success (figure 2). There is clearly some kind of assortative mating going on, in which individuals with the potential to invest highly are forming families with those having the potential to invest highly. This need to be extremely choosy in selecting a mate may reinforce low-fertility patterns.
Childlessness is commonly an outcome of failing to find or retain a suitable partner. Low socioeconomic status men are more likely to be childless, although if they do find a partner they are just as likely to reproduce as higher socioeconomic status males [70]. Low socioeconomic status women in Addis Ababa, Ethiopia were also much more likely to fail to reproduce, an effect largely driven by failure to marry [100]. Having a ‘high bar’ in mate choice decisions could also create childlessness even in wealthy women. A strategy of choosiness can be selected for, but some casualities of that strategy will be individuals who wait too long to find a suitable mate. Kokko et al. [101,102] show that high population densities and longer life-expectancy further promote increased choosiness as well as delayed reproduction in animal populations, and a similar situation may be occurring in modern urban settings [94,103]. Late reproduction makes help from grandparents less likely, as they simply get too old, and modern education and employment patterns mean that more and more parents do not live close to kin. This lack of kin support in child-rearing increases the costs of reproduction and focuses yet more attention on the nuclear family as the key unit of investment.
6. Conclusion
Studies of the human family, unable to harness the power of the experimental method, face important methodological challenges in quantifying life-history trade-offs. Nevertheless, available evidence suggests that fertility limitation can be understood as a parental strategy to improve the chances of offspring success. Whether or not such strategies actually maximize parental inclusive fitness is more difficult to determine, with recent declines in fertility associated with modernization particularly difficult to reconcile with adaptive models. Yet, anthropologists and historical demographers agree that this shift to low fertility is also associated with an extension in childhood [104, p. 139]. Offspring remain dependent on parents for longer, and parents invest more time and more resources in individual offspring, than ever before. Models of modern fertility decline solely based on cultural diffusion of social norms or novel contraceptive technologies may regard the concurrence of this shift with low-fertility norms as coincidental, but a fall in fertility rates may also be interpreted as a strategic shift from high fertility to high investment in fewer offspring. Increases in socioeconomic status within modern populations, and between populations at varying levels of development, only serve to intensify the benefits of fertility reduction on offspring success.
Acknowledgements
This review was supported by the UK Economic and Social Research Council (ESRC). We thank Alexandra Alvergne, Monique Borgerhoff Mulder, Rebecca Sear, Richard Webb and two anonymous reviewers for critical comments on earlier versions of this manuscript.
Footnotes
One contribution of 14 to a Theme Issue ‘Evolution and human behavioural diversity’.
References
- 1.Wells J. C. K., Stock J. T. 2007. The biology of the colonizing ape. Am. J. Phys. Anthropol. 134, 191–222 10.1002/ajpa.20735 (doi:10.1002/ajpa.20735) [DOI] [PubMed] [Google Scholar]
