Abstract
Mutations in α, β, or γ subunits of the epithelial sodium channel (ENaC) can downregulate ENaC activity and cause a severe salt-losing syndrome with hyperkalemia and metabolic acidosis, designated pseudohypoaldosteronism type 1 in humans. In contrast, mice with selective inactivation of αENaC in the collecting duct (CD) maintain sodium and potassium balance, suggesting that the late distal convoluted tubule (DCT2) and/or the connecting tubule (CNT) participates in sodium homeostasis. To investigate the relative importance of ENaC-mediated sodium absorption in the CNT, we used Cre-lox technology to generate mice lacking αENaC in the aquaporin 2–expressing CNT and CD. Western blot analysis of microdissected cortical CD (CCD) and CNT revealed absence of αENaC in the CCD and weak αENaC expression in the CNT. These mice exhibited a significantly higher urinary sodium excretion, a lower urine osmolality, and an increased urine volume compared with control mice. Furthermore, serum sodium was lower and potassium levels were higher in the genetically modified mice. With dietary sodium restriction, these mice experienced significant weight loss, increased urinary sodium excretion, and hyperkalemia. Plasma aldosterone levels were significantly elevated under both standard and sodium-restricted diets. In summary, αENaC expression within the CNT/CD is crucial for sodium and potassium homeostasis and causes signs and symptoms of pseudohypoaldosteronism type 1 if missing.
Sodium reabsorption in the kidney is essential for maintaining fluid and electrolyte homeostasis as well as regulation of blood pressure (BP). Renal sodium reabsorption is under tight control of aldosterone in the late distal convoluted tubule (DCT2), the connecting tubule (CNT), and the collecting duct (CD).1 Sodium enters the aldosterone-sensitive epithelial cell through the epithelial sodium channel (ENaC) at the apical plasma membrane, and sodium is extruded to the interstitial fluid via the basolateral Na+-K+-ATPase in exchange for potassium. In the DCT2, sodium is also absorbed through the thiazide-sensitive NaCl co-transporter (TSC).2 The critical role of ENaC in sodium homeostasis has been emphasized by identification of gain-of-function mutations in the C-terminus of the β or the γ subunit in patients with Liddle syndrome, a severe form of hypertension caused by sodium retention.3,4 Pseudohypoaldosteronism type 1 (PHA-1), conversely, is a severe salt-wasting syndrome characterized by urinary loss of sodium and reduced potassium excretion despite elevated levels of aldosterone. In humans, a life-threatening form of the disease is inherited as an autosomal recessive trait and is caused by loss-of-function mutations in any of the three ENaC subunits.5 Clinical symptoms of the disease are weight loss and dehydration, hypovolemia and hypotension, hyponatremia, hyperkalemia, and metabolic acidosis accompanied by elevated plasma aldosterone levels.6 Complete knockout (KO) of each of the ENaC subunits resulted in an early and lethal PHA-1 phenotype.7–9 Previously, a CD-specific conditional KO for αENaC was generated, and, surprising, these mice were able to maintain water, sodium, and potassium balance even after 1 week of salt restriction, 23 hours of water deprivation, or 4 days of potassium loading.10 In this study, we investigated the implication of the CNT and CD for the ENaC-mediated sodium reabsorption using mice that express the Cre recombinase from the Aqp2 promoter (Aqp2::iCre) and conditional alleles of αENaC (Scnn1aloxlox).11,12 Aquaporin 2 (AQP2) is a water channel expressed along the CNT and CD.13,14 Our data indicate that αENaC expression within the CNT is important for sodium and potassium balance.
RESULTS
Inactivation of αENaC in the CD and the CNT
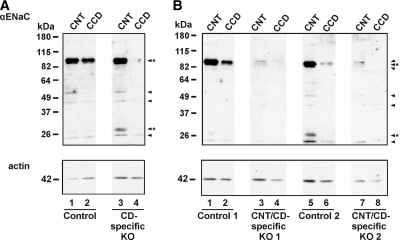
KO and control mice were born consistent with Mendelian inheritance (Scnn1alox/−/Aqp2::iCre 25.9%, Scnn1alox/+/Aqp2::iCre 29.4%, Scnn1alox/+ 21.8%, and Scnn1alox/− 22.8%; n = 197). To verify the deletion of αENaC expression in these CNT/CD-specific KO mice (Scnn1alox/-/Aqp2::iCre), we dissected the CNT and the CCD and analyzed these by Western blot (Figure 1). The previously described CD-specific KO mice (Scnn1alox/lox/HoxB7::Cre) were used as positive (and negative) control.10 In the CNT and the CCD of all control groups, a band at approximately 95 kD corresponding to full-length αENaC protein was observed (Figure 1A, lanes 1 and 2, and B, lanes 1 and 2 and lanes 5 and 6). This band was absent in the CCD of the CD-specific KO mice (Figure 1A, lane 4)10 and in the CCD of the CNT/CD-specific KO mice (Figure 1B, lanes 4 and 8). We observed a faint band corresponding to full-length αENaC protein in the CNT of the CNT/CD-specific KO mice (Figure 1B, lanes 3 and 7), suggesting that the inactivation of αENaC in the CNT was not complete. We further observed a band just above 26 kD in the CNT of the CD-specific KO mice (Figure 1A, lane 3) and in the CNT of the control littermates of the CNT/CD-specific KO mice (Figure 1B, lanes 1 and 5) that most likely corresponds to a cleavage product of the α subunit.15,16 It was not observed in the CNT of the CNT/CD-specific KO mice (Figure 1B, lanes 3 and 7).
Figure 1.
Loss of αENaC expression in CD and partial loss in CNT of CNT/CD-specific KO mice. (A and B) Western blot of microdissected CNT and CCD from CD-specific KO mice and their corresponding controls (A) and from the CNT/CD-specific KO mice and littermate controls (B). Anti-αENaC antibody recognizes a band at approximately 95 kD corresponding to full-length αENaC and a band just above 26 kD that may correspond to a cleaved fragment of αENaC (arrowheads*). Some unspecific bands are also observed (arrowheads). The blots are reprobed with anti-actin antibody to examine the amount of protein loaded on the gels.
