Abstract
Objective:
To investigate if a central cholinesterase inhibitor will reduce falling frequency in subjects with Parkinson disease (PD) with advanced postural instability.
Background:
Falling due to postural instability is a significant problem in advancing PD, and is minimally impacted by dopaminergic therapy. Anticholinergic medications increase falling in the elderly. Further, CNS cholinergic neuron loss occurs in PD. We hypothesized that acetylcholine augmentation may reduce frequent falling in subjects with PD.
Methods:
We enrolled 23 subjects with PD who reported falling or nearly falling more than 2 times per week. In a randomized, placebo-controlled, crossover design, subjects were given 6 weeks of donepezil or placebo with a 3-week washout between phases. The primary outcomes were daily falls and near falls reported on postcards. Secondary outcomes included scores on the Activities of Balance Confidence Scale, Berg Balance Scale, Clinical Global Impression of Change, Folstein Mini-Mental State Examination, and the motor section of the Unified Parkinson's Disease Rating Scale.
Results:
Fall frequency per day on placebo was 0.25 ± 0.08 (SEM) compared with 0.13 ± 0.03 on donepezil (p < 0.05). The frequency of near falls was not significantly different between phases. The secondary outcomes did not differ; however, there was a trend to improvement on the subject-completed Global Impression of Change scale.
Conclusions:
Subjects with PD fell approximately half as often during the 6 weeks on donepezil than on placebo. Larger trials of cholinergic augmentation are warranted in subjects with PD with frequent falls.
Classification of evidence:
This study provides Class II evidence that donepezil (maximum 10 mg per day) significantly reduced the number of falls in patients with PD (0.13 falls/day, SEM = 0.03) than when taking placebo (0.25 falls/day, SEM = 0.08, p = 0.049).
GLOSSARY
- NBM
= nucleus basalis of Meynert;
- MMSE
= Mini-Mental State Examination;
- OHSU
= Oregon Health & Sciences University;
- PD
= Parkinson disease;
- PPN
= pedunculopontine nucleus;
- UPDRS
= Unified Parkinson's Disease Rating Scale.
Postural instability is a cause of significant morbidity that worsens as Parkinson disease (PD) advances and rarely improves with dopaminergic or surgical therapy. Falls are highly prevalent in PD. It is estimated that up to two-thirds of patients with PD experience falls each year,1,2 with approximately 40% of falls leading to injury.3 Even when falls do not result in injury, fear of falling often leads to limitations of daily activities and further deterioration of quality of life.4 While the problem of falling in PD is complex and risk of falls only partially predictable from the clinical examination,5 there are few treatment options. Clinicians can prevent some falls by treating orthostasis, painful arthritis, and visual impairments and by optimizing the environment for patients. Physical therapy with balance rehabilitation remains a mainstay of treatment but more options are needed.
We hypothesized that increasing central acetylcholine could improve balance and reduce falls in subjects with PD. It is known that medications with anticholinergic properties are associated with impaired balance, increased falls, and increased rates of bony fractures in the elderly.6–10 PD itself can be considered an acetylcholine-deficient state based on cholinergic cell loss in the nucleus basalis of Meynert (NBM) and in the pedunculopontine nucleus (PPN)-laterodorsal tegmental complex.11–13 Loss of PPN cells may be correlated with increasing balance impairment and disability as reflected in Hoehn & Yahr scores.14 Furthermore, cholinergic forebrain projections (from NBM) are important in maintenance of normal cognition, and dementia is associated with increased risk of falling.15 Thus, we initiated a controlled trial of an acetylcholinesterase inhibitor, donepezil (Aricept, Pfizer, New York, NY), in subjects with PD who fall frequently.
METHODS
Standard protocol approvals, registrations, and patient consents.
This trial (NCT00912808 in ClinicalTrials.gov) was approved by the Oregon Health & Sciences University (OHSU) Institutional Review Board and all subjects provided written informed consent for participation.
Design.