- 2.Jones J. H. 2009. The force of selection on the human life cycle. Evol. Hum. Behav. 30, 305–314 10.1016/j.evolhumbehav.2009.01.005 (doi:10.1016/j.evolhumbehav.2009.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lack D. 1947. The significant of clutch size. Ibis 89, 302–352 10.1111/j.1474-919X.1947.tb04155.x (doi:10.1111/j.1474-919X.1947.tb04155.x) [DOI] [Google Scholar]
- 4.Roff D. A. 2002. Life history evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 5.Bongaarts J. 1975. Why high birth rates are so low. Popul. Dev. Rev. 1, 289–296 10.2307/1972225 (doi:10.2307/1972225) [DOI] [Google Scholar]
- 6.Cleland J. 2001. The effects of improved survival on fertility: a reassessment. Popul. Dev. Rev. 27, 60–92 [Google Scholar]
- 7.Kaplan H., Hill K., Lancaster J., Hurtado A. M. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. Issues News Rev. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [DOI] [Google Scholar]
- 8.Borgerhoff Mulder M., et al. 2009. Intergenerational wealth transmission and the dynamics of inequality in small-scale societies. Science 326, 682–688 10.1126/science.1178336 (doi:10.1126/science.1178336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mace R. 1998. The coevolution of human wealth and inheritance strategies. Phil. Trans. R. Soc. Lond. B 353, 389–397 10.1098/rstb.1998.0217 (doi:10.1098/rstb.1998.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly R. 1995. The foraging spectrum. Washington, DC: Smithsonian Institution Press [Google Scholar]
- 11.Bentley G., Paine R. R., Boldsen J. L. 2001. Fertility changes with the prehistoric transition to agriculture. In Reproductive ecology and human evolution (ed. Ellison P. T.). Hawthorne, NY: Aldine de Gruyter [Google Scholar]
- 12.Lee R. 2003. The demographic transition: three centuries of fundamental change. J. Econ. Perspect. 17, 167–190 10.1257/089533003772034943 (doi:10.1257/089533003772034943) [DOI] [Google Scholar]
- 13.Pérrusse D. 1993. Cultural and reproductive success in industrial societies: testing the relationship at the proximate and ultimate levels. Behav. Brain Sci. 16, 267–323 10.1017/S0140525X00029939 (doi:10.1017/S0140525X00029939) [DOI] [Google Scholar]
- 14.Vining D. R. J. 1986. Social versus reproductive success: the central theoretical problem of human sociobiology. Behav. Brain Sci. 9, 167–216 10.1017/S0140525X00021968 (doi:10.1017/S0140525X00021968) [DOI] [Google Scholar]
- 15.Newson L., Postmes T., Lea S. E. G., Webley P. 2005. Why are modern families small? Toward an evolutionary and cultural explanation for the demographic transition. Personal. Soc. Psychol. Rev. 9, 360–375 10.1207/s15327957pspr0904_5 (doi:10.1207/s15327957pspr0904_5) [DOI] [PubMed] [Google Scholar]
- 16.Richerson P., Boyd R. 2005. Not by genes alone: how culture transformed human evolution. Chicago, IL: University of Chicago Press [Google Scholar]
- 17.Kaplan H. 1996. A theory of fertility and parental investment in traditional and modern human societies. Yearb. Phys. Anthropol. 39, 91–135 [Google Scholar]
- 18.Kaplan H., Lancaster J. B., Tucker W. T., Anderson K. G. 2002. Evolutionary approach to below replacement fertility. Am. J. Hum. Biol. 14, 233–256 10.1002/ajhb.10041 (doi:10.1002/ajhb.10041) [DOI] [PubMed] [Google Scholar]
- 19.Mace R. 2007. The evolutionary ecology of human family size. In The Oxford handbook of evolutionary psychology (eds Dunbar R., Barrett L.), pp. 383–396 Oxford, UK: Oxford University Press [Google Scholar]
- 20.Notestein F. 1953. Economic problems of population change. In Proc. Eighth Int. Conf. Agricultural Economics, pp. 13–31 London, UK: Oxford University Press [Google Scholar]