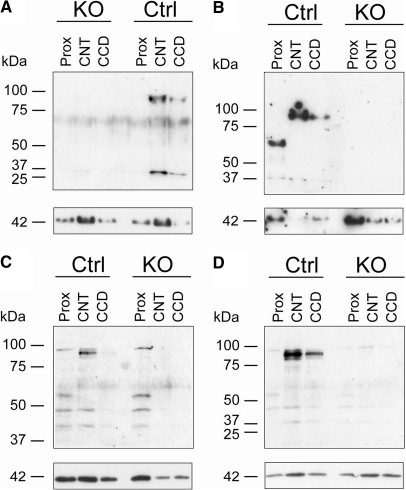
Western blots of microdissected tubules from sodium-restricted CNT/CD-specific KO mice and littermate control mice showed that the approximately 95 kD band corresponding to full-length αENaC protein was absent in the CCD of the CNT/CD-specific KO mice (Figure 2). In the CNT of the CNT/CD-specific KO mice, this band was weakly expressed or absent (Figure 2). Thus, on a standard or salt-restricted diet (Figures 1 and 2), αENaC protein is barely detectable in the CNT and not detected in the CCD of KO mice.
Figure 2.
Sodium-restricted CNT/CD-specific KO mice show near-loss of αENaC protein expression in CNT. (A through D) Western blots of microdissected proximal tubules (Prox), CNT, and CCD from four different KO and control mice that are subjected to sodium-deficient diet. Anti-αENaC antibody recognizes a band at approximately 95 kD corresponding to full-length αENaC (top blots). The blots are reprobed with anti-actin antibody (bottom blots).
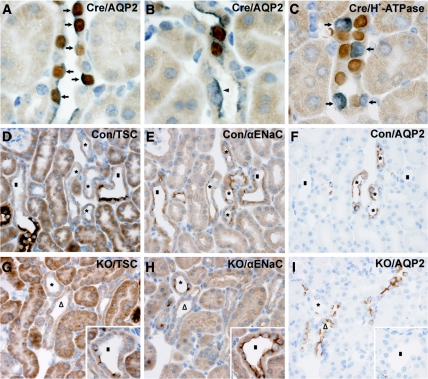
To investigate further the recombination efficiency, we performed immunohistochemistry on kidneys from sodium-deprived CNT/CD-specific KO mice and control mice. Double labeling showed almost complete co-localization of AQP2 and Cre recombinase in the principal cells along the CD (Figure 3A). Cellular counting in the CCD revealed that 96% of the AQP2-positive cells showed co-staining with the Cre recombinase. In the CNT, cellular counting showed that approximately 70% of AQP2-positive cells were co-labeled with Cre recombinase (Figure 3B). Double labeling of Cre recombinase and H+-ATPase (marker for intercalated cells) revealed no Cre expression in the intercalated cells (Figure 3C).
Figure 3.
Residual expression of αENaC in CNT of CNT/CD-specific KO mice. (A through I) Immunohistochemistry using whole kidney sections from sodium-restricted control (D through F) and KO mice (A through C and G through I). (A and B) Double labeling of whole kidney sections from a KO mouse is performed with polyclonal primary antibodies that recognize Cre recombinase (brown) and AQP2 (gray-blue). Along the CD, nuclear Cre recombinase staining is observed in AQP2-expressing cells (A, arrows). Within the CNT, some AQP2-expressing cells are negative for Cre recombinase (arrowhead, B). (C) Double labeling with primary antibodies recognizing Cre recombinase (brown) and H+-ATPase (gray-blue) shows no co-localization of the two proteins (arrows). (D through I) Serial kidney sections from a control mouse (D through F) and KO mouse (G through I) incubated with anti-TSC antibody (D and G), anti-αENaC antibody (E and H), and anti-AQP2 antibody (F and I). In control mice, αENaC labeling is observed in the CNT (AQP2 positive and TSC negative, D through F, *) including the early CNT, which is identified adjacent to the transition from the TSC-positive DCT to the TSC-negative CNT (D through F, ■). In the KO mice, αENaC is absent from part of the CNT (G through I, △) but present in other parts (G through I, *), including the early CNT (G through I, inset, ■).
Within the CNT, immunolabeling was performed with antibodies recognizing αENAC, AQP2, and TSC. The anti-TSC antibody was used as a marker of the DCT.2 In control mice, αENaC was expressed along the early and late CNT (Figure 3, D through F). The early CNT, which was identified at the transition from the TSC-positive DCT to the TSC-negative CNT, contained none or only a few AQP2-positive cells in both control and KO mice (Figure 3, D through I). Consistent with this, αENaC was also detected in such tubules, which were both AQP2 and TSC negative and likely represent early parts of the CNT with undetectable AQP2 expression. In KO mice, αENaC was partly absent in the CNT (Figure 3, G through I) with some expression remaining in both late and early CNT. Approximately 70% of all early CNT cells (both CNT and intercalated cells) in the control were αENaC positive, in comparison with only 50% in the KO situation. In the late CNT, the percentage of αENaC-positive CNT cells (control 63%, KO 18%; identified as TSC negative and AQP2 positive) was reduced by approximately 70% in the KO. In summary, the CNT/CD-specific αENaC KO mice have reduced numbers of αENaC-expressing cells in both the late and early CNT, with a more severe reduction (up to 70%) in the late CNT, and corresponding to an increase in AQP2-expressing cells in the late CNT.
CNT/CD-Specific αENaC KO Mice Exhibit a PHA-1 Phenotype under Standard Salt Diet
Following a standard salt diet, mice did not exhibit reduced body weight. In contrary, the urinary sodium excretion was significantly higher in the KO mice (P < 0.05; Table 1), whereas the urinary potassium excretion was unchanged. Food and, thus, sodium intake were not altered (Table 1). The increased urine excretion (P < 0.01; Table 1) was accompanied by lower urinary osmolality (P < 0.01; Table 1) and higher water intake (P < 0.05; Table 1). The CNT/CD-specific αENaC KO mice presented with significantly lower serum sodium concentrations (P < 0.05; Table 1) and hyperkalemia revealed by significantly higher blood potassium concentrations (P < 0.05; Table 1). Plasma aldosterone was measured in CNT/CD-specific αENaC KO and control mice that were homozygous for a specific renin allele (Ren-2−/−),17 and the CNT/CD-specific αENaC KO mice presented with significantly higher plasma aldosterone levels (P < 0.05; Table 2). BP was slightly reduced in the KO mice, without any significant difference (Table 1). No significant changes were observed in the heart rate (HR; Table 1).
