Abstract
G protein-coupled receptors (GPCRs) have key roles in cardiovascular regulation and are important targets for the treatment of hypertension. GTPase-activating proteins, such as RGS2, modulate downstream signaling by GPCRs. RGS2 displays regulatory selectivity for the Gαq subclass of G proteins, and mice lacking RGS2 develop hypertension through incompletely understood mechanisms. Using total body RGS2-deficient mice, we used a kidney crosstransplantation strategy to examine separately the contributions of RGS2 actions in the kidney from those in extrarenal tissues with regard to BP regulation. Loss of renal RGS2 was sufficient to cause hypertension, whereas the absence of RGS2 from all extrarenal tissues including the peripheral vasculature did not significantly alter BP. Accordingly, these results suggest that RGS2 acts within the kidney to modulate BP and prevent hypertension. These data support a critical role for the renal epithelium and/or vasculature as the final determinants of the intra-arterial pressure in hypertension.
The role of G protein-coupled receptors (GPCRs) in hypertension and cardiovascular diseases is well established.1 Moreover, pharmacologic antagonists of GPCRs, such as β-adrenergic and angiotensin receptors, are cornerstones of therapy in the treatment of hypertension and its complications.2 Signaling by GPCRs is triggered by ligand-induced conformational changes in the receptor that promote exchange of guanosine 5′-diphosphate for guanosine 5′-triphosphate on the Gα subunit of the G protein complex,3 followed by dissociation of Gα from the Gβγ dimer. The dissociated subunits can then interact with effector molecules to propagate the signal. The duration and intensity of signaling are further regulated by GTPase-activating proteins.4 The regulators of G protein signaling (RGSs) are a family of proteins with GTPase-activating protein activity.4 Among these, RGS2 displays regulatory selectivity for the Gαq subclass of G proteins.5 Many key cardiovascular hormones such as angiotensin II, endothelin-1, thromboxane A2, and norepinephrine activate receptors that couple to Gαq.
A specific role for RGS2 in maintaining normal vascular tone and BP was established using genetically modified mice.6,7 RGS2-deficient mice have hypertension6,7 along with abnormal vascular contraction and relaxation responses.7 In addition to its actions to influence the contractile state of vascular smooth muscle, regulated expression of RGS2 has been described in other tissues that are important for BP regulation including the central nervous system8 and the kidney.9 Here, we use a kidney crosstransplantation strategy to distinguish contributions of RGS2 actions in the kidney from extrarenal tissues to the regulation of BP and the development of hypertension. Our studies indicate that RGS2 effects within the kidney are critical for regulation of BP, suggesting that altered renal epithelial and/or vascular functions are responsible for hypertension in this genetic model.
To determine the relative contributions of RGS2 in renal versus extrarenal tissues to the pathogenesis of hypertension, we used a kidney crosstransplantation strategy. By varying the genotype of the transplant donor and recipient, we generated four groups of animals in which renal function was provided entirely by the single transplanted kidney. The wild-type group consisted of wild-type mice transplanted with kidneys from wild-type donors, having normal expression of RGS2 in the kidney transplant and in all systemic tissues. For the systemic knockout (KO) group, RGS2-deficient recipients were transplanted with kidneys from wild-type donors; these animals lack RGS2 in all tissues except the kidney. Kidney KO animals are wild-type recipients of RGS2-deficient kidneys lacking expression of RGS2 only in renal parenchyma and vasculature but with normal expression of receptors in all systemic, nonrenal tissues including peripheral vessels. Finally, the total KO group consists of RGS2-deficient recipients of RGS2-deficient kidneys and therefore completely lacks RGS2 in all tissues.
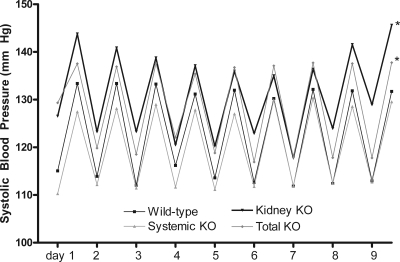
The absence of RGS2 did not significantly affect the normal diurnal variation in BP in any of the groups (Figure 1). Among the transplanted animals, mean systolic BP levels for the period of baseline recording in the wild-type group (123 ± 2 mmHg; n = 7) were in a range similar to previous measurements in nontransplanted, wild-type C57BL/6 mice,10 supporting our previous observations that the surgical procedure and the presence of only a single transplanted kidney do not significantly alter baseline levels of BP.10 By contrast (Figure 2), BP levels were significantly increased in the total KO animals completely lacking RGS2 (129 ± 2 mmHg; n = 6) compared with the wild-type controls (P = 0.04). Thus, elimination of RGS2 in all tissues in the total KO group recapitulates the original phenotype of elevated BP described in Rgs2−/− mice.6,7,11
Figure 1.