The trial was a randomized, crossover, double-blind study of 15 weeks duration. Each drug phase, donepezil or identical placebo, lasted 6 weeks, with a 3-week washout period in between. In each drug phase, subjects were instructed to take 1 tablet (5 mg of donepezil or placebo) for 3 weeks and to increase to 2 tablets (10 mg) for the remaining 3 weeks. Drug and placebo tablets were identical in appearance and were provided by Pfizer. If intolerable side effects occurred during titration, the dose was halved until the effect abated and the titration schedule was resumed, or the subject chose to withdraw.
Subjects.
Eligible adult subjects were those diagnosed with probable idiopathic PD, defined as manifesting 2 of 3 cardinal features (tremor, rigidity, bradykinesia), without any other historical or physical signs to suggest another diagnosis, and were recruited from the OHSU Movement Disorders Clinic. All subjects were responsive to levodopa replacement therapy. The inclusion criterion specified a baseline frequency of falling or nearly falling 2 or more times per week. A fall was defined as an event which results in a person coming to rest inadvertently on the ground or other lower level, and other than a consequence of the following: sustaining a violent blow, loss of consciousness, sudden onset of paralysis, as in a stroke or an epileptic seizure. A near fall was defined as an involuntary or uncontrolled descent where the potential for a fall existed but the outcome was not a fall. All subjects were ambulatory about the home either independently or with a walker or cane, thus Hoehn & Yahr stage 5 excluded participation in the trial.
Subjects were excluded if they had freezing or non-CNS contributors to falls such as orthostasis, arthritic impairments, or neuropathy, and if subjects were currently using cholinesterase inhibitors or drugs with anticholinergic or sedative-hypnotic properties. Subjects were also excluded if they had a Folstein score <25 or unstable medical or psychiatric problems.
Measurements.
The primary outcomes were fall and near-fall frequency determined using daily event recording by the subjects onto postcards which accumulated data for 1 week of monitoring, and collected for 6 weeks per phase. Postcards were mailed back to the investigator weekly. Secondary outcomes included change from baseline scores in each of the following scales during each phase.
Subject-completed global impression of improvement: a 7-point scale that is completed by the subject to indicate treatment response. This scale is anchored at 4 (no change), with 1 indicating very much improved; 2, much improved; 3, minimally improved. Conversely, 5 indicates minimally worse; 6, much worse; or 7, very much worse.
The Activities of Balance Confidence Scale16: measures confidence that respondents will not lose their balance or become unsteady in the course of 16 activities of daily living. Each item is self-rated from 0% (no confidence) to 100% (complete confidence) and in a previous study, patients with PD scored a mean of 68.7% ± 2.9% compared with healthy controls who on average scored 93.2% ± 1.3%.17
The Berg Balance Scale18: composed of 14 tasks that assess balance such as sitting to standing, standing unsupported, standing with feet together, retrieving an object from the floor, and standing on one foot. In most items, the subject is asked to maintain a given position for a specific time. Points are lost if time or distance requirements are not achieved, or if external support or assistance from the examiner is needed (maximum score is 56).
Motor Unified Parkinson's Disease Rating Scale (UPDRS) (part III): one section from a comprehensive evaluation of parkinsonism, and consists of rating the severity of the motor signs by clinical observation. Fourteen items including speech, hand movements, leg agility, posture, gait, and upright stability are rated using a 5-point scale with 0 = normal or no deficits to 4 = highest severity of abnormality. The scores in this section can vary from 0 to a total of 108.
Folstein Mini-Mental State Examination (MMSE)19: this is a 30-point questionnaire that estimates the severity of cognitive impairment using questions or tasks to assess mathematical, memory, and orientation abilities, with scores ranging from 0 to 30 (no errors).
Analysis.
Means and standard deviations were calculated to describe the subject baseline characteristics. Paired t tests evaluated the difference between baseline and end of treatment frequency of falls. Changes posttreatment from baseline in secondary measures were also compared between the donepezil and placebo phases with paired t tests or Wilcoxon signed rank tests when data were nonparametric. SPSS was used for the analysis.