Table 1.
Urinary and blood parameters from mice kept on a standard-salt diet
| Parameter | KO | Control |
|---|---|---|
| Body weight (g) | ||
| females | 21.30 ± 0.66 (n = 13) | 22.10 ± 0.65 (n = 24) |
| males | 27.70 ± 0.89 (n = 13) | 28.20 ± 1.08 (n = 15) |
| Food intake (g/g body wt per 24 h) | 0.13 ± 0.01 (n = 8) | 0.12 ± 0.01 (n = 10) |
| Na+ intake (mmol/24 h) | 0.27 ± 0.03 (n = 8) | 0.26 ± 0.03 (n = 10) |
| Urinary Na+ (mmol/24 h) | 0.23 ± 0.01 (n = 31)a | 0.19 ± 0.01 (n = 46) |
| Urinary Na+ (mM) | 136 ± 11 (n = 31) | 155 ± 7 (n = 46) |
| Urinary K+ (mmol/24 h) | 0.33 ± 0.04 (n = 31) | 0.28 ± 0.03 (n = 46) |
| Urinary K+ (mM) | 213 ± 38 (n = 31) | 242 ± 23 (n = 46) |
| Urinary osm (mosm/kgH2O) | 1809 ± 119 (n = 26)b | 2442 ± 133 (n = 39) |
| Urine output (ml/g body wt per 24 h) | 0.085 ± 0.008 (n = 31)b | 0.055 ± 0.004 (n = 46) |
| Water intake (ml/g body wt per 24 h) | 0.25 ± 0.03 (n = 31)a | 0.16 ± 0.01 (n = 46) |
| Serum osm (mosm/kgH2O) | 309 ± 4 (n = 5) | 313 ± 34 (n = 6) |
| Serum Na+ (mM) | 145.8 ± 1.0 (n = 5)a | 149.8 ± 1.1 (n = 6) |
| Plasma/serum K+ (mM) | 6.3 ± 0.2 (n = 10)a | 5.6 ± 0.1 (n = 16) |
| Mean BP (mmHg) | 127 ± 2 (n = 13) | 135 ± 3 (n = 19) |
| Mean HR (beats/min) | 632 ± 18 (n = 13) | 630 ± 13 (n = 19) |
Data are means ± SEM.
aP < 0.05.
bP < 0.01.
Table 2.
Plasma aldosterone (pg/ml) in mice kept on a standard-salt diet or a sodium-deficient diet
| Diet | KO | Control |
|---|---|---|
| Standard diet (Ren-2−/− gene mice) | 1915 ± 396 (n = 5)a | 381 ± 55 (n = 8) |
| Sodium-deficient diet, 4 days (Ren-2−/− gene mice) | 3085 ± 400 (n = 4)b | 1064 ± 213 (n = 6) |
| Sodium-deficient diet, 15 days (Ren-2+/− gene mice) | 20,984 ± 2775 (n = 7)c | 1218 ± 304 (n = 7) |
Data are means ± SEM.
aP < 0.05.
bP < 0.01.
cP < 0.001.
Sodium-Deficient Diet Induces Severe Renal Sodium Loss
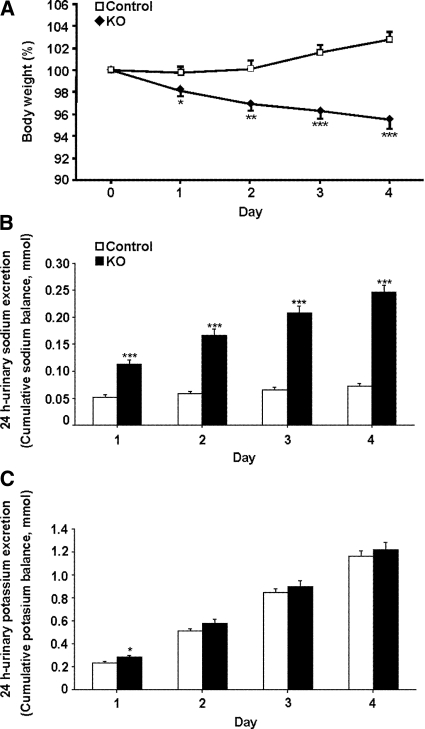
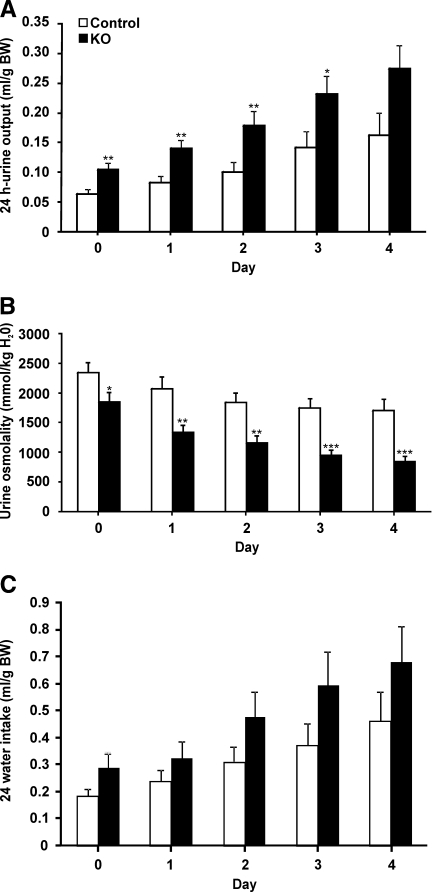
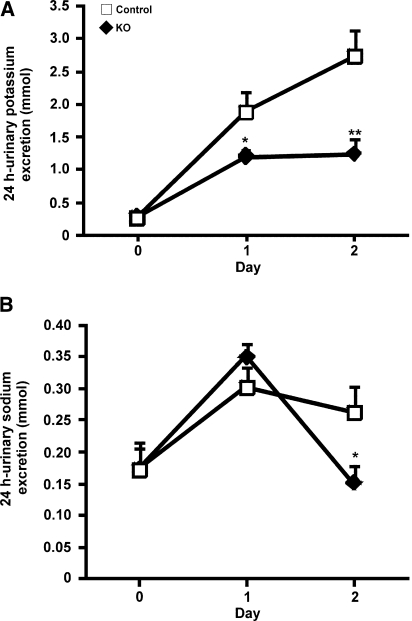
The CNT/CD-specific αENaC KO and control mice were challenged with a sodium-deficient diet for 4 consecutive days. Already after 1 day, the KO mice lost significant body weight, whereas the control mice kept or even gained body weight (P < 0.001; Figure 4A). No difference was observed in food intake (KO [n = 8] 0.15 ± 0.01 versus control [n = 10] 0.14 ± 0.01 g/g body wt), and loss of body weight was paralleled by a severe urinary sodium loss (Figure 4B). The cumulative sodium balance showed that the control mice were able to retain their sodium, whereas the KO mice continued to excrete sodium (KO 0.25 ± 0.014 mmol; control 0.07 ± 0.005 mmol; P < 0.001, day 4; Figure 4B). After 1 day of sodium-deficient diet, the urinary potassium excretion was significantly increased in the KO mice (P < 0.05), a difference that vanished during the subsequent days (Figure 4C). The KO mice continued to have a significant higher urinary excretion during the first 3 days on the sodium-deficient diet (Figure 5A). The urine osmolality was significantly lower in the KO mice on the sodium-deficient diet (P < 0.001, day 4; Figure 5B). Water intake was not different (Figure 5C). After 4 days, a significant increase was found in blood potassium levels (KO [n = 8] 6.4 ± 0.2 versus control [n = 10] 5.8 ± 0.2 mM; P < 0.05, day 4) and plasma aldosterone levels (P < 0.01; Table 2).