Daytime and nighttime systolic BPs measured by radiotelemetry are elevated in kidney KO and total KO groups. Diurnal variation was preserved in all groups. *P = 0.04.
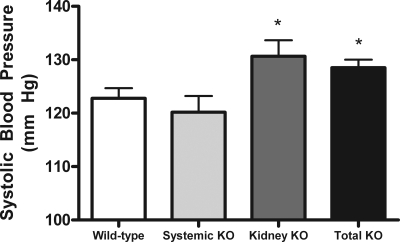
Figure 2.
Mean systolic BPs are significantly increased in the kidney KO and total KO groups compared with wild-type controls. *P = 0.04. In the systemic KO group, transplantation of a wild-type kidney into a RGS2-deficient mouse generated a normal BP.
BP levels in the systemic KO group (120 ± 3 mmHg; n = 6) were not different from wild-type controls. Thus, deletion of RGS2 from all extrarenal tissues including the central nervous system and peripheral vasculature is not sufficient to cause hypertension. On the other hand, BP levels in the kidney KO group (131 ± 3.0 mmHg; n = 7) were significantly increased compared with the wild-type controls (P = 0.046) and comparable with those of the total KO group. This finding is consistent with the view that the kidney is a major determinant of the chronic level of BP and indicates that the absence of signaling pathways linked to RGS2 in the kidney and its vasculature is sufficient to increase BP. The patterns of BP differences between the groups were similar when daytime and nighttime BPs were examined separately (not shown). Furthermore, feeding a high-salt (6% NaCl) diet for 7 days did not significantly affect BP in any of the groups except the total KO group, in which an increase in BP from 131 ± 3 mmHg on the regular (0.4% NaCl) diet to 137 ± 11 mmHg on the high-salt diet was observed, which approached statistical significance (P = 0.0503).
At the end of the studies, kidneys and hearts were harvested, and organ weights were determined. As shown in Table 1, whereas the heart-to-body weight ratio was numerically highest in the kidney KO group, there were no significant differences across the four groups. Thus, the relatively modest differences in BP between the groups did not generate appreciable differences in heart weight. Likewise, there was no systematic evidence of cardiac hypertrophy in the Rgs2−/− recipients, which had experienced life-long RGS2 deficiency before transplantation.
Table 1.
Organ weights in transplant groups
| Transplant Group | Body Weight (g) | Kidney Weight (mg) | Heart Weight (mg) | Kidney Weight/Body Weight (mg/g) | Heart Weight/Body Weight (mg/g) |
|---|---|---|---|---|---|
| Wild-type | 26.7 ± 0.6 | 201.4 ± 8 | 126.0 ± 5 | 7.6 ± 0.3 | 4.7 ± 0.1 |
| Systemic KO | 27.7 ± 1.6 | 262.9 ± 11 | 137.1 ± 9 | 9.1 ± 0.6a | 4.9 ± 0.1 |
| Kidney KO | 30.5 ± 0.8 | 263.8 ± 29 | 196.1 ± 34 | 8.7 ± 1.1 | 6.5 ± 1.3 |
| Total KO | 27.3 ± 0.9 | 215.2 ± 11 | 139.2 ± 6 | 7.9 ± 0.3 | 5.1 ± 0.1 |
aP = 0.03 compared with wild-type.
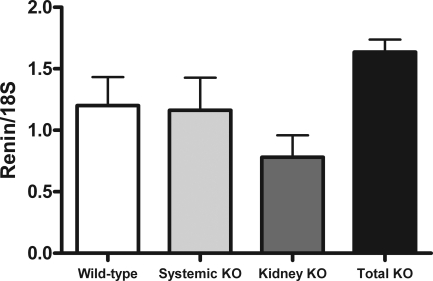
Because the renin-angiotensin system (RAS) is a key regulator of BP homeostasis and Gq-linked GPCRs may influence responsiveness to angiotensin II, we measured mRNA expression of renin, a key rate-limiting enzyme regulating the activity of the RAS. As shown in Figure 3, renin mRNA levels in the transplanted kidneys were not significantly different between the groups. In particular, there was no evidence for enhanced renin expression in the kidneys of the kidney KO and total KO groups with the highest BP, indicating that activation of the systemic RAS was not a mechanism driving the elevated BP in these groups.
Figure 3.
There are no significant differences in renin mRNA expression in transplanted kidneys among the experimental groups.