- 21.Bongaarts J., Watkins S. C. 1996. Social interactions and contemporary fertility transitions. Popul. Dev. Rev. 22, 616–620 10.2307/2137804 (doi:10.2307/2137804) [DOI] [Google Scholar]
- 22.Coale A. J., Watkins S. J. 1986. The decline of fertility in Europe. Princeton, NJ: Princeton University Press [Google Scholar]
- 23.Blurton-Jones N. 1986. Bushman birth spacing: a test of optimal interbirth intervals. Ethol. Sociobiol. 7, 91–105 10.1016/0162-3095(86)90002-6 (doi:10.1016/0162-3095(86)90002-6) [DOI] [Google Scholar]
- 24.Gibson M. A., Mace R. 2006. An energy-saving development initiative increases birth rate and childhood malnutrition in rural Ethiopia. PLoS Med. 3, e87. 10.1371/journal.pmed.0030087 (doi:10.1371/journal.pmed.0030087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobcraft J. N., McDonald J. W., Rutstein S. O. 1985. Demographic determinants of infant and early child mortality: a comparative analysis. Popul. Stud. 39, 363–385 10.1080/0032472031000141576 (doi:10.1080/0032472031000141576) [DOI] [Google Scholar]
- 26.Gabler S., Voland E. 1994. Fitness of twinning. Hum. Biol. 66, 699–713 [PubMed] [Google Scholar]
- 27.Sear R., Shanley D., McGregor I. A., Mace R. 2001. The fitness of twin mothers: evidence from rural Gambia. J. Evol. Biol. 14, 433–443 10.1046/j.1420-9101.2001.00287.x (doi:10.1046/j.1420-9101.2001.00287.x) [DOI] [Google Scholar]
- 28.Draper P., Hames R. 2000. Birth order, sibling investment, and fertility among Ju/'Hoansi (!Kung). Hum. Nat. 11, 116–156 10.1007/s12110-000-1016-0 (doi:10.1007/s12110-000-1016-0) [DOI] [PubMed] [Google Scholar]
- 29.Pennington R., Harpending H. 1988. Fitness and fertility among Kalahari !Kung. Am. J. Phys. Anthropol. 77, 303–319 10.1002/ajpa.1330770304 (doi:10.1002/ajpa.1330770304) [DOI] [PubMed] [Google Scholar]
- 30.Hill K., Hurtado A. M. 1996. Ache life history: the ecology and demography of a foraging people. New York, NY: Aldine de Gruyter [Google Scholar]
- 31.Meij J. J., van Bodegom D., Ziem J. B., Amankwa J., Polderman A. M., Kirkwood T. B. L., de Craen A. J. M., Zwaan B. J., Westendorp R. G. J. 2009. Quality–quantity tradeoff of human offspring under adverse environmental conditions. J. Evol. Biol. 22, 1014–1023 10.1111/j.1420-9101.2009.01713.x (doi:10.1111/j.1420-9101.2009.01713.x) [DOI] [PubMed] [Google Scholar]
- 32.Strassmann B. I., Gillespie B. 2002. Life-history theory, fertility and reproductive success in humans. Proc. R. Soc. Lond. B 269, 553–562 10.1098/rspb.2001.1912 (doi:10.1098/rspb.2001.1912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borgerhoff Mulder M. 1998. Brothers and sisters. How sibling interactions affect optimal parental allocations. Hum. Nat. 9, 119–162 [DOI] [PubMed] [Google Scholar]
- 34.Gillespie D., Russell A., Lummaa V. 2008. When fecundity does not equal fitness: effects of an offspring quantity versus quality trade-off in pre-industrial humans. Proc. R. Soc. B 275, 713–722 10.1098/rspb.2007.1000 (doi:10.1098/rspb.2007.1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penn D. J., Smith K. R. 2007. Differential fitness costs of reproduction between the sexes. Proc. Natl Acad. Sci. USA 104, 553–558 10.1073/pnas.0609301103 (doi:10.1073/pnas.0609301103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voland E., Dunbar R. I. M. 1995. Resource competition and reproduction. Hum. Nat. 6, 33–49 10.1007/BF02734134 (doi:10.1007/BF02734134) [DOI] [PubMed] [Google Scholar]
- 37.Hagen E. H., Hames R. B., Craig N. M., Lauer M. T., Price M. E. 2001. Parental investment and child health in a Yanomamo village suffering short-term food stress. J. Biosoc. Sci. 33, 503–528 10.1017/S002193200100503X (doi:10.1017/S002193200100503X) [DOI] [PubMed] [Google Scholar]