Figure 4.
Loss of body weight and sodium in sodium-deprived CNT/CD-specific KO mice. (A) KO (n = 18) and control mice (n = 23) are subjected to a sodium-deficient diet for 4 days, and body weight is measured daily. Body weight is presented as percentage of the initial weight. (B and C) Cumulative sodium (B) and potassium (C) balance in KO mice (n = 18, ■) and control mice (n = 23, □) subjected to a sodium-deficient diet for up to 4 days. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 5.
Increased urine output and reduced osmolality in sodium-deprived CNT/CD-specific KO mice. (A through C) Urine excretion (A), urine osmolality (B), and water intake (C) are measured daily in KO mice (n = 18, ■) and control mice (n = 23, □) subjected to a sodium-deficient diet for up to 4 days. *P < 0.05; **P < 0.01; ***P < 0.001.
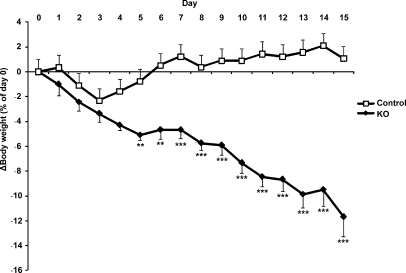
When the mice were followed for a period of 15 days upon salt-deprivation in standard cages, the KO mice lost weight continuously. The weight loss reached more than 10% of their initial weight (P < 0.001; Figure 6). Moreover, plasma aldosterone levels were significantly elevated (P < 0.001; Table 2).
Figure 6.
Continuous loss of body weight in sodium-deprived CNT/CD-specific KO mice. KO (n = 7) and control mice (n = 7) are subjected to a sodium-deficient diet for 15 days in standard cages. Body weight is measured daily and is presented as percentage of the initial body weight (at day 0; **P < 0.01, ***P < 0.001).
CNT/CD-Specific αENaC KO Mice Are not Able to Eliminate a Potassium Load
The ability of the KO mice to eliminate a potassium load was tested by challenging the animals with a diet containing 5% potassium for 2 consecutive days. The KO mice had a significant lower urinary potassium excretion (P < 0.01, day 2; Figure 7A) and significantly higher levels of potassium in the blood (P < 0.05; Table 3), whereas the serum sodium concentration was significantly reduced (P < 0.05; Table 3). After 1 day of a high-potassium diet, urinary sodium excretion was not affected but was significantly reduced after day 2 (P < 0.05, day 2; Figure 7B). Fractional excretion of sodium FE(Na+) was not different (Table 3), but KO mice showed significantly lower urinary chloride excretion (KO [n = 11] 1.1 ± 0.2 versus control [n = 11] 2.5 ± 0.4 mmol; P < 0.05, day 2), whereas no difference in serum chloride concentration was observed (Table 3). The concentration of bicarbonate was significantly lower in the KO mice (Table 3), indicating metabolic acidosis. Serum osmolality (Table 3), urine osmolality (KO [n = 11] 1198 ± 54 mmol/kg H2O; control [n = 11] 1306 ± 62 mmol/kg H2O), urine output (KO [n = 11] 0.14 ± 0.03 ml/g body wt per 24 hours; control [n = 11] 0.21 ± 0.04 ml/g body wt per 24 hours), and water intake (KO [n = 11] 0.28 ± 0.05 ml/g body wt per 24 hours; control [n = 11] 0.37 ± 0.04 ml/g body wt per 24 hours) did not reveal significant differences after 2 days of a high-potassium diet.
Figure 7.
Retention of potassium in CNT/CD-specific KO mice upon potassium loading. KO mice (n = 11) and control mice (n = 11) are subjected to a high-potassium diet for 2 days. (A and B) Urinary potassium excretion (A) and urinary sodium excretion (B) are measured daily. *P < 0.05; **P < 0.01.
Table 3.
