Abstract
Metallothionein (MT) localizes in the intermembrane space of liver mitochondria as well as in the cytosol and nucleus. Incubation of intact liver mitochondria with physiological, micromolar concentrations of MT leads to the import of MT into the mitochondria where it inhibits respiration. This activity is caused by the N-terminal β-domain of MT; in this system, the isolated C-terminal α-domain is inactive. Free zinc inhibits respiration at concentrations commensurate with the zinc content of either MT or the isolated β-domain, indicating that MT inhibition involves zinc delivery to mitochondria. Respiratory inhibition of uncoupled mitochondria identifies the electron transfer chain as the primary site of inhibition. The apoform of MT, thionein, is an endogenous chelating agent and activates zinc-inhibited respiration with a 1:1 stoichiometry ([zinc binding sites]/[zinc]). Carbamoylation of the lysines of MT significantly attenuates the inhibitory effect, suggesting that these residues are critical for the passage of MT through the outer mitochondrial membrane. Such an import pathway has been proposed for other proteins that also lack a mitochondrial targeting sequence, e.g., apocytochrome c, and possibly Cox17, a mitochondrial copper chaperone that is the only protein known so far to exhibit significant primary sequence homology to MT. The presence and respiratory inhibition of MT in liver, but not heart, mitochondria suggest a hitherto unknown biological modulating activity of MT in cellular respiration and energy metabolism in a tissue-specific manner.
Zinc has a multitude of known cellular functions (1), but knowledge regarding its functions in mitochondria is still scant. Mitochondrial zinc constitutes a significant fraction of total cellular zinc. It has been estimated that in B cell-rich islets 32% of the total zinc is in mitochondria (2). The concentration of zinc in rat liver mitochondria is lower than that in the cytosol (3), indicating the existence of mechanisms to maintain this gradient and to regulate the amount of mitochondrial zinc, which seems to be quite variable. Thus, orally administered zinc salts increase zinc in rat liver mitochondria (4), and mitochondria of epithelial prostate cells are particularly rich in zinc (5), but its concentration remains lower than in the cytosol. Molecules that transport zinc to mitochondria and distribute it within them, as well as the control of these processes, remain to be identified and defined. So far, metallothionein (MT) is the only protein that has been implicated in cellular zinc distribution (6). We became interested in the function of MT in relation to mitochondria because commercial preparations of cadmium-containing MT have previously been shown to inhibit oxygen consumption of intact mitochondria (7, 8). Addition of zinc to isolated mitochondria inhibits respiration, an effect that has been traced to multiple sites in the electron transport chain (9). Simpkins et al. (7) compared the inhibitory effects of MT and zinc and concluded that the effect of zinc alone occurs at concentrations higher by orders of magnitude than those thought to be available based on the known MT–zinc binding constant. They conjectured that the MT effect should not be attributed to its bound metals. However, we have shown recently that there is a thermodynamic and/or kinetic driving force for zinc transfer from MT when zinc acceptor sites are available, and further, that redox reactions modulate zinc transfer (10). Thus, the proposition that the concentration of zinc at equilibrium with MT determines its availability is apparently invalid. Our studies of the effect of defined, zinc-containing MT isoforms on respiration of rat liver mitochondria now reveal that MT localizes to the mitochondrial intermembrane space (IMS) and inhibits the respiratory chain at concentrations at least one order of magnitude lower than those reported previously. This effect is caused by release of zinc from the N-terminal β-domain of MT. Addition of thionein (T), the metal-free form of MT, to zinc-inhibited mitochondria restores respiration. These results suggest that, among other possible functions, the MT/T system is capable of affecting respiration through controlling the availability of zinc in the IMS.
Materials and Methods
Materials.
N,N,N′,N′-Tetrakis(2-pyridylmethyl) ethylenediamine (TPEN), 2,4-dinitrophenol, sodium succinate, and ADP were obtained from Sigma. Rabbit liver Cd,Zn-MT-1 and -2 were provided by G. J. Xu (Shanghai Institute of Biochemistry, China) and converted to the zinc-containing forms (11). Human Zn7-MT-3 and rat Zn7-MT-1 were provided by M. Vašák (University of Zurich, Switzerland).
Preparation and Characterization of Zn-MT, T, and MT-Domain Peptides.
MT isoforms were equilibrated with incubation buffer [0.25 M sucrose/20 mM KCl/5 mM MgCl2/10 mM K2HPO4/KH2PO4 (pH 7.4)] by using Centricon-3 centrifugal microconcentrators (Amicon). Rabbit liver T was prepared from cadmium MT-1 and stored in liquid nitrogen (12). T was lyophilized from its frozen solution in 10 mM HCl, dissolved in incubation buffer, and its concentration (ɛ220 = 76,000 M−1⋅cm−1) and sulfhydryl content were determined spectrophotometrically immediately before use. The two MT-domain peptides were reconstituted with zinc (13). Lysines of MT and the β-domain peptide were modified by carbamoylation (14).
Preparation of Mitochondria.