- 38.Hagen E. H., Barrett C., Price M. E. 2006. Do human parents face a quantity-quality tradeoff?: evidence from a Shuar community. Am. J. Phys. Anthropol. 130, 405–418 10.1002/ajpa.20272 (doi:10.1002/ajpa.20272) [DOI] [PubMed] [Google Scholar]
- 39.Desai S. 1995. When are children from large families disadvantaged? Evidence from cross-national analyses. Popul. Stud. 49, 195–210 10.1080/0032472031000148466 (doi:10.1080/0032472031000148466) [DOI] [Google Scholar]
- 40.Mace R. 1996. Biased parental investment and reproductive success in Gabbra pastoralists. Behav. Ecol. Sociobiol. 38, 75–81 10.1007/s002650050219 (doi:10.1007/s002650050219) [DOI] [PubMed] [Google Scholar]
- 41.Gibson M. A., Gurmu E. Submitted Land inheritance establishes sibling competition for marriage and reproduction in rural Ethiopia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faurie C., Russell A. F., Lummaa V. 2009. Middleborns disadvantaged? Testing birth-order effects on fitness in pre-industrial Finns. PLoS ONE 4, e5680. 10.1371/journal.pone.0005680 (doi:10.1371/journal.pone.0005680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Low B. S. 1991. Reproductive life in 19th-century Sweden: an evolutionary perspective on demographic phenomena. Ethol. Sociobiol. 12, 411–448 10.1016/0162-3095(91)90024-K (doi:10.1016/0162-3095(91)90024-K) [DOI] [Google Scholar]
- 44.Quinlan R. J. 2007. Human parental effort and environmental risk. Proc. R. Soc. B 274, 121–125 10.1098/rspb.2006.3690 (doi:10.1098/rspb.2006.3690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winterhalder B., Leslie P. 2002. Risk-sensitive fertility: the variance compensation hypothesis. Evol. Hum. Behav. 23, 59–82 10.1016/S1090-5138(01)00089-7 (doi:10.1016/S1090-5138(01)00089-7) [DOI] [Google Scholar]
- 46.Kramer K. L. 2005. Children's help and the pace of reproduction: cooperative breeding in humans. Evol. Anthropol. 14, 224–237 10.1002/evan.20082 (doi:10.1002/evan.20082) [DOI] [Google Scholar]
- 47.Sear R., Mace R. 2008. Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18 10.1016/j.evolhumbehav.2007.10.001 (doi:10.1016/j.evolhumbehav.2007.10.001) [DOI] [Google Scholar]
- 48.Hudson R., Trillmich F. 2008. Sibling competition and cooperation in mammals: challenges, developments and prospects. Behav. Ecol. Sociobiol. 62, 299–307 10.1007/s00265-007-0417-z (doi:10.1007/s00265-007-0417-z) [DOI] [Google Scholar]
- 49.Mock D., Parker G. A. 1997. The evolution of sibling rivalry. New York, NY: Oxford University Press [Google Scholar]
- 50.Quataert D. 2000. The Ottoman Empire, 1700–1922. Cambridge, UK: Cambridge University Press [Google Scholar]
- 51.Lawson D. W., Mace R. 2009. Trade-offs in modern parenting: a longitudinal study of sibling competition for parental care. Evol. Hum. Behav. 30, 170–183 10.1016/j.evolhumbehav.2008.12.001 (doi:10.1016/j.evolhumbehav.2008.12.001) [DOI] [Google Scholar]
- 52.Sear R. 2007. The impact of reproduction on Gambian women: does controlling for phenotypic quality reveal costs of reproduction? Am. J. Phys. Anthropol. 132, 632–641 10.1002/ajpa.20558 (doi:10.1002/ajpa.20558) [DOI] [PubMed] [Google Scholar]
- 53.van Noordwijk A. J., de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142 10.1086/284547 (doi:10.1086/284547) [DOI] [Google Scholar]