Functional data from mice subjected to a high-potassium diet for 48 hours
| Parameter | KO | Control |
|---|---|---|
| Serum Na+ (mM) | 147.6 ± 1.4 (n = 8)a | 151.5 ± 0.7 (n = 11) |
| Serum K+ (mM) | 7.7 ± 0.8 (n = 9)a | 5.2 ± 0.2 (n = 11) |
| Serum HCO3− (mM) | 12.8 ± 1.1 (n = 7)b | 18.3 ± 0.5 (n = 10) |
| Serum osm (mosm/kgH2O) | 329.0 ± 4.7 (n = 8) | 325.0 ± 1.5 (n = 9) |
| Serum creatinine (mM) | 19.1 ± 2.3 (n = 7)a | 12.1 ± 1.5 (n = 10) |
| Serum Cl− (mM) | 114.4 ± 1.4 (n = 7) | 114.8 ± 0.7 (n = 10) |
| FE(Na+) (%) | 0.27 ± 0.05 (n = 7) | 0.25 ± 0.02 (n = 10) |
Data are means ± SEM.
aP < 0.05.
bP < 0.001.
DISCUSSION
Constitutive KO of either β or γENaC causes an early lethal phenotype as a result of disturbances in the electrolyte balance.7,9 αENaC deficiency also induces death shortly after birth, with a lung, skin, and kidney phenotype.8,18 Mice in which αENaC is deleted specifically in the CD are viable and do not show disturbances in sodium and potassium balance even when subjected to challenging diets.10 This suggested that the CNT is important in controlling ENaC-mediated sodium reabsorption in the kidney. To investigate this further, we generated mice in which the αENaC gene was deleted in the CD and partly in the CNT. These mice are viable until adulthood and exhibit normal BP, although they show increased urinary sodium excretion, urine output, and plasma aldosterone, leading to hyponatremia and hyperkalemia under standard diet. After sodium restriction, the mice develop a severe salt-losing phenotype and show a continuous life-threatening reduction of body weight.
Partial Inactivation of αENaC in the CNT Is Sufficient to Induce a Severe Salt-Losing Syndrome
Whereas αENaC was deleted efficiently in the CCD principal cells, approximately 30% of the late CNT cells are not targeted and still express αENaC protein. This may explain the remaining αENaC expression in microdissected CNT of some animals under normal and sodium-deprived diet. Similar to our findings, CNT/CD-specific mineralocorticoid receptor (MR) KO mice (using the same Aqp2::iCre transgene) showed complete deletion of MR in the CD, whereas deletion of the MR protein in the early and late CNT was equally partial, thus following the AQP2 expression pattern in these segments.11
The absence of AQP2 expression in the early CNT is therefore consistent with previous observations. In vasopressin-deficient Brattleboro rats, the initial portion of the CNT lacks detectable levels of AQP2, whereas long-term vasopressin treatment induced its expression throughout the CNT.19 Moreover, in TRPV5::EGFP transgenic mice, some tubule segments were enhanced green fluorescent protein and calbindin positive but negative for AQP2 and TSC, thus likely representing early CNT.20
We observed that the Cre recombinase protein was expressed along the CD, consistent with CNT/CD-specific MR KO mice.11 Immunohistochemistry also showed that αENaC was not expressed anymore in the early part of the CCD in the CNT/CD-specific αENaC KO mice (Supplemental Figure 1).
The CNT Is Critical for the ENaC-Mediated Sodium Reabsorption
The increased urinary sodium excretion, hyponatremia, and hyperkalemia observed in the KO mice indicate impaired sodium reabsorption in the CNT and are therefore consistent with the absence of ENaC in this segment. Moreover, the KO mice exhibited decreased urine osmolality and increased urine output/water intake. The changes in water balance could be explained by a reduced renal urine-concentrating ability, leading to increased urine output and causing increased water intake. The renal effect can be due to impaired ENaC-mediated sodium reabsorption in the CNT and CD, which leads to a reduced driving force for osmotic reabsorption of water in these segments. Apparently, the urine-concentrating ability would be affected only when both the CNT and the CD are targeted because no change in water balance was seen in the CD-specific αENaC KO mice.10 An impaired urine-concentrating ability was previously described in aldosterone synthase–deficient mice.21 The changes in water balance could also be due to the activation of the renin-angiotensin-aldosterone system as shown by elevated plasma aldosterone concentrations, which could lead to a stimulation of thirst and result in increased water intake and subsequently increased urine output.
It was previously shown that CD-specific αENaC KO mice do not exhibit a phenotype even after challenging diets.10 In contrast, we observed that sodium restriction caused a continuous loss of body weight and sodium in the KO mice. The body weight of the KO mice did not stabilize after 15 days of sodium restriction. A similar weight loss in response to a low-sodium diet was reported in transgenic mice expressing low levels of βENaC.22 Moreover, the severe salt loss in humans with the recessive form of PHA-1 does not improve with age.6 The CNT/CD-specific αENaC KO mice were hyperkalemic in contrast to the CD-specific αENaC KO mice. Both CNT/CD-specific and CD-specific αENaC KO mice decreased their urine osmolality upon sodium restriction, but only the CNT/CD-specific αENaC KO mice exhibited a significant lower urine osmolality compared with their littermate controls. Challenging the CD-specific αENaC KO mice with a potassium-rich diet for 4 days resulted in increased sodium and potassium in both plasma and urine but not different from the control.10 In contrast, the CNT/CD-specific αENaC KO mice were not able to excrete a potassium load, as shown by significantly higher potassium concentration in blood and decreased urinary potassium excretion. The potassium-loaded CNT/CD-specific αENaC KO mice also showed indications of metabolic acidosis. Thus, in contrast to CD-specific αENaC KO mice, the CNT/CD-specific αENaC KO mice exhibited symptoms of PHA-1, supporting that the CNT is critical for the ENaC-mediated sodium reabsorption. This is consistent with previous suggestions that the late DCT and CNT, rather than the CD, are the main regulators of ENaC-mediated sodium and potassium homeostasis.23,24 The majority (90%) of the sodium delivered to aldosterone-sensitive distal segments is reabsorbed in the CNT and DCT.25 Moreover, the CNT exhibit a higher ENaC activity compared with the CD of aldosterone-infused rats.26 The apical ENaC expression has also been shown to be more pronounced in the CNT compared with the CD in mice on a moderately low-sodium diet (0.05%).27 That a partial gene deletion in the CNT was sufficient to induce a severe salt-losing syndrome also shows that the functional reserve in the CNT is limited because the remaining αENaC-positive CNT cells were unable to compensate fully for the loss of αENaC in the other cells.