RESULTS
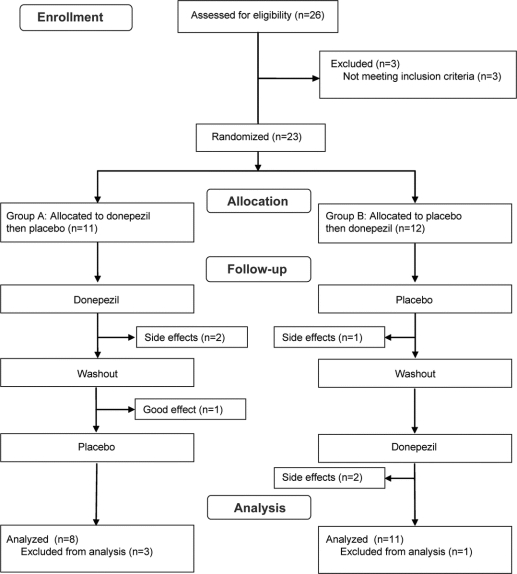
A total of 26 subjects were screened; 3 were not randomized due to failing inclusion/exclusion criteria. For subjects with intolerable adverse events during drug titration phase, the dose was halved until the adverse effects abated and the titration schedule was resumed. Four dropped out before the second phase (2 on active drug, 1 each during placebo phase and washout) and were excluded from the analysis. Two additional subjects withdrew before the end of the second crossover period, but were included in the analysis, leaving 19 subjects in whom the primary outcomes were measured (figure 1).
Figure 1 Consort diagram of subject participation
Baseline characteristics.
Average age at enrollment in the study was 68.3 years (SD 10.8), and participants were mostly male (n = 15). Six subjects had deep brain stimulator implants, all located in the subthalamic nucleus location. The mean duration of illness was 10 years (SD 5.6) and the baseline severity of motor signs as measured by the UPDRS III was 24.7 (SD 8.6) with modified Hoehn & Yahr staging indicating moderate baseline disease (mean 3.2, SD 0.4) The average baseline Mini-Mental State Examination Score was 27.6 (SD 4.5). Confidence in balance at baseline was 51% (SD 0.2) and measured balance on the Berg scale was 41.6 (maximum of 56, SD 7.4).
Efficacy analysis.
Subjects with PD fell less on donepezil (0.13 falls/day, SEM = 0.03) than when taking placebo (0.25 falls/day, SEM = 0.08, p = 0.049) (figure 2, table). The 5 subjects with the most frequent falls (at least 3 per week) showed the most reduction after 6 weeks on donepezil. Those on drug did not differ in near falls when compared to those on placebo; however, the 2 participants with the greatest number of near falls (over 5 per week) showed the greatest improvement, with an average reduction of 2.5 near falls per week. Absolute risk reduction was 0.12 falls/day (95% confidence interval −0.09 to 0.33) with 8.3 people needing treatment to prevent a fall. No carryover effect was detected.

Figure 2 Fall frequency is reduced during donepezil use
The effect appears to be more pronounced in those with higher rates of falling.
Table Outcomes during donepezil and placebo phases
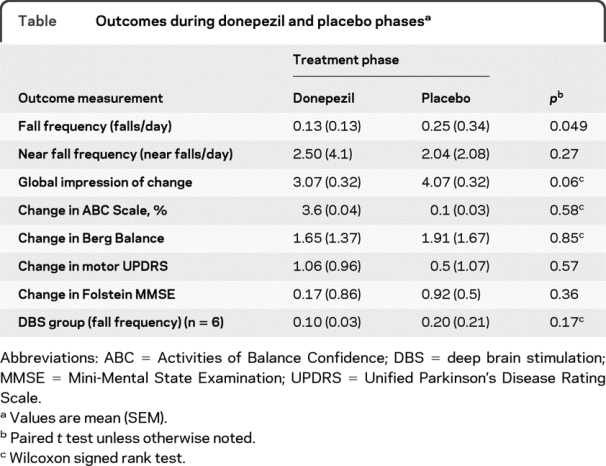
There was a trend toward improvement in subject-scored global impression of change with +1.07 on donepezil and +0.07 on placebo. However, there were no differences between the placebo or donepezil treatment in the change from baseline in the Activities of Balance Confidence Scale, Berg Balance Scale, UPDRS III, or Folstein MMSE scores (table).