- 54.von Rueden C., Gurven M., Kaplan H. 2008. The multiple dimensions of male social status in an Amazonian society. Evol. Hum. Behav. 29, 402–415 10.1016/j.evolhumbehav.2008.05.001 (doi:10.1016/j.evolhumbehav.2008.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monden C. W. S., Smits J. 2009. Maternal height and child mortality in 42 developing countries. Am. J. Hum. Biol. 21, 305–311 10.1002/ajhb.20860 (doi:10.1002/ajhb.20860) [DOI] [PubMed] [Google Scholar]
- 56.Sear R. 2006. Height and reproductive success: how a Gambian population compares to the West. Hum. Nat. 17, 405–418 10.1007/s12110-006-1003-1 (doi:10.1007/s12110-006-1003-1) [DOI] [PubMed] [Google Scholar]
- 57.Braveman P. A., Cubbin C., Susan E., Chideya S., Marchi K. S., Metzler M., Posner S. 2005. Socioeconomic status in health research: one size does not fit all. J. Am. Med. Assoc. 294, 2879–2888 10.1001/jama.294.22.2879 (doi:10.1001/jama.294.22.2879) [DOI] [PubMed] [Google Scholar]
- 58.Reiches M. W., Ellison P. T., Lipson S. F., Sharrock K. C., Gardiner E., Duncan L. G. 2009. Pooled energy budget and human life history. Am. J. Hum. Biol. 21, 421–429 10.1002/ajhb.20906 (doi:10.1002/ajhb.20906) [DOI] [PubMed] [Google Scholar]
- 59.Omariba D. W. R., Boyle M. 2007. Family structure and child mortality in sub-Saharan Africa: cross-national effects of polygyny. J. Marriage Family 69, 528–543 10.1111/j.1741-3737.2007.00381.x (doi:10.1111/j.1741-3737.2007.00381.x) [DOI] [Google Scholar]
- 60.Pettifor R. A., Perrins C. M., McCleery R. H. 2001. The individual optimization of fitness: variation in reproductive output, including clutch size, mean nestling mass and offspring recruitment, in manipulated broods of great tits Parus major. J. Anim. Ecol. 70, 62–69 10.1046/j.1365-2656.2001.00465.x (doi:10.1046/j.1365-2656.2001.00465.x) [DOI] [Google Scholar]
- 61.Laland K. N., Brown G. R. 2006. Niche construction, human behaviour, and the adaptive-lag hypothesis. Evol. Anthropol. 15, 95–104 10.1002/evan.20093 (doi:10.1002/evan.20093) [DOI] [Google Scholar]
- 62.Bentley G. 1999. Aping our ancestors: comparative aspects of reproductive ecology. Evol. Anthropol. 7, 175–185 (doi:10.1002/(SICI)1520-6505(1999)7:5<175::AID-EVAN3>3.0.CO;2-4) [DOI] [Google Scholar]
- 63.Ellison P. T. 2003. Energetics and reproductive effort. Am. J. Hum. Biol. 15, 342–351 10.1002/ajhb.10152 (doi:10.1002/ajhb.10152) [DOI] [PubMed] [Google Scholar]
- 64.Kaplan H., Gangestad S. 2005. Life history theory and evolutionary psychology. In The handbook of evolutionary psychology (ed. Buss D. M.), pp. 68–95 Hoboken, NJ: Wiley [Google Scholar]
- 65.Mathews P., Sear R. 2008. Life after death: an investigation into how mortality perceptions influence fertility preferences using evidence from an internet-based experiment. J. Evol. Psychol. 6, 155–172 10.1556/JEP.6.2008.3.1 (doi:10.1556/JEP.6.2008.3.1) [DOI] [Google Scholar]
- 66.Hrdy S. B. 1999. Mother Nature: a history of mothers, infants and natural selection. New York, NY: Pantheon; [DOI] [PubMed] [Google Scholar]
- 67.Lycett J., Dunbar R. I. M. 1999. Abortion rates reflect the optimization of parental investment strategies. Proc. R. Soc. Lond. B 266, 2355–2358 10.1098/rspb.1999.0931 (doi:10.1098/rspb.1999.0931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphries M. H., Boutin S. 2000. The determinants of optimal litter size in free-ranging red squirrels. Ecology 81, 2867–2877 10.1890/0012-9658(2000)081[2867:TDOOLS]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[2867:TDOOLS]2.0.CO;2) [DOI] [Google Scholar]
- 69.Borgerhoff Mulder M. 2000. Optimizing offspring: the quantity-quality trade off in agropastoral Kipsigis. Evol. Hum. Behav. 21, 391–410 10.1016/S1090-5138(00)00054-4 (doi:10.1016/S1090-5138(00)00054-4) [DOI] [PubMed] [Google Scholar]