CNT/CD-Specific Deletion of αENaC Is More Severe than Inactivation of the MR in the Same Segments
The actions of aldosterone are mediated through the MR. After binding of aldosterone to the MR, the hormone is translocated to the nucleus, where it controls the transcription of ENaC and other genes. In contrast to the CNT/CD-specific αENaC KO mice, no disturbance in electrolyte and water balance was observed in CNT/CD-specific MR KO mice subjected to a normal-salt diet.11 Thus, partial deletion of αENaC in the CNT induces a more severe phenotype than inactivation of MR in the same segments. This may not be surprising because the MR protein is an upstream effector on sodium absorption compared with ENaC as an effector. Upon sodium restriction, the CNT/CD-specific MR KO mice also showed loss of body weight, increased sodium and urinary excretion, and significantly higher plasma aldosterone levels,11 similar to CNT/CD-specific αENaC KO mice.
In summary, gradual gene deletion in the CNT was sufficient to induce a severe salt-losing syndrome, confirming that the CNT is crucial for maintaining sodium and potassium balance.
CONCISE METHODS
Generation of Transgenic Mice
To inactivate the Scnn1a gene in the CNT cells, we used mice expressing Cre recombinase under the control of the regulatory elements of the mouse Aqp2 gene11 and Scnn1aloxlox conditional KO mice and the Scnn1a+/− mice.8,12 CNT/CD-specific αENaC KO mice (Scnn1alox/−/Aqp2::iCre; KO group) and heterozygous (Scnn1alox/+/±Aqp2::iCre and Scnn1alox/−; control group) littermates were obtained by interbreeding Scnn1a+/−/Aqp2::iCre mice with Scnn1aloxlox mice. Genotyping of the mice was performed by PCR analysis of tail biopsies described previously.12 To genotype Scnn1a+/− and Aqp2::iCre mice, the following primers were used: Scnn1a+/−: primer #1: 5′-TTAAGGGTGCACACAGTGACGGC-3′, #2: 5′-TTTGTCACGTCCTGCACGACGCG-3′, and #3: 5′-AACTCCAGAAGGTCAGCTGGCTC-3′, and Aqp2::iCre: #4: 5′-AAGTGCCCACAGTCTAGCCTCT-3′, #5: 5′-CCTGTTGTTCAGCTTGCACCAG-3′, and #6: 5′-GGAGAACGCTATGGACCGGAGT-3′.
Microdissection of Nephron Segments
Microdissection of kidneys was performed from KO mice (n = 2) and control littermates (Scnn1alox/+and Scnn1alox/+/Aqp2::iCre; n = 2, approximately 3 months of age). As additional controls, we included CD-specific KO mice (Scnn1aloxlox/HoxB7::iCre; n = 2) and their littermate controls (Scnn1aloxlox; n = 2, approximately 11 months of age).10 Microdissection was also performed on kidneys from KO mice (n = 4) and control littermates (Scnn1alox/+; n = 4, 2 to 2.5 months of age) that were subjected to a sodium-deficient diet in standard cages for 6 to 17 days. The experiment was performed four times and each time with one KO mouse and one control mouse in parallel. The kidney was perfused with DMEM/F-12 (1:1; 21041 medium; Invitrogen) completed with 40 μg/ml liberase blendzyme TM 2 or 30 μg/ml blendzyme (Roche Applied Science). Thin pyramids cut along the corticomedullary axis were incubated at 37 or 30°C for 40 minutes in the perfusion medium. The action of enzyme was stopped by washing the pyramids with ice-cold DMEM/F-12 (1:1) without blendzyme. Then, the medulla was removed under microscope and the CNT and CCD were microdissected in ice-cold DMEM/F-12 (1:1) without blendzyme. Pools of 10 to 20 microdissected tubules in 5 μl of DMEM/F12 (1:1) were then transferred to 5 μl of 2× concentrated protein sample buffer (9.6% [wt/vol] SDS, 13.8% [wt/vol] sucrose, 0.026% [wt/vol] bromphenol blue, and 4.2% [vol/vol] β-mercaptoethanol).
Western Blot Analysis of Microdissected Nephron Segments
Samples were heated at 95°C for 5 minutes and loaded and electrophoresed on an 8% SDS-PAGE. Proteins were then transferred to Protran nitrocellulose membrane (Schleicher & Schuell) or Amersham Hybond-ECL nitrocellulose membrane (Amersham), and Western blots were performed according to standard procedures. The membrane was first probed with affinity-purified αENaC antibody (1:100, 1:500, or 1:2000 dilution10); after stripping, anti-actin antibody (1:200 dilution; Sigma) was used. Blots were revealed with SuperSignal reagent (Pierce) or Amersham ECL Western blotting Detection Reagents (Amersham).
Immunohistochemistry
Kidneys from mice that were sodium-restricted for 4 consecutive days (Scnn1alox/−/Aqp2::iCre [n = 2] and Scnn1alox/+/Aqp2::iCre [n = 3]) were fixed by intravascular perfusion of 3% paraformaldehyde and subjected to paraffin embedding and sectioning (2-μm-thick sections).
Double Labeling
Double-labeling experiments were performed using (1) rabbit polyclonal Cre recombinase antibody (1:8000 dilution; Covance) and biotinylated rabbit polyclonal AQP2 antibody (7661AP; 1:250 dilution) and (2) Cre recombinase antibody (1:8000 dilution) and biotinylated rabbit polyclonal H+-ATPase antibody (H7659AP; 1:100 dilution28). Sections were incubated overnight at 4°C with Cre antibody before undergoing incubation with horseradish peroxidase–conjugated goat anti-rabbit secondary antibody and visualization by 3,3′-diaminobenzidine (brown color). Sections were then incubated in 3.5% H2O2 in methanol to remove any remaining peroxidase from the first staining. After blocking for endogenous biotin (Biotin Blocking System; DakoCytomation), sections were then incubated overnight at 4°C with either biotinylated AQP2 or H+-ATPase antibodies. Labeling was visualized by use of Strepavidin horseradish peroxidase (Sigma) and Vector SG substrate (blue-gray color; Vector Laboratories).