Of the 4 subjects who dropped out before phase II, 3 did so because of side effects of the study medication (2 on donepezil due to insomnia, nausea; 1 on placebo due to increased tremor). One subject on active drug during the first phase worsened during the washout and refused to submit to the second phase. Side effects such as nausea, abnormal sweating, insomnia, headache, poor appetite, or weight loss were noted in 35% on donepezil, but were mild or transient in most. Interestingly, no fractures or other serious adverse events occurred during the course of this study despite nearly 200 falls.
DISCUSSION
Donepezil reduced fall frequency in frequently falling subjects with PD by approximately half (from 0.25 to 0.13 falls per day). Subjects who fell the most often at baseline demonstrated the most improvement, perhaps related to a floor effect; subjects with fewer falls had less room for improvement given the short duration of the study. Subjects who had deep brain stimulators appeared to respond as well as those treated medically, although the small number of subjects with DBS prevented subgroup analysis.
The reason that near falls were not affected by donepezil is unclear. One explanation may be that the working definition for near falls was ineffective in practice; near falls may be too subjective for subjects to accurately assess and remember. Perhaps the lack of reduction in near falls with donepezil treatment suggests that subjects are better able to recover from postural perturbations such that falls are avoided (i.e., would-be falls become only near falls). Another possibility is that a near fall is a different phenomenon, affected differently by adding acetylcholine to the CNS, than is a fall.
Our secondary outcomes do not suggest the etiology of the benefits of donepezil. The Berg Balance test did not reveal improvement in measures of balance. The subjects' balance confidence did not improve. The motor UPDRS did not suggest changes in the parkinsonism and MMSE did not indicate changes in cognition. The lack of change in secondary measures of balance, PD symptoms, and mental status could be due to lack of sensitivity of these clinical measures5 or because falls were reduced for other reasons not tested. For example, patients with PD have impaired postural responses to external perturbations that are not improved with levodopa.20 Improvement in automatic postural responses may not be detected with our secondary measures.21
Why should donepezil improve falls? A strong rationale for this study was the evidence that drugs with anticholinergic properties are associated with falls. A review of several studies in other patient groups have documented that anticholinergics are associated with increased falls in the community-dwelling elderly or hospitalized patients.6–10
By what mechanisms might augmentation of cholinergic neurotransmission reduce falls? First, the cortical projections from the cholinergic nucleus basalis of Meynert are heavily damaged in PD.11 The nucleus basalis of Meynert has been implicated to be important for cognition and attentional processes.22,23 The role of optimal cognitive input in the success of balance maintenance and avoidance of falls is becoming increasingly apparent.24 While it is recognized that cognitive deficits and falling are associated, the cause is difficult to infer. It could be postulated that attentional processes to the surrounding environment are important to maintenance of balance and avoidance of falls. Impaired attentional processes have been described in PD25,26 and attentional processes may be affected significantly by cholinergic manipulation.27,28 Our tests did not examine frontal executive function and attention, which may have yielded clues about the importance of these processes in fall reduction.
While attentional processes may have been improved with supplemental acetylcholine, structures mediating balance and upright stability must also be considered. The cerebellum influences balance, posture, and muscle tone as well as coordination of movement. Recent studies suggest that cholinergic input is important to the cerebellum,29,30 and may involve cholinergic projections from the PPN, the lateral paragigantocellular nucleus, and other smaller nuclei.31 Preliminary animal evidence suggests that cholinergic input to Purkinje cells is important for optimal vestibular righting reflexes32 and modulation of oculomotor reflexes.33 Other human studies have suggested the archicerebellum is affected by acetylcholine and levels may decline with aging.34 In PD, the degeneration of the PPN and locus coeruleus and loss of their projections to the cerebellum may account for at least some of the balance abnormalities that are a source of significant disability for many.