- 70.Nettle D., Pollet T. V. 2008. Natural selection on male wealth in humans. Am. Nat. 172, 658–666 [DOI] [PubMed] [Google Scholar]
- 71.Wilson C., Pison G. 2004. More than half the population lives where fertility is below the replacement level. Popul. Societies 405, 1–4 [Google Scholar]
- 72.Kaplan H., Lancaster J. B., Bock J., Johnson S. 1995. Fertility and fitness among Albuquerque men: a competitive labour market theory. In Human reproductive decisions: biological and social perspectives (ed. Dunbar R. I. M.), pp. 96–136 London, UK: Macmillan [Google Scholar]
- 73.Mueller U. 2001. Is there a stabilizing selection around average fertility in modern human populations? Popul. Dev. Rev. 27, 469–498 10.1111/j.1728-4457.2001.00469.x (doi:10.1111/j.1728-4457.2001.00469.x) [DOI] [Google Scholar]
- 74.Clark G., Cummins N. 2009. Urbanization, mortality, and fertility in Malthusian England. Am. Econ. Rev. 99, 242–247 10.1257/aer.99.2.242 (doi:10.1257/aer.99.2.242) [DOI] [PubMed] [Google Scholar]
- 75.Livi-Bacci M. 1986. Social-group forerunners of fertility control in Europe. In The decline of fertility in Europe (eds Coale A., Watkins S. C.), pp. 182–200 Princeton, NJ: Princeton University Press [Google Scholar]
- 76.Lawson D. W., Mace R. 2010. Optimizing modern family size: trade-offs between fertility and the economic costs of reproduction. Hum. Nat. 21, 39–61 10.1007/s12110-010-9080-6 (doi:10.1007/s12110-010-9080-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weeden J., Abrams M. J., Green M. C., Sabini J. 2006. Do high-status people really have fewer children? Education, income, and fertility in the contemporary U.S. Hum. Nat. 17, 377–392 10.1007/s12110-006-1001-3 (doi:10.1007/s12110-006-1001-3) [DOI] [PubMed] [Google Scholar]
- 78.Barkow J. H., Burley N. 1980. Human fertility, evolutionary biology, and the demographic transition. Ethol. Sociobiol. 1, 163–180 10.1016/0162-3095(80)90006-0 (doi:10.1016/0162-3095(80)90006-0) [DOI] [Google Scholar]
- 79.Boyd R., Richerson P. J. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 80.Montgomery M. R., Casterline J. B. 1996. Social learning, social influences, and new models of fertility. Popul. Dev. Rev. 22, 151–175 10.2307/2808010 (doi:10.2307/2808010) [DOI] [Google Scholar]
- 81.Blake J. 1989. Family size and achievement. Los Angeles, CA: University of California [Google Scholar]
- 82.Downey D. B. 1995. When bigger is not better: family size, parental resources and children's educational performance. Am. Soc. Rev. 60, 746–761 10.2307/2096320 (doi:10.2307/2096320) [DOI] [Google Scholar]
- 83.Lawson D. W. 2009. The behavioural ecology of modern families: a longitudinal study of parental investment and child development. PhD thesis, University College London, UK [Google Scholar]
- 84.Steelman L., Powell B., Werum R., Carter S. 2002. Reconsidering the effects of sibling configuration: recent advances and challenges. Ann. Rev. Sociol. 28, 243–269 10.1146/annurev.soc.28.111301.093304 (doi:10.1146/annurev.soc.28.111301.093304) [DOI] [Google Scholar]
- 85.Lawson D. W., Mace R. 2008. Sibling configuration and childhood growth in contemporary British families. Int. J. Epidemiol. 37, 1408–1421 10.1093/ije/dyn116 (doi:10.1093/ije/dyn116) [DOI] [PubMed] [Google Scholar]
- 86.Cooney T. M., Uhlenberg P. 1992. Support from parents over the life course: the adults perspective. Social Forces 71, 63–84 10.2307/2579966 (doi:10.2307/2579966) [DOI] [Google Scholar]