Male Wistar rats (Harlan–Sprague–Dawley), weighing 180–250 g, were killed in a carbon dioxide chamber. Isolation of liver and heart mitochondria were based on the methods of Johnson and Lardy (15) and Tyler and Gonze (16), respectively. All steps were performed at 4°C. The mitochondrial pellet was resuspended in 200–400 μl of sucrose solution (0.25 M, pH 7.4). The concentration of mitochondrial protein (and protein in submitochondrial fractions, see below) was quantified with Coomassie blue (ref. 17; with BSA as a standard) after treating the mitochondria with 0.5% (vol/vol) Triton X-100 in water, and was typically about 60–90 mg/ml. The integrity of the isolated mitochondria was determined polarographically with a Clark-type oxygen electrode. The P:O (ADP/oxygen consumption) and respiratory control (state 3/state 4 respiration) ratios of different liver mitochondrial preparations were in the ranges of 2.5–2.9 and 6.5–8.5, respectively.
For the localization studies (see below), liver and heart mitochondria were further purified by gradient centrifugation at 285,000 × g for 2 h at 4°C by using 50% (wt/vol) Accudenz (Accurate Scientific, Westbury, NY) in 0.125 M sucrose/10 mM Tris⋅HCl (pH 7.4) according to Rickwood et al. (18).
Preparation of Submitochondrial Fractions.
Rat liver (30 g) was washed with isolation buffer [70 mM sucrose/220 mM d-mannitol/0.5 mg/ml BSA (fraction V, Sigma)/2 mM Hepes-KOH (pH 7.4)], minced, and homogenized with 2 vol of isolation buffer. The homogenate was diluted with 3 vol of isolation buffer and spun at 660 × g for 10 min. The supernatant was then spun at 9,770 × g for 15 min, and the pellet was resuspended in 15 ml of isolation buffer and also spun at 9,770 × g for 15 min. The washing process was repeated twice. The pellet containing mitochondria was resuspended at a concentration of 100 mg/ml protein in a minimum of isolation buffer. Submitochondrial fractions were prepared according to the method of Greenawalt (19). All submitochondrial fractions contained 0.2 mM phenylmethylsulfonyl fluoride (in 2-propanol) and were kept at −20°C. Alcohol dehydrogenase activity with ethanol as substrate (20) was measured to assess cytosolic contamination of mitochondria, intermembrane fraction, and other submitochondrial fractions. The specific activity in the supernatant was 0.146 units/mg protein.
Western Blotting.
Samples were diluted with 125 mM Tris⋅HCl (pH 6.8) containing 10% (vol/vol) glycerol, 0.01% (wt/vol) bromophenol blue, 0.25 mg/ml CdCl2, and 1% (wt/vol) SDS. After SDS/PAGE (21), the 15% acrylamide gels were incubated with transfer buffer [192 mM glycine/25 mM Tris⋅HCl/20% (vol/vol) methanol (pH 8.5)] at room temperature for 20 min. Proteins were then blotted onto a nitrocellulose membrane (Schleicher & Schuell) at 4°C for 2 h using a Bio-Rad transblotting apparatus at 200 mA. The membrane was incubated with 5% (wt/vol) fat-free milk in 10 mM Tris⋅HCl/100 mM NaCl/0.1% (vol/vol) Tween-20 (pH 7.5; TBST) overnight at 4°C. An affinity-purified monoclonal antibody against rat MT-1 (22) that reacts primarily with monomeric MT was used at 1:2,000 dilution in TBST with 5% (wt/vol) fat-free milk for 2 h. Then the membrane was washed three times with TBST, for 15 min per wash. The secondary antibody was an anti-mouse IgG coupled to horseradish peroxidase (StressGen Biotechnologies, Victoria, BC, Canada) used according to a protocol for enhanced chemiluminescent detection (Amersham Pharmacia). Western blots were quantified by densitometric scanning with a ChemiImager 4000 instrument (Alpha Innotech, San Leandro, CA).
For comparison of liver and heart mitochondria, an antibody against ubiquinol–cytochrome c oxidoreductase core 1 protein (Molecular Probes) served as a control for the amount of mitochondrial protein loaded on the gel. Conditions of electrophoresis, blotting, and detection were essentially the same as described above except that the sample buffer contained 5% (vol/vol) β-mercaptoethanol and that samples were boiled for 3 min before they were loaded on the gel.
Import of MT into Mitochondria.
Intact coupled mitochondria were suspended in 3 ml of incubation buffer (see above) at a concentration of 60 mg/ml mitochondrial protein; succinate was added to a final concentration of 3 mM to initiate respiration and incubated for 1 min; ADP was added to a final concentration of 0.4 mM and incubated for 2 min; and finally Zn7-MT-3 was added to a final concentration of 5 μM and incubated for 3–5 min, all under gentle stirring at room temperature. Mitochondria were then collected by centrifugation at 10,000 × g for 10 min and washed twice with 15 ml of isolation buffer, and the mitochondria were then used for preparation of submitochondrial fractions as described above. A preparation in which succinate, ADP, and then isolation buffer were successively added to mitochondria served as a control. The mitochondrial protein fractions were incubated in the SDS sample buffer, boiled for 3 min, and then separated by SDS/PAGE as described above. The gels were incubated with transfer buffer containing 2 mM CaCl2 for 20 min and blotted for 80 min as described above. Rabbit antiserum obtained by immunization against a unique polypeptide of MT-3 (23) was a gift of M. Vašák. It was further purified by precipitation with an acetone extract of rat liver cellular protein (24) and used in Western blots to detect MT-3. The membrane was blocked with 5% (wt/vol) fat-free milk for 3 h at room temperature and washed as described above, and purified anti-MT-3 antibody [1:5,000 dilution in TBST buffer containing 2.5% (wt/vol) BSA and 2.5% (wt/vol) fat-free milk] was added, and then incubated overnight at room temperature. After three washes, the secondary antibody, goat anti-rabbit IgG coupled to horseradish peroxidase (New England Biolabs), was used.