The PPN cholinergic projections include not only deep cerebellar nuclei, but also thalamic nuclei, the substantial nigra pars compacta, the subthalamic nucleus, and the globus pallidus interna.35 Descending projections also include several midbrain, pontine, and medullary areas as well as the spinal cord.36 A role for the PPN in gait and postural stability has been suggested by animal studies and in humans using deep brain stimulation though results are not conclusive.37 However, it is likely that cholinergic supplementation affects not only cortical areas important for balance, but also throughout the neuraxis based on projections from the PPN that may play an important role in the maintenance of posture, balance, and gait. It is noteworthy that in a recent PET investigation of acetylcholinesterase activity in subjects with PD, a history of falling was associated with thalamic cholinergic hypofunction, likely due to PPN degeneration.38
The limitations of this study include the small number of subjects and lack of objective measurement to quantify falls. One difficulty in conducting this trial was screening for subjects with PD with postural instability that could not be associated with proprioceptive loss in the feet, arthritic foot conditions, and orthostasis, common in the elderly. The most difficult condition to exclude was coexisting freezing of gait. In this study, 2 subjects who claimed to experience infrequent freezing and did not demonstrate significant freezing during screening manifested moderately severe ON gait freezing during subsequent study visits. These 2 subjects did not benefit from donepezil in fall reduction.
While overall fall frequency declined on drug, there were clearly some subjects who did not improve. Understanding the differences between responders and nonresponders may help us further understand causes of postural instability. Future investigations of these findings should include larger studies and ideally, objective measures. The possibility of improved cognition can be explored further with more detailed psychometric testing. Testing the components of postural control with computerized dynamic posturography may better illuminate the mechanisms for fall reduction.39 Cholinesterase inhibitors have been used to treat cognitive decline in subjects with PD and have been tolerated well by the majority.40 Reduced falls may be another reason to initiate this therapy but further studies are needed.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Brenna Lobb.
DISCLOSURE
Dr. Chung has received research support from a VA Career Development Award. B.M. Lobb receives support from the Department of Veterans Affairs. Dr. Nutt has received funding for travel from Novartis and Teva Pharmaceutical Industries Ltd.; has received speaker honoraria from Novartis; has served as a consultant for XenoPort Inc., IMPAX Laboratories, Inc., Neurogen Inc., Synosia Therapeutics, and NeuroDerm, Ltd.; and has received research support from Schering-Plough Corp, the NIH (NINDS R01 NS 21062 [PI] and UL1-RR024140 [PI]), the Veterans Administration (PADRECC [Co-PI]), and the National Parkinson Foundation. Dr. Horak serves on scientific advisory boards for the Movement Disorders Society, Novartis, and the MS Society; serves as an Associate Editor for Cerebellum and on the editorial boards of Gait and Posture and the Journal of Biomechanics; holds/has filed patents re: Device for conditioning balance and motor coordination and instrumented mobility system to objectively measure balance and gait; serves as Chief Scientific Officer and member of the board of APDM, Inc.; and receives research support from the NIH (NIDCD R01 DC004082-07 [PI], NIA R37 AG006457 [PI], and RC1 NS068678 [PI]), the National MS Society, Parkinson's Alliance, and the Kinetics Foundation.
The AAN Provides a New Resource for Your Patients
Written by Ronald DeVere, MD, Director of the Taste and Smell Disorders Clinic in Austin, Texas and Marjorie Calvert, Food Consultant at the clinic, Navigating Smell and Taste Disorders includes causes, treatment options, and 36 recipes and additional tips that will make food appealing again. “More than 200,000 people visit doctors each year for smell and taste problems, which often are the first sign of neurologic disorders, such as Alzheimer's disease, Parkinson's disease, head injury, or multiple sclerosis,” said DeVere.