- 87.Coall D. A., Meier M., Hertwig R., Wänke M., Höpflinger F. 2009. Grandparental investment: the influence of reproductive timing and family size. Am. J. Hum. Biol. 21, 455–463 10.1002/ajhb.20894 (doi:10.1002/ajhb.20894) [DOI] [PubMed] [Google Scholar]
- 88.Keister L. A. 2003. Sharing the wealth: the effect of siblings on adults' wealth ownership. Demography 40, 521–542 [DOI] [PubMed] [Google Scholar]
- 89.Li L., Manor O., Power C. 2004. Early environment and child-to-adult growth trajectories in the 1958 British birth cohort. Am. J. Clin. Nutr. 80, 185–192 [DOI] [PubMed] [Google Scholar]
- 90.Davey Smith G., Hart C., Upton M., Hole D., Gillis C., Watt G., Hawthorne V. 2000. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J. Epidemiol. Commun. Health 54, 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nettle D. 2003. Intelligence and class mobility in the British population. Br. J. Psychol. 94, 551–556 10.1348/000712603322503097 (doi:10.1348/000712603322503097) [DOI] [PubMed] [Google Scholar]
- 92.Keister L. A. 2004. Race, family structure, and wealth: the effect of childhood family on adult asset ownership. Sociol. Perspect. 47, 161–187 10.1525/sop.2004.47.2.161 (doi:10.1525/sop.2004.47.2.161) [DOI] [Google Scholar]
- 93.Hill S. E., Reeve H. K. 2005. Low fertility in humans as the evolutionary outcome of snowballing resource games. Behav. Ecol. 16, 398–402 10.1093/beheco/ari001 (doi:10.1093/beheco/ari001) [DOI] [Google Scholar]
- 94.McNamara J. M., Houston A. I. 2006. State and value: a perspective from behavioural ecology. In Social information transmission and human biology (eds Wells J. C. K., Strickland S. S., Laland K. N.), pp. 59–88 London, UK: CRC Press [Google Scholar]
- 95.Rogers A. R. 1990. Evolutionary economics of human reproduction. Ethol. Sociobiol. 11, 479–495 10.1016/0162-3095(90)90022-X (doi:10.1016/0162-3095(90)90022-X) [DOI] [Google Scholar]
- 96.Eloundou-Enyegue P. M., Williams L. B. 2006. Family size and schooling in sub-Saharan African settings: a reexamination. Demography 43, 25–52 10.1353/dem.2006.0002 (doi:10.1353/dem.2006.0002) [DOI] [PubMed] [Google Scholar]
- 97.Gibson M. A., Sear R. 2010. Does wealth increase parental investment biases in child education? Evidence from two African populations on the cusp of the fertility transition. Curr. Anthropol. 51, 693–701 10.1086/655954 (doi:10.1086/655954) [DOI] [Google Scholar]
- 98.Gibson M. A., Lawson D. W. In press ‘Modernisation’ increases parental investment and sibling resource competition: evidence from a rural development initiative in Ethiopia. Evol. Hum. Behav. [Google Scholar]
- 99.Grawe N. 2010. Bequest receipt and family size effects. Econ. Inquiry 48, 156–162 10.1111/j.1465-7295.2008.00208.x (doi:10.1111/j.1465-7295.2008.00208.x) [DOI] [Google Scholar]
- 100.Gurmu E., Mace R. 2008. Fertility decline driven by poverty: the case of Addis Ababa (Ethiopia). J. Biosoc. Sci. 40, 339–358 10.1017/S002193200700260X (doi:10.1017/S002193200700260X) [DOI] [PubMed] [Google Scholar]
- 101.Kokko H., Brooks R., McNamara J. M., Houston A. I. 2002. The sexual selection continuum. Proc. R. Soc. Lond. B 269, 1331–1340 10.1098/rspb.2002.2020 (doi:10.1098/rspb.2002.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kokko H., Rankin D. J. 2006. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Phil. Trans. R. Soc. B 361, 319–334 10.1098/rstb.2005.1784 (doi:10.1098/rstb.2005.1784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mace R. 2008. Reproducing in cities. Science 319, 764–766 10.1126/science.1153960 (doi:10.1126/science.1153960) [DOI] [PubMed] [Google Scholar]
- 104.Stearns P. N. 2006. Childhood in world history. Oxford, UK: Oxford University Press [Google Scholar]