Polarographic Determination of Oxygen.
A Clark-type oxygen electrode, model DO-166PC Flow from Lazar Research Laboratories (Los Angeles) was used at 25 ± 2°C. Aliquots containing 500 μg of mitochondrial protein in 450 μl incubation buffer were added to the cell and incubated for 4 min with magnetic stirring. Succinate in incubation buffer was then added to a final concentration of 3 mM. After 1 min, ADP dissolved in incubation buffer was added to a final concentration of 0.4 mM. Zinc was added as an aqueous solution of zinc sulfate. The final volume was 470 μl. The electrode was calibrated with nitrogen-purged water and incubation buffer based on the oxygen content of water (25). The rate of oxygen consumption was calculated from the recorder tracing and is expressed as nmol atomic oxygen per min per mg of protein (26).
Determination of Zinc.
The total amounts of zinc in the purified liver and heart mitochondria were measured by atomic absorption spectrophotometry (Perkin–Elmer Model 4100ZL).
Results
Localization of MT in Mitochondria.
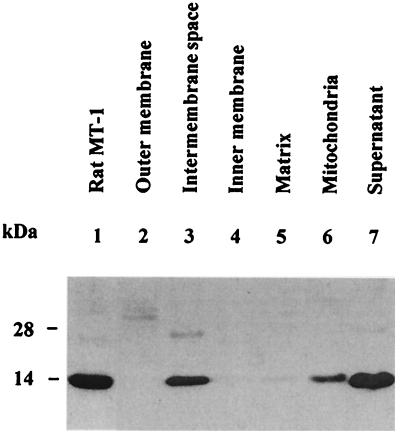
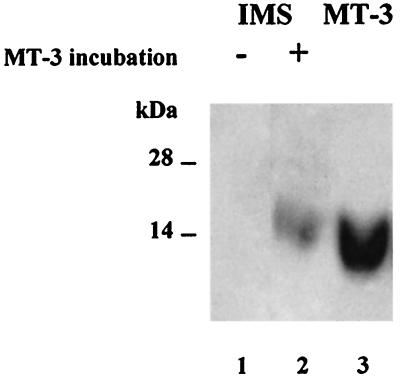
Submitochondrial fractions were prepared and assayed by Western blotting with a monoclonal anti-MT antibody (27) to determine whether MT is present in rat liver mitochondria. MT turns out to be a constituent of the IMS fraction; it is absent from both the outer and inner membrane and the matrix (Fig. 1). The presence of MT in the IMS fraction is not caused by cytosol contamination. In this fraction, the specific activity of alcohol dehydrogenase, a typical cytosolic enzyme, is less than 5% of that in the supernatant. However, the amount of MT detectable is about 50% of that in the supernatant. Mitochondria were incubated with MT-3 followed by Western blot analysis with a polyclonal anti-MT-3 antibody that does not crossreact with either MT-1 or MT-2. Mitochondria take up MT-3, which then localizes in the IMS fraction (Fig. 2).
Figure 1.
Western blot detection of MT in subcellular and submitochondrial fractions from rat liver. Lane 1, rat Zn7-MT-1; lanes 2–5, isolated submitochondrial fractions; lane 6, total mitochondrial protein obtained in the presence of 1% (vol/vol) Triton X-100; lane 7, supernatant from the centrifugation of mitochondria. Proteins (20 μg of total protein in each lane except for MT-1, which was 4.5 μg) were separated on 15% SDS/PAGE and transferred to a nitrocellulose membrane. The relative amounts of MT were determined with the monoclonal antibody II-10a.
Figure 2.
Western blot detection of MT-3 imported into isolated rat liver mitochondria. Lane 1, IMS fraction (200 μg); lane 2, IMS fraction (200 μg) after incubating mitochondria with human MT-3; lane 3, MT-3 (0.2 μg). For details see Materials and Methods.
The Effects of MT, MT-Domain Peptides, and Zinc on Respiration.
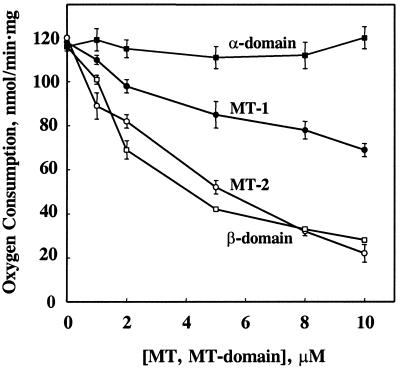
Zn7-MT isoforms inhibit mitochondrial respiration in the presence of succinate and ADP (coupled, or so-called “state” 3 mitochondria; Fig. 3) where MT-2 inhibits more strongly than MT-1. The effect of MT-3 is similar to that of MT-1 (data not shown). Half-maximum inhibition is at 4 μM and >10 μM for MT-2 and MT-1, respectively, regardless of whether MT is added after succinate or after ADP.† Thus, a relatively small change in MT-2 concentration significantly affects respiration.
Figure 3.