On the basis of these previous studies using RGS2-deficient mice, it was concluded that the mechanism of hypertension in RGS2-deficient mice was likely related to exaggerated GPCR signaling in vascular smooth muscle cells leading to chronic vasoconstriction and consequent increases in peripheral vascular resistance.6,7 This conclusion was consistent with other studies suggesting that alterations in vascular signaling pathways are necessary and sufficient to mediate hypertension.12–19 However, previous work by Guyton20 suggests that chronic peripheral vasoconstriction alone should not be sufficient to cause hypertension. This view is on the basis of the idea that the sodium excretory capacity of the kidney provides a compensatory system with virtually infinite gain for countermanding elevations in BP from other causes including changes in peripheral vascular resistance. As a corollary of this hypothesis, abnormal sodium handling by the kidney would be required to maintain chronic elevation of arterial pressure irrespective of the nature of the initial stimulus.20 Work from the Lifton laboratory21 showing that virtually all of the Mendelian syndromes characterized by high or low BP in humans are caused by genetic variants affecting renal sodium handling further highlights the power of these renal pathways to affect BP. Along the same lines, we have shown that actions of type 1 angiotensin receptors in the kidney alone mediate the major actions of the RAS to promote hypertension.22
To separately examine the contributions of renal and extrarenal actions of RGS2 in BP control, kidney transplantation was carried out between genetically matched C57BL/6 wild-type and RGS2-deficient mice homozygous for a targeted disruption of the Rgs2 gene locus.23 Except for the presence or absence of RGS2, the donors and recipients are genetically identical, so there is no rejection and no need for immunosuppressive therapy. The major finding in our study is that the pool of RGS2 in the kidney is required for maintenance of normal systemic BP. This is clearly illustrated in the kidney KO group animals, in which the lack of RGS2 only in the kidney and its vasculature is sufficient to recapitulate the phenotype of hypertension seen in Rgs2−/− mice with global deficiency of RGS2. Conversely, the systemic KO group has normal BP despite the absence of RGS2 from extrarenal tissues, including key areas that potentially affect BP homeostasis including the brain, the heart, the peripheral vasculature, and the adrenal gland. In this case, providing normal levels of RGS2 expression at key sites within the kidney rescues the hypertensive phenotype.
Although these studies strongly support the importance of renal RGS2 in maintaining normal levels of BP, they are inadequate to distinguish the precise functions of RGS2 responsible for producing this hypertensive phenotype. Specifically, these experiments cannot distinguish between actions of RGS2 to modulate renal epithelial functions versus regulation of vasomotor tone in the renal vasculature. RGS2 is highly expressed within the kidney in a number of cell types including epithelium and vascular smooth muscle cells. The actions of GPCRs coupled to Gαq such as type 1 angiotensin receptors affect fluid and solute reabsorption by epithelial cells along the nephron, thereby modulating BP.22 RGS2 may act to attenuate these effects and promote natriuresis. In addition, GPCRs expressed along the renal vasculature regulate renal blood flow and thereby have secondary effects to influence renal sodium handling.24 For example, renal vasoconstriction caused by angiotensin II reduces medullary blood flow, thus blunting the kidney's excretory capacity for sodium.25 RGS2 would attenuate these actions, and these effects may be exaggerated in RGS2-deficient mice, potentially promoting hypertension. Therefore, RGS2 in the kidney may affect BP through direct actions on epithelial function and/or renal vascular resistance, and abrogation of these actions causes hypertension. However, with our current data, we cannot dissect which of these precise compartments plays the dominant role or the exact mechanism(s) involved. Future studies will address these questions.
CONCISE METHODS
Experimental Animals
A null mutation in the Rgs2 gene23 was back-crossed onto the C57BL/6 genetic background, and inbred C57BL/6-Rgs2+/+ and −/− male mice were used as kidney transplant donors and recipients. The experimental procedures described below were approved by the respective IACUCs of the Durham Veterans Affairs and Duke University Medical Centers.
Renal Crosstransplantation in RGS2-deficient Mice
Transplantation of a single mouse kidney with bilateral native nephrectomy was performed as we have described previously.10 Overall surgical mortality was approximately 20%.
Measurement of BP in Conscious Mice
We used a radiotelemetry system (Data Sciences International/Transoma Medical, St. Paul, MN) to monitor BP in conscious mice as described previously.10 The pressure-sensing catheter was implanted via the left carotid artery as described,10 6 to 8 days after transplantation. The mice were allowed to recover for 7 days after surgery to regain their normal circadian rhythms before BP measurements were initiated; the mice were housed in a separate light cycle-controlled “monitoring” room in the animal facility where quiet is maintained and no other activities are permitted. The BP data were collected continuously with sampling every 5 minutes at 10-second intervals26 during the prescribed time periods using Dataquest A.R.T. software (Data Sciences International/Transoma Medical, St. Paul, MN).