Single Labeling of Serial Kidney Sections
Serial paraffin sections were incubated with (1) rabbit polyclonal αENaC antibody (1:800 dilution10), rabbit polyclonal AQP2 antibody (1:3000 dilution), and mouse monoclonal Calbindin D-28K antibody (1:20,000 dilution; Research Diagnostics) and (2) rabbit polyclonal TSC antibody (1:1000 dilution29), rabbit polyclonal αENaC antibody (1:800 dilution10), and rabbit polyclonal AQP2 antibody (1:3000 dilution). Labeling was visualized by use of peroxidase-conjugated secondary antibody and 3,3′-diaminobenzidine. Sections were counterstained with hematoxylin. Light microscopy was carried out using a Leica DMRE microscope.
Quantification of αENaC-Positive Cells in the CNT
Cell counting was performed on kidney sections from Scnn1alox/−/Aqp2::iCre mice and Scnn1alox/+/Aqp2::iCre mice that were labeled with rabbit polyclonal αENaC antibodies and peroxidase-conjugated secondary antibodies. Counting was performed on electronic images taken with a ×25 objective. The number of αENaC-positive (labeled) and αENaC-negative (unlabeled) cells with a distinct nucleus was counted in the early CNT, adjacent to the transition from DCT to CNT (the transition was identified on serial sections labeled with TSC antibodies). The total number of cells counted was 65 in the Scnn1alox/−/Aqp2::iCre mice (n = 2) and 81 in the Scnn1alox/+/Aqp2::iCre mice (n = 2). Cellular counting was also performed in the late CNT (tubules were identified on serial sections labeled with AQP2 and TSC antibodies). The number of cells counted was 330 in the Scnn1alox/−/Aqp2::iCre mice (n = 2) and 339 in the Scnn1alox/+/Aqp2::iCre mice (n = 3). The fraction of ENaC-positive cells was calculated from the number of positive cells divided by the total number of cells counted for each animal.
Quantification of Cre Recombinase–Positive Cells in the CCD and the CNT
The cellular counting was performed on kidney sections from Scnn1alox/−/Aqp2::iCre mice and Scnn1alox/+/Aqp2::iCre mice that were double labeled with rabbit polyclonal Cre recombinase antibody and biotinylated rabbit polyclonal AQP2 antibody. Counting was performed on electronic images taken with a ×63 objective. The number of AQP2-positive/Cre recombinase–positive and AQP2-positive/Cre recombinase–negative cells with a distinct nucleus was counted in the CCD (313 cells, n = 5 mice) and in the CNT (465 cells, n = 5 mice). The fraction of Cre recombinase–positive cells was calculated from the number of AQP2-positive/Cre recombinase–positive cells divided by the total number of AQP2-positive cells counted for each animal.
Experimental Protocols
Sodium-Deficient Diet in Metabolic Cages
For each metabolic cage study, experimental mice and controls from the same litter were used. Six- to 12-week-old mice were placed in individual metabolic cages (Tecniplast, Buguggiate, Italy) and fed a standard-salt diet (0.23% sodium; Institut National de la Recherche Agronomique, Unité de Préparation des Aliments Expérimentaux, Jouy en Josas, France) for 2 days, followed by 4 consecutive days on a sodium-deficient diet (0% sodium; Institut National de la Recherche Agronomique, Unité de Préparation des Aliments Expérimentaux). During the experiment, the animals had free access to food and water. The diet was given as a mixture of food in gelatin and water (100 g food/60 ml water). Blood was collected from the tail vein of conscious mice at the end of the diet. The experiment was also performed with 8- to 12-week-old mice fed a standard-salt diet (0.17% sodium, given as powder food; Ssniff Spezialdiäten GmbH, Soest, Germany) for 2 days followed by 3 days on a sodium-deficient diet (<0.01% sodium; Ssniff Spezialdiäten GmbH).
Sodium-Deficient Diet in Standard Cages
Experimental (n = 7) and control littermate mice (n = 7, 8 to 12 weeks old) were fed a standard-salt diet (0.17% sodium; Ssniff Spezialdiäten GmbH), and the body weight was measured at day 0 to determine the reference weight. Then, mice were fed a sodium-deficient diet (<0.01% sodium; Ssniff Spezialdiäten GmbH) with free access to water, and their body weight was monitored daily (at the same time) for 15 consecutive days.
Blood Collection for Aldosterone Measurements
Control and Scnn1alox/lox/Aqp2::iCre (KO) mice (8 to 12 weeks old) that were homozygous for a specific renin allele (Ren-2−/−) were kept in standard cages with free access to food and water. Thirteen mice (eight control and five experimental) were fed a standard-salt diet (0.17% sodium; Ssniff Spezialdiäten GmbH) and 10 mice (six control and four KO) were fed a sodium-deficient diet (<0.01% sodium; Ssniff Spezialdiäten GmbH) for 4 consecutive days. At the end of experiment, blood samples were collected after decapitation. Plasma aldosterone levels were measured according to standard procedures using a RIA (Coat-A-Count RIA kit; Siemens Medical Solutions Diagnostics, Ballerup, Denmark). Mouse samples with values >1200 pg/ml were further diluted using a serum pool with a low aldosterone concentration (<50 pg/ml).
High-Potassium Diet
Data from two separate experiments were pooled. In the first series of experiments, mice were 4 months old; in the second series, mice were 2.5 to 12 months old. Experimental mice (n = 5 for first experiment; n = 6 for second experiment) and control mice (n = 6 for first experiment; n = 5 for second experiment) were placed in individual metabolic cages and fed a standard diet for 2 consecutive days (0.59% potassium in the first experiment and 0.95% potassium in the second experiment). This was followed by 2 days on a 5% potassium diet (the potassium was added as KCl). During the experiment, the animals had free access to food and water. The diets were given as a mixture of food in gelatin and water (100 g food/60 ml water). After the diet, blood was collected from the eye.
Urine and Serum/Plasma Analysis
Urine and serum/plasma osmolarity as well as sodium, potassium, chloride, creatinine, and bicarbonate composition were analyzed at the Laboratoire Central de Chimie Clinique (Centre Hospitalier Universitaire Vaudoise, Lausanne, Switzerland). The potassium values were corrected for the degree of hemolysis.