Inhibition of state 3 mitochondrial respiration by MT isoforms and zinc-reconstituted MT-domain peptides. Mitochondria corresponding to 500 μg of protein were injected into the electrochemical cell (450 μl) and incubated for 3 min. Succinate and ADP were then added to final concentrations of 3 mM and 0.5 mM, respectively. MT (up to 15 μl) was added when about half of the ADP was consumed (after about 1 min). The data for rabbit Zn7-MT-1 (●) and Zn7-MT-2 (○) were from two preparations of mitochondria. Incubation buffer (2–15 μl) or BSA (15 μg in 10 μl of buffer) had no effect. Zn-reconstituted α-domain (■) and β-domain (□) of human MT-2 were also equilibrated with incubation buffer. Addition of 10 μl of buffer served as a control. Standard errors are based on at least two measurements.
The MT molecule contains two zinc-binding clusters, one in the α- (4-zinc) and the other in the β- (3-zinc) domain. We have tested the zinc-containing, synthetic domains of MT-2, whose structures and properties were characterized recently (13), for their capacity to affect respiration. The α-domain is essentially inactive toward state 3 respiration, but the β-domain is as potent an inhibitor as the intact protein (half-maximum inhibition at 3 μM, Fig. 3).
Zinc sulfate dissolved in water and adjusted to pH 7.4 inhibits respiration with half-maximum inhibition that occurs at 4 μM.‡ Quantitative comparison of the effects of MT-2, the domains, and zinc indicates their similarity on a molar basis, suggesting that only one of the seven zinc atoms of MT or of the three zinc atoms of the β-domain is released. Hence, the effect of MT on mitochondrial respiration is caused by the release of that zinc.
Effects on Uncoupled Mitochondria.
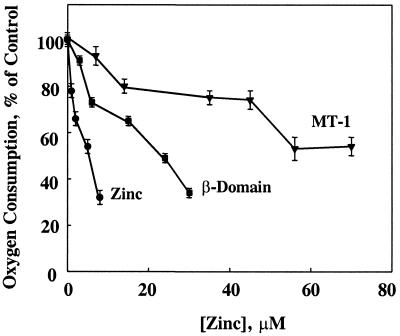
Uncoupled, i.e., nonphosphorylating, mitochondria served to determine whether the effects of MT and zinc are caused by inhibition of the respiratory chain. Both zinc and MT inhibit mitochondrial oxygen consumption stimulated by carbonyl cyanide m-chlorophenylhydrazone (m-Cl-CCP) (Fig. 4). Half-maximum inhibition occurs at 7 μM free zinc, 21 μM zinc in the form of the β-domain, and >50 μM in the form of MT-1. Thiol reagents reverse uncoupling by m-Cl-CCP (31); hence, 2,4-dinitrophenol was also used as an uncoupler. Zinc and MT inhibit 2,4-dinitrophenol-uncoupled respiration in a manner similar to that seen with m-Cl-CCP-uncoupled respiration (data not shown). This rules out that the inhibition of uncoupling is caused by a reaction of m-Cl-CCP with the thiols of MT. The results demonstrate that, indeed, MT inhibits the respiratory chain and, moreover, are in accord with the above observation that the effect of MT or the β-domain is brought about by one zinc atom released by either molecule.
Figure 4.
Inhibition of uncoupled mitochondrial respiration by rabbit MT-1 (▾), zinc-reconstituted β-domain (■), and zinc (●). The order of addition was mitochondria, succinate, m-Cl-CCP, and MT, MT-domain peptide, or zinc. The final concentration of m-Cl-CCP was 2 μM.
Effect of T and Other Chelating Agents on Zinc-Inhibited and Normal Respiration.
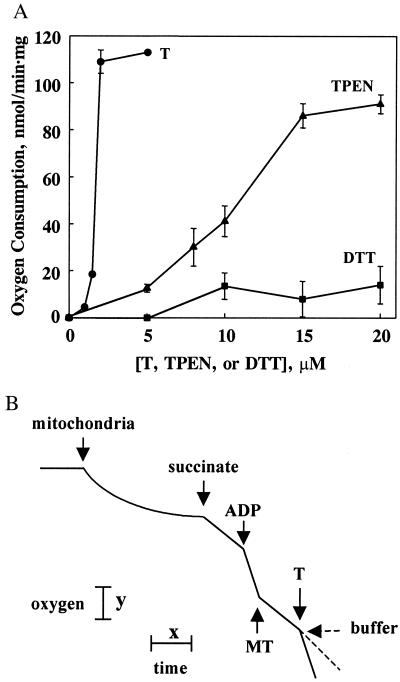
We have noted previously that T reverses the often very strong inhibition of enzymes by zinc (32). Hence, we examined whether T could similarly reverse the zinc or MT inhibition of mitochondrial respiration. Indeed, T (2 μM) reverses the inhibition of respiration by 10 μM zinc at a stoichiometry close to 1:1 (Fig. 5A). TPEN [Kd(Zn) = 2.6 × 10−16 M (33)], a membrane-permeant chelating agent, also reverses zinc-inhibited state 3 respiration. However, this chelating agent, which features nitrogen donor atoms and binds zinc three orders of magnitude more tightly than does T (Kd(Zn) = 1.4 × 10−13 M), is not as effective as T. The reactivation by T is caused by its zinc-chelating capacity, not the reducing capacity of its thiol groups, as indicated by the fact that DTT does not significantly affect zinc-inhibited respiration (Fig. 5A). T also activates mitochondrial state 3 respiration that has been inhibited by MT (Fig. 5B). Because MT turns out to be a constitutive protein of the IMS fraction of liver mitochondria, it is reasonable to expect that it might also inhibit normal mitochondrial respiration. Therefore, T was added to the respiration assay before ADP. This increased state 3 respiration by 20% and 40% at 2 and 4 μM T, respectively. TPEN also increased respiration 20%, but at 20 μM. Both experiments demonstrate that endogenous zinc partially inhibits native rat liver mitochondrial respiration.