“An enlightening guide. . . this patient-oriented approach should be hailed as a groundbreaking book. It is highly recommended for any patients suffering from these often undiagnosed and untreated disorders and the relatives who help care for them.”
—Alan R. Hirsch, MD, neurological director at the Smell and Taste Treatment and Research Foundation in Chicago
Invite your patients to visit www.aan.com/view/smellandtaste for more information about this invaluable resource. Available from all major booksellers.
Address correspondence and reprint requests to Dr. Kathryn Chung, OP-32, 3181 SW Sam Jackson Park Road, Portland, OR 97239 chungka@ohsu.edu
Editorial, page 1226
e-Pub ahead of print on September 1, 2010, at www.neurology.org.
Study funding: This was an investigator-initiated project funded with an unrestricted grant from Pfizer Inc., who did not design or monitor the study or receive the data or influence the writing of the manuscript. Supported by a Veterans Administration Career Development Award, US Public Health Service Grant (ULIRR024140-02), and the NIH (R01-NS21062 and NIA AG006457). Pfizer Inc. provided drug and matched placebo.
Disclosure: Author disclosures are provided at the end of the article.
Received December 28, 2009. Accepted in final form June 14, 2010.
REFERENCES
- 1.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 2002;72:721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburn A, Stack E, Pickering RM, Ward CD. A community-dwelling sample of people with Parkinson's disease: characteristics of fallers and non-fallers. Age Ageing 2001;30:47–52. [DOI] [PubMed] [Google Scholar]
- 3.Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. J Neurosci Nurs 2000;32:222–228. [DOI] [PubMed] [Google Scholar]
- 4.Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson's disease. Mov Disord 2003;18:496–502. [DOI] [PubMed] [Google Scholar]
- 5.Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol 2001;248:950–958. [DOI] [PubMed] [Google Scholar]
- 6.Aizenberg D, Sigler M, Weizman A, Barak Y. Anticholinergic burden and the risk of falls among elderly psychiatric inpatients: a 4-year case-control study. Int Psychogeriatr 2002;14:307–310. [DOI] [PubMed] [Google Scholar]
- 7.Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc 2002;50:1629–1637. [DOI] [PubMed] [Google Scholar]
- 8.Thapa PB, Gideon P, Cost TW, Milam AB, Ray WA. Antidepressants and the risk of falls among nursing home residents. N Engl J Med 1998;339:875–882. [DOI] [PubMed] [Google Scholar]
- 9.Ray WA, Griffin MR, Malcolm E. Cyclic antidepressants and the risk of hip fracture. Arch Intern Med 1991;151:754–756. [PubMed] [Google Scholar]
- 10.Berdot S, Bertrand M, Dartigues JF, et al. Inappropriate medication use and risk of falls: a prospective study in a large community-dwelling elderly cohort. BMC Geriatr 2009;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA 1987;84:5976–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspar P, Gray F. Dementia in idiopathic Parkinson's disease: a neuropathological study of 32 cases. Acta Neuropathol 1984;64:43–52. [DOI] [PubMed] [Google Scholar]
- 13.Whitehouse PJ, Hedreen JC, White CL III, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol 1983;13:243–248. [DOI] [PubMed] [Google Scholar]
- 14.Rinne JO, Ma SY, Lee MS, Collan Y, Roytta M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinson's disease is related to disability of the patients. Parkinsonism Relat Disord 2008;14:553–557. [DOI] [PubMed] [Google Scholar]
- 15.van DC, Gruber-Baldini AL, Zimmerman S, et al. Dementia as a risk factor for falls and fall injuries among nursing home residents. J Am Geriatr Soc 2003;51:1213–1218. [DOI] [PubMed] [Google Scholar]
- 16.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995;50A:M28–M34. [DOI] [PubMed] [Google Scholar]