BP Measurements
The BP and HR were measured in KO (n = 13) and control mice (n = 19). The mice were kept on a normal-salt diet containing 0.23% of sodium with free access to tap water and were analyzed at the age of 4 to 6 months. BP and HR were recorded intra-arterially using a computerized data-acquisition system (Notocord Systems SA, Croissy, France).17 Briefly, for placement of the intra-arterial catheter, a mouse was anesthetized via inhalation of 1 to 2% halothane with oxygen. The right carotid artery was exposed for a length of approximately 4 mm. A silicone/PE10 catheter filled with 0.9% NaCl solution containing heparin (300 IU/ml) was inserted into the artery. After ligation, the catheter was subcutaneously tunneled to exit at the back of the neck and fixed with a piece of scotch tape and dental cement. The mouse was allowed 3 to 4 hours to recover from the anesthesia and placed in a Plexiglas tube for partial restriction of its movements. Thirty minutes later, the arterial line was connected to a pressure transducer; BP and HR were then monitored every 20 seconds for 15 to 20 minutes using the Notocord computerized data-acquisition system at a sampling rate of 500 Hz. Once BP measurement was completed, blood was sampled from the arterial catheter for analysis of serum sodium and potassium concentrations.
Statistical Analysis
Results are presented as mean ± SEM. Data were analyzed by one-way ANOVA and unpaired t test. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
Financial support for this study was provided by the Swiss National Science Foundation, the Danish Medical Research Council, the Danish National Research Foundation, and the Leducq Foundation.
We are very grateful to Johannes Loffing (University of Fribourg/University of Zürich, Switzerland) for help with perfusion of the mouse kidneys. We also thank Nicole Fowler Jaeger, Anne-Marie Mérillat, Sandrine Egli, and Inger-Merete Paulsen for excellent technical assistance and Friedrich Beermann for critically reading the manuscript. We also thank Søren Nielsen (Water and Salt Research Center, University of Aarhus, Aarhus, Denmark) for providing the antibodies against AQP2 and H+-ATPase and Mark Knepper (National Institutes of Health, Bethesda, MD) for providing the antibody against TSC.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Verrey F, Hummler E, Schild L, Rossier BC: Mineralocorticoid action in the aldosterone-sensitive distal nephron. In: The Kidney Physiology and Pathophysiology, 4th Ed., edited by Alpern RJ, Hebert SC. Academic Press, 2008, pp 889–924 [Google Scholar]
- 2. Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B: Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Jr, Ulick S, Milora RV, Findling JW: Liddle's syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP: Hypertension caused by a truncated epithelial sodium channel gamma subunit: Genetic heterogeneity of Liddle syndrome. Nat Genet 11: 76–82, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP: Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Kuhnle U: Pseudohypoaldosteronism: mutation found, problem solved? Mol Cell Endocrinol 133: 77–80, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B: Role of gammaENaC subunit in lung liquid clearance and electrolyte balance in newborn mice: Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest 102: 1634–1640, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC: Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 9. McDonald FJ, Yang B, Hrstka RF, Drummond HA, Tarr DE, McCray PB, Jr, Stokes JB, Welsh MJ, Williamson RA: Disruption of the beta subunit of the epithelial Na+ channel in mice: Hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci U S A 96: 1727–1731, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC: Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ronzaud C, Loffing J, Bleich M, Gretz N, Grone HJ, Schutz G, Berger S: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Hummler E, Merillat AM, Rubera I, Rossier BC, Beermann F: Conditional gene targeting of the Scnn1a (alphaENaC) gene locus. Genesis 32: 169–172, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW: Cellular and subcellular immunolocalization of vasopressin- regulated water channel in rat kidney. Proc Natl Acad Sci U S A 90: 11663–11667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kishore BK, Mandon B, Oza NB, DiGiovanni SR, Coleman RA, Ostrowski NL, Wade JB, Knepper MA: Rat renal arcade segment expresses vasopressin-regulated water channel and vasopressin V2 receptor. J Clin Invest 97: 2763–2771, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ergonul Z, Frindt G, Palmer LG: Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR: Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Wang Q, Hummler E, Nussberger J, Clement S, Gabbiani G, Brunner HR, Burnier M: Blood pressure, cardiac, and renal responses to salt and deoxycorticosterone acetate in mice: Role of Renin genes. J Am Soc Nephrol 13: 1509–1516, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Charles RP, Guitard M, Leyvraz C, Breiden B, Haftek M, Haftek-Terreau Z, Stehle JC, Sandhoff K, Hummler E: Postnatal requirement of the epithelial sodium channel for maintenance of epidermal barrier function. J Biol Chem 283: 2622–2630, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Coleman RA, Wu DC, Liu J, Wade JB: Expression of aquaporins in the renal connecting tubule. Am J Physiol Renal Physiol 279: F874–F883, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Hofmeister MV, Fenton RA, Praetorius J: Fluorescence isolation of mouse late distal convoluted tubules and connecting tubules: Effects of vasopressin and vitamin D3 on Ca2+ signaling. Am J Physiol Renal Physiol 296: F194–F203, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Makhanova N, Lee G, Takahashi N, Sequeira Lopez ML, Gomez RA, Kim HS, Smithies O: Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol 290: F61–F69, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Pradervand S, Barker PM, Wang Q, Ernst SA, Beermann F, Grubb BR, Burnier M, Schmidt A, Bindels RJ, Gatzy JT, Rossier BC, Hummler E: Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci U S A 96: 1732–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meneton P, Loffing J, Warnock DG: Sodium and potassium handling by the aldosterone-sensitive distal nephron: The pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Loffing J, Korbmacher C: Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflugers Arch 458: 111–135, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Malnic G, Klose RM, Giebisch G: Micropuncture study of renal potassium excretion in the rat. Am J Physiol 206: 674–686, 1964 [DOI] [PubMed] [Google Scholar]
- 26. Frindt G, Palmer LG: Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B: Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B: Human cortical distal nephron: Distribution of electrolyte and water transport pathways. J Am Soc Nephrol 13: 836–847, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.