Figure 5.
(A) Reactivation of zinc-inhibited state 3 respiration by T (●), TPEN (▴), and DTT (■). Mitochondrial respiration was first inhibited by zinc (10 μM). Respiration rates were normalized to that of a buffer control. The order of addition was mitochondria, succinate, ADP, zinc, and then T, TPEN, or DTT. (B) Reactivation of MT-2-inhibited state 3 respiration by T. Respiration was about 55% inhibited by 6.5 μM rabbit Zn7-MT-2 and then fully activated by T (2 μM). Buffer as a control had no effect. (Bars: x = 1 min, y = 22 nmol of oxygen atoms.)
Effect of Lysine-Modified MT.
The localization and functional studies show that MT is imported into the mitochondrial IMS, where it releases zinc, which then inhibits respiration. To establish which factors determine the entrance of MT through the outer mitochondrial membrane, we have chemically modified the conserved lysines of MT by carbamoylation. This modification changes the charge of the lysine side chains without affecting the capacity of MT to bind zinc (14). Chemically modified MT-1 (10 μM) or its β-domain (8 μM) shows 25% and 35% less inhibition of state 3 respiration than either parent molecule, implicating the participation of the MT lysines in mitochondrial uptake through the outer membrane.
Differential Presence and Inhibition of MT in Rat Liver and Heart Mitochondria.
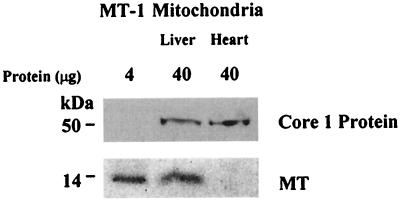
Heart mitochondria lack MT although its concentration in the cytosol is similar to that of the liver (34). Both rat liver and heart mitochondria were prepared and purified by density gradient centrifugation. MT is clearly detected in liver mitochondria but is completely lacking in heart mitochondria (Fig. 6). Moreover, whereas zinc inhibits respiration of both liver and heart mitochondria, MT-2 inhibits respiration of liver mitochondria, but not that of heart mitochondria (Table 1). It may well be that the lower zinc concentration of heart mitochondria underlies their higher respiratory rate. The tissue-specific factors that determine these differences remain to be identified.
Figure 6.
Western blot detection of MT in mitochondria of rat liver and heart. Mitochondria isolated by differential centrifugation were purified by density gradient centrifugation as described in Materials and Methods. Ubiquinol–cytochrome c oxidoreductase core 1 protein served as a control for the presence of the same amount of total mitochondrial protein on the membrane.
Table 1.
Rat liver and heart mitochondria: Zinc content and the effect of zinc and MT on respiration
| Tissue | Zinc, ng/mg protein | Oxygen consumption, nmol/min⋅mg protein | Inhibition
of respiration, %*
|
|
|---|---|---|---|---|
| Zn | MT | |||
| Liver | 53 ± 2 | 109 ± 8 | 46 ± 1 | 60 ± 3 |
| Heart | 39 ± 2 | 155 ± 10 | 29 ± 3 | ND |
Respiration was measured at state 3 with succinate and ADP. Means and standard errors are from at least two experiments. ND, not detectable.
Either 5 μM zinc or MT-2 has been used in the respiration assay.
Discussion
Zinc and Mitochondrial Function.
Hunter (35) first described the inhibition of oxidative phosphorylation by zinc 45 years ago. Zinc inhibits all four complexes of the respiratory chain (9), but only complexes I (36) and III (30, 37, 38) are inhibited with high affinity, i.e., Ki,app < 1 μM. Two zinc-binding sites in complex III have been identified by crystallographic methods (39). Overall, little attention has been paid to the possible physiological significance of these findings, largely because it was unclear how cellular zinc could elicit effects at the micromolar concentrations reported in these investigations when its availability as the free zinc ion was thought to be only in the nanomolar or picomolar range (40–42). The demonstration that MT inhibits respiration (7, 8), the observation that this inhibition occurs at micromolar concentrations of Zn7-MT, and studies on the zinc transfer potential of MT (10) now point to a mechanism by which MT transports zinc into mitochondria to exert the above described effects. Together with the observed T-mediated activation of zinc-inhibited respiration, these findings recognize and demonstrate that MT and T modulate mitochondrial respiration in a zinc-dependent manner.
MT and Mitochondrial Function.
MT is thought to be primarily a cytosolic protein that translocates to the nucleus during the early S phase of the cell cycle (43) or during differentiation (44). Immunohistochemical staining has localized it either at the outer membrane of mitochondria (45) or inside mitochondria when copper is in excess under pathological conditions (46). The presence of MT in mitochondria under physiological conditions has not been reported. In fact, the absence of MT from heart mitochondria has been stated explicitly (34). We have confirmed the absence of MT from heart mitochondria, but have established that it is present in the IMS of liver mitochondria.