- 17.Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson's disease. Mov Disord 2003;18:496–502. [DOI] [PubMed] [Google Scholar]
- 18.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health 1992;83(suppl 2):S7–S11. [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 20.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol 1996;75:2380–2396. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs JV, Horak FB, Tran VK, Nutt JG. Multiple balance tests improve the assessment of postural stability in subjects with Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev 2006;51:145–160. [DOI] [PubMed] [Google Scholar]
- 23.Harati H, Barbelivien A, Cosquer B, Majchrzak M, Cassel JC. Selective cholinergic lesions in the rat nucleus basalis magnocellularis with limited damage in the medial septum specifically alter attention performance in the five-choice serial reaction time task. Neuroscience 2008;153:72–83. [DOI] [PubMed] [Google Scholar]
- 24.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 2002;16:1–14. [DOI] [PubMed] [Google Scholar]
- 25.Woodward TS, Bub DN, Hunter MA. Task switching deficits associated with Parkinson's disease reflect depleted attentional resources. Neuropsychologia 2002;40:1948–1955. [DOI] [PubMed] [Google Scholar]
- 26.Dujardin K, Degreef JF, Rogelet P, Defebvre L, Destee A. Impairment of the supervisory attentional system in early untreated patients with Parkinson's disease. J Neurol 1999;246:783–788. [DOI] [PubMed] [Google Scholar]
- 27.Balducci C, Nurra M, Pietropoli A, Samanin R, Carli M. Reversal of visual attention dysfunction after AMPA lesions of the nucleus basalis magnocellularis (NBM) by the cholinesterase inhibitor donepezil and by a 5-HT1A receptor antagonist WAY 100635. Psychopharmacology 2003;167:28–36. [DOI] [PubMed] [Google Scholar]
- 28.Ceravolo R, Volterrani D, Frosini D, et al. Brain perfusion effects of cholinesterase inhibitors in Parkinson's disease with dementia. J Neural Transm 2006;113:1787–1790. [DOI] [PubMed] [Google Scholar]
- 29.Bencherif B, Endres CJ, Musachio JL, et al. PET imaging of brain acetylcholinesterase using [11C]CP-126,998, a brain selective enzyme inhibitor. Synapse 2002;45:1–9. [DOI] [PubMed] [Google Scholar]
- 30.Graham A, Court JA, Martin-Ruiz CM, et al. Immunohistochemical localisation of nicotinic acetylcholine receptor subunits in human cerebellum. Neuroscience 2002;113:493–507. [DOI] [PubMed] [Google Scholar]
- 31.de LS, Hersh LB, Saper CB. Cholinergic innervation of the human cerebellum. J Comp Neurol 1993;328:364–376. [DOI] [PubMed] [Google Scholar]
- 32.Pompeiano O. Noradrenergic and cholinergic modulations of corticocerebellar activity modify the gain of vestibulospinal reflexes. Ann NY Acad Sci 1992;656:519–536. [DOI] [PubMed] [Google Scholar]
- 33.Tan HS, Collewijn H. Cholinergic modulation of optokinetic and vestibulo-ocular responses: a study with microinjections in the flocculus of the rabbit. Exp Brain Res 1991;85:475–481. [DOI] [PubMed] [Google Scholar]
- 34.Podruchny TA, Connolly C, Bokde A, et al. In vivo muscarinic 2 receptor imaging in cognitively normal young and older volunteers. Synapse 2003;48:39–44. [DOI] [PubMed] [Google Scholar]
- 35.Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol 1994;344:210–231. [DOI] [PubMed] [Google Scholar]
- 36.Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 1995;47:1–29. [DOI] [PubMed] [Google Scholar]
- 37.Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain 2007;130:1596–1607. [DOI] [PubMed] [Google Scholar]
- 38.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 2009;73:1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, eds. Handbook of Physiology. New York: Oxford University Press; 1996:255–292. [Google Scholar]
- 40.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004;351:2509–2518. [DOI] [PubMed] [Google Scholar]