Experimental Protocol
Baseline BPs were measured on 10 consecutive days while the animals ingested a conventional diet containing 0.4% sodium chloride. BP response to a high-sodium diet (6% sodium chloride; Harlan Teklad, Madison, WI) was also assessed over a 1-week period. The animals were sacrificed 6 weeks after the initial surgery, and the hearts and kidneys were removed, weighed, and snap frozen in liquid nitrogen.
RNA Isolation and Analysis
Total RNA was extracted from the transplanted kidneys of mice from each group (RNEasy; Qiagen) using standard techniques. Renin mRNA levels were measured using real-time quantitative PCR, which was performed using the fluorogenic 5′-exonuclease assay27 with primers and dual-labeled probe (5′-FAM and 3′-TAMRA) on the basis of previously published sequences28 as described previously.29 Gene expression was quantified using the ΔΔCt method for relative quantitation.30
Statistical Analysis
The values for each parameter within a group are expressed as the means ± the SEM. For comparisons between groups, statistical significance was assessed using a t test or ANOVA followed by Tukey's test for multiple comparisons. A paired t test was used for comparisons within groups.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health Grants PO1 HL077378-01 (MEM, RHK, and TMC) and RO1 HL55309 (MEM), the Medical Research Service of the Veterans Administration (TMC), the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research at Duke (TMC), and American Heart Association Fellow-to-Faculty Transition Award (SBG).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “RGS2 Proteins Regulate Blood Pressure,” on pages 1809–1810.
REFERENCES
- 1. Rockman HA, Koch WJ, Lefkowitz RJ: Seven-transmembrane-spanning receptors and heart function. Nature 415: 206–212, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ: Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Gilman AG: G proteins: Transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Ross EM, Wilkie TM: GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem 69: 795–827, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR: RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci U S A 94: 14389–14393, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin AJ, Steinberg TH, Wyss JM, Mecham RP, Blumer KJ: Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J Clin Invest 111: 445–452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, Mendelsohn ME: Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med 9: 1506–1512, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Iadecola C, Davisson RL: Hypertension and cerebrovascular dysfunction. Cell Metab 7: 476–484, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crowley SD, Coffman TM: In hypertension, the kidney breaks your heart. Curr Cardiol Rep 10: 470–476, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM: Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hercule HC, Tank J, Plehm R, Wellner M, da Costa Goncalves AC, Gollasch M, Diedrich A, Jordan J, Luft FC, Gross V: Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp Physiol 92: 1014–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM: Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest 110: 203–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris DM, Cohn HI, Pesant S, Zhou RH, Eckhart AD: Vascular smooth muscle G (q) signaling is involved in high blood pressure in both induced renal and genetic vascular smooth muscle-derived models of hypertension. Am J Physiol Heart Circ Physiol 293: H3072–H3079, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Keys JR, Zhou RH, Harris DM, Druckman CA, Eckhart AD: Vascular smooth muscle overexpression of G protein-coupled receptor kinase 5 elevates blood pressure, which segregates with sex and is dependent on Gi-mediated signaling. Circulation 112: 1145–1153, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Mendelsohn ME: In hypertension, the kidney is not always the heart of the matter. J Clin Invest 115: 840–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME: High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci U S A 105: 6702–6707, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F: Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S: G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME: Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Guyton AC: Blood pressure control: Special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Lifton RP, Wilson FH, Choate KA, Geller DS: Salt and blood pressure: New insight from human genetic studies. Cold Spring Harbor Symp Quant Biol 67: 445–450, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oliveira-Dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, Mariathasan S, Sasaki T, Wakeham A, Ohashi PS, Roder JC, Barnes CA, Siderovski DP, Penninger JM: Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Natl Acad Sci U S A 97: 12272–12277, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zuber AM, Singer D, Penninger JM, Rossier BC, Firsov D: Increased renal responsiveness to vasopressin and enhanced V2 receptor signaling in RGS2−/− mice. J Am Soc Nephrol 18: 1672–1678, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Cowley AW, Jr.: Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol 273: R1–R15, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Butz GM, Davisson RL: Long-term telemetric measurement of cardiovascular parameters in awake mice: A physiological genomics tool. Physiol Genomics 5: 89–97, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Heid CA, Stevens J, Livak KJ, Williams PM: Real time quantitative PCR. Genome Res 6: 986–994, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Kim HS, Lee G, John SW, Maeda N, Smithies O: Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proc Natl Acad Sci U S A 99: 4602–4607, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM: Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 54: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong ML, Medrano JF: Real-time PCR for mRNA quantitation. Biotechniques 39: 75–85, 2005 [DOI] [PubMed] [Google Scholar]