Clearly, it is important to establish how MT enters mitochondria, because the protein lacks a mitochondrial targeting sequence. The outer mitochondrial membrane is permeable to molecules up to a size of 12 kDa (47). However, there is no example of a protein such as MT that enters mitochondria through a protein complex controlling this permeability. MT would presumably be imported into mitochondria by a mechanism akin to that used to import apocytochrome c. This process is unique thus far and is based on the interaction of critical lysine residues with phospholipids, direct translocation of the apoprotein through the membrane, and trapping it in the IMS by incorporation of heme into the apoenzyme (48). Additional support for such a mechanism of translocation derives from the fact that structurally, MT is homologous to the Cox17 metallochaperone that also lacks a mitochondrial targeting sequence and transfers copper into mitochondria for the reconstitution of cytochrome c oxidase (49). The molecular size of MT is quite similar to that of Cox17. Moreover, its sequence aligns with that of Cox17 at lysine and cysteine residues. The mechanisms of trapping Cox17 in the IMS and releasing copper are unknown. Experimental support for a mechanism of uptake involving the lysines of MT stems from the observation that chemical modification of the evolutionarily conserved lysines significantly reduces the capacity of the molecule to inhibit mitochondrial respiration (see above). Once MT crosses the outer mitochondrial membrane, it could bind to ATP (50), whose concentration in the IMS is high because of the dynamic compartmentalization of adenine nucleotides (51), providing a mechanism for retention of MT in the IMS. Concomitantly, zinc is transferred to an inhibitory site in the respiratory chain of the inner mitochondrial membrane so that it can elicit the effects that are the subject of this investigation. Zinc transfer could be triggered by an oxidative mechanism (10), or by lowering the pH from 7.4 to 6.0 in actively respiring mitochondria in the IMS, or by a combination of both. In this regard, it may be significant that the β- but not the α-domain is active in the respiration assay, and that in this pH range, binding of zinc to the β-domain is weaker than to the α-domain (13). The delivery of zinc to an inhibitory site on the respiratory chain and the reactivation by T is analogous to the action of zinc and T on cytosolic enzymes (32). Thus, zinc strongly inhibits a significant number of enzymes that are not zinc metalloenzymes, whereas T reverses this inhibition.
Implications.
Import of MT into the IMS and inhibition of respiration reveals a zinc-dependent biological activity in the MT/T system regarding mitochondrial function. It is not clear whether MT/T acts as a general shuttle to control the zinc content of mitochondria or is imported into mitochondria to modulate specific mitochondrial zinc-dependent functions. The absence of MT in heart mitochondria indicates that mitochondrial MT is not simply a result of equilibration with cytosolic MT. If it were, then MT should have been detected in mitochondria of transgenic mouse hearts where the cardiac concentration of MT is higher than that in the liver (34). Incubation of isolated heart mitochondria with MT neither affects their respiration nor effects import of MT into mitochondria. A specific mechanism would seem to allow access of MT to liver mitochondria, but would preclude its access to heart mitochondria.
We have limited the present discussion to the effects of zinc on the respiratory chain, because so little is known about other zinc-dependent mitochondrial functions; to date, few mitochondrial proteins have been identified as zinc proteins.
Uncoupler proteins separate fuel oxidation from the generation of energy in the form of ATP. Completely uncoupled mitochondria do not synthesize ATP, and the energy generated from the oxidation of fuel is dissipated as heat. An endogenous inhibitor of the respiratory chain that could serve as a pacemaker of energy production has not been identified as yet. Zinc could be such an agent. Its availability could be controlled strictly by the redox-linked MT/T system (52). Inhibition of the respiratory chain slows metabolism, i.e., zinc can override the respiratory control exercised by ADP. When modulated by an inhibitor of the respiratory chain, coupled mitochondria could continue to phosphorylate ADP to ATP, albeit at a slower pace.
In this context, it could be significant that mice whose MT-1/-2 genes have been disrupted become mildly obese (53), supporting the postulated roles of MT and T as modulators of energy metabolism. In such mice, the total amount of cellular zinc does not change, but the lack of T would make more free zinc available, and even a slight increase in the amount of available zinc could affect mitochondrial function significantly (Fig. 4), and thereby affect cellular energy metabolism. The metabolic response of MT-1/-2 knock-out mice to endotoxin, as well as studies on cultured hepatocytes from these mice, have also suggested such a role (54, 55). Indeed, the very large number of physiological states, conditions, and agents that induce MT/T—including starvation, the acute phase response, cold, heat, or stresses (56, 57), and the highly dynamic nature of the MT/T system, including changes of the MT concentration during the cell cycle (27)—would seem to support its proposed global role in energy metabolism, and in particular, in fine-tuning mitochondrial energy metabolism by modulating reversible zinc binding. These circumstances further support the contention that the primary function of MT is not metal detoxification, but the control of cellular zinc distribution, translocation, and availability (6).
There is increasing awareness of the central role of mitochondria in controlling the output of reactive oxygen species, thereby determining life and death of a cell. Pathologically high levels of zinc can inhibit respiration completely, leading to apoptosis as documented for neurodegeneration, where zinc derived from zinc-containing neurons in the brain enters target cells and induces cell death through formation of reactive oxygen species (58). Knowledge of the involvement of MT/T in mitochondrial respiration will help decipher the critical roles of metals such as zinc and copper in energetics-related pathogenesis of neurodegenerative diseases (59, 60).
Acknowledgments
We thank Prof. Milan Vašák for providing human MT-3, rat MT-1 protein, and the anti-MT-3 antibody. We appreciate the invaluable discussions with Profs. James F. Riordan and Richard E. McCarty. This work was supported by the Endowment for Research in Human Biology, Inc.
Abbreviations
- MT
metallothionein
- IMS
intermembrane space
- T
thionein
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine
- m-Cl-CCP
carbonyl cyanide m-chlorophenylhydrazone
Footnotes
In the absence of ADP, incubation of mitochondria with zinc activates oxygen consumption (data not shown; ref. 7). Apparently, incubation allows enough time for zinc to reach mitochondrial sites where it can affect the rate of succinate oxidation. Alternatively, activation by zinc or MT could be caused by an effect on mitochondrial swelling and ensuing uncoupling, which occurs on a time scale longer (>5 min) than that of the effects on the respiratory chain (28,29). In the absence of ADP, the effect of MT depends on the order of addition. In succinate-powered mitochondria, MT inhibits the rate of oxygen consumption. However, if added before succinate, MT substantially activates this rate at concentrations below 4 μM (data not shown). Such an activation by Cd,Zn-MT was also reported by Simpkins et al. (7), who did not specify the order of additions, however.
Little effect was noticed with zinc sulfate from a 500 μM stock solution prepared in incubation buffer that contained phosphate, presumably because of the formation of insoluble zinc phosphate. The chemical form of zinc is critical and may be the reason for the wide variations noted when zinc was tested for its effects on mitochondrial respiration under different assay conditions (7, 30).
References
- 1.Vallee B L, Falchuk K H. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Andersson T, Berggren P-O, Flatt P R. Horm Metab Res. 1980;12:275–276. doi: 10.1055/s-2007-996266. [DOI] [PubMed] [Google Scholar]
- 3.Thiers R E, Vallee B L. J Biol Chem. 1957;226:911–927. [PubMed] [Google Scholar]
- 4.Yamaguchi M, Kura M, Okada S. Chem Pharm Bull. 1981;29:2370–2374. doi: 10.1248/cpb.29.2370. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Franklin R B, Costello L C. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Vallee B L. Neurochem Int. 1995;27:23–33. doi: 10.1016/0197-0186(94)00165-q. [DOI] [PubMed] [Google Scholar]
- 7.Simpkins C O, Zhao H-L, Torrence C A. Life Sci. 1994;55:221–226. doi: 10.1016/0024-3205(94)00883-3. [DOI] [PubMed] [Google Scholar]
- 8.Simpkins C, Balderman S, Mensah E. J Surg Res. 1998;80:16–21. doi: 10.1006/jsre.1998.5383. [DOI] [PubMed] [Google Scholar]
- 9.Skulachev V P, Chistyakov V V, Jasaitis A A, Smirnova E G. Biochem Biophys Res Commun. 1967;26:1–6. doi: 10.1016/0006-291x(67)90242-2. [DOI] [PubMed] [Google Scholar]
- 10.Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vašák M. Methods Enzymol. 1991;205:41–44. doi: 10.1016/0076-6879(91)05082-7. [DOI] [PubMed] [Google Scholar]
- 12.Jacob C, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang L-J, Vašák M, Vallee B L, Maret W. Proc Natl Acad Sci USA. 2000;97:2503–2508. doi: 10.1073/pnas.97.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng J. Methods Enzymol. 1991;205:433–437. doi: 10.1016/0076-6879(91)05127-h. [DOI] [PubMed] [Google Scholar]
- 15.Johnson D, Lardy H. Methods Enzymol. 1967;10:94–96. [Google Scholar]
- 16.Tyler D D, Gonze J. Methods Enzymol. 1967;10:75–77. [Google Scholar]
- 17.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Rickwood D, Ford T, Graham J. Anal Biochem. 1982;123:23–31. doi: 10.1016/0003-2697(82)90618-2. [DOI] [PubMed] [Google Scholar]
- 19.Greenawalt J W. Methods Enzymol. 1974;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- 20.Wagner F W, Burger A R, Vallee B L. Biochemistry. 1983;22:1857–1863. doi: 10.1021/bi00277a018. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli A K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Nagel W, Hartmann H-J, Weser U. Immunol Lett. 1990;26:291–296. doi: 10.1016/0165-2478(90)90162-j. [DOI] [PubMed] [Google Scholar]
- 23.Mizzen C A, Cartel N J, Yu W H, Fraser P E, McLachlan D R. J Biochem Biophys Methods. 1996;32:77–83. doi: 10.1016/0165-022x(95)00044-r. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 25.Gevantman L H. In: Handbook of Chemistry and Physics. 81st Ed. Lide D R, editor. Boca Raton, FL: CRC; 2000. pp. 8-86–8-89. [Google Scholar]
- 26.Estabrook R W. Methods Enzymol. 1967;10:41–47. [Google Scholar]
- 27.Nagel W W, Vallee B L. Proc Natl Acad Sci USA. 1995;92:579–583. doi: 10.1073/pnas.92.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpkins C, Lloyd T, Li S, Balderman S. J Surg Res. 1998;75:30–34. doi: 10.1006/jsre.1997.5241. [DOI] [PubMed] [Google Scholar]
- 29.Wudarczyk J, Debska G, Lenartowicz E. Arch Biochem Biophys. 1999;363:1–8. doi: 10.1006/abbi.1998.1058. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner D, von Jagow G. FEBS Lett. 1972;20:229–232. doi: 10.1016/0014-5793(72)80802-0. [DOI] [PubMed] [Google Scholar]
- 31.Heytler P G. Biochemistry. 1963;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- 32.Maret W, Jacob C, Vallee B L, Fischer E H. Proc Natl Acad Sci USA. 1999;96:1936–1940. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arslan P, Di Virgilio F, Beltrame M, Tsien R Y, Pozzan T. J Biol Chem. 1985;260:2719–2727. [PubMed] [Google Scholar]
- 34.Zhou Z, Kang Y J. Am J Pathol. 2000;156:1653–1662. doi: 10.1016/S0002-9440(10)65036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter F E, Ford L. J Biol Chem. 1955;216:357–369. [PubMed] [Google Scholar]
- 36.Brown A M, Kristal B S, Effron M S, Shestopalov A I, Ullucci P A, Sheu K-F R, Blass J P, Cooper A J L. J Biol Chem. 2000;275:13441–13447. doi: 10.1074/jbc.275.18.13441. [DOI] [PubMed] [Google Scholar]
- 37.Lorusso M, Cocco T, Sardanelli A M, Minuto M, Bonomi F, Papa S. Eur J Biochem. 1991;197:555–561. doi: 10.1111/j.1432-1033.1991.tb15944.x. [DOI] [PubMed] [Google Scholar]
- 38.Link T A, von Jagow G. J Biol Chem. 1995;270:25001–25006. doi: 10.1074/jbc.270.42.25001. [DOI] [PubMed] [Google Scholar]
- 39.Berry E A, Zhang Z, Bellamy H D, Huang L. Biochim Biophys Acta. 2000;1459:440–448. doi: 10.1016/s0005-2728(00)00182-1. [DOI] [PubMed] [Google Scholar]
- 40.Peck E J, Ray W J. J Biol Chem. 1971;246:1160–1167. [PubMed] [Google Scholar]
- 41.Atar D, Backx P H, Appel M M, Gao W D, Marban E. J Biol Chem. 1995;270:2473–2477. doi: 10.1074/jbc.270.6.2473. [DOI] [PubMed] [Google Scholar]
- 42.Simons T J B. J Membr Biol. 1991;123:63–71. doi: 10.1007/BF01993964. [DOI] [PubMed] [Google Scholar]
- 43.Tsujikawa K, Imai T, Kakutani M, Kayamori Y, Mimura T, Otaki N, Kimura M, Fukuyama R, Shimizu N. FEBS Lett. 1991;283:239–242. doi: 10.1016/0014-5793(91)80597-v. [DOI] [PubMed] [Google Scholar]
- 44.Apostolova M D, Ivanova I A, Cherian M G. Toxicol Appl Pharmacol. 1999;159:175–184. doi: 10.1006/taap.1999.8755. [DOI] [PubMed] [Google Scholar]
- 45.Yamada M, Hayashi S, Hozumi I, Inuzuka T, Tsuji S, Takahashi H. Brain Res. 1996;735:257–264. doi: 10.1016/0006-8993(96)00586-0. [DOI] [PubMed] [Google Scholar]
- 46.Sakurai H, Nakajima K, Kamada H, Satoh H, Otaki N, Kimura M, Kawano K, Hagino T. Biochem Biophys Res Commun. 1993;192:893–898. doi: 10.1006/bbrc.1993.1499. [DOI] [PubMed] [Google Scholar]
- 47.Pfaff E, Klingenberg M, Ritt E, Vogell W. Eur J Biochem. 1968;5:222–232. doi: 10.1111/j.1432-1033.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 48.Stuart R A, Neupert W. Biochimie (Paris) 1990;72:115–121. doi: 10.1016/0300-9084(90)90136-5. [DOI] [PubMed] [Google Scholar]
- 49.Amaravadi R, Glerum D M, Tzagaloff A. Hum Genet. 1997;99:329–333. doi: 10.1007/s004390050367. [DOI] [PubMed] [Google Scholar]
- 50.Jiang L-J, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:9146–9149. doi: 10.1073/pnas.95.16.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gellerich F N, Schlame M, Bohnensack R, Kunz W. Biochim Biophys Acta. 1987;890:117–126. doi: 10.1016/0005-2728(87)90012-0. [DOI] [PubMed] [Google Scholar]
- 52.Maret W. J Nutr. 2000;130:1455S–1458S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- 53.Beattie J H, Wood A M, Newman A M, Bremner I, Choo K H A, Michalska A E, Duncan J S, Trayhurn P. Proc Natl Acad Sci USA. 1998;95:358–363. doi: 10.1073/pnas.95.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Philcox J C, Sturkenboom M, Coyle P, Rofe A M. J Nutr. 2000;130:1901–1909. doi: 10.1093/jn/130.8.1901. [DOI] [PubMed] [Google Scholar]
- 55.Rofe A M, Philcox J C, Coyle P. Biol Trace Elem Res. 2000;75:87–97. doi: 10.1385/BTER:75:1-3:87. [DOI] [PubMed] [Google Scholar]
- 56.Samson S L, Gedamu L. Prog Nucleic Acid Res Mol Biol. 1998;59:257–288. doi: 10.1016/s0079-6603(08)61034-x. [DOI] [PubMed] [Google Scholar]
- 57.Andrews G K. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 58.Sensi S L, Yin H Z, Carriedo S G, Rao S S, Weiss J H. Proc Natl Acad Sci USA. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beal M F. Trends Neurosci. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- 60.Bush A I. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]