Abstract
It is not clear whether interstitial fibroblasts or tubular epithelial cells are primarily responsible for the profibrotic effects of NF-κB activation during renal fibrogenesis. Here, we crossed mice carrying a conditional IκB dominant-negative transgene (IκBdN) with mice transgenic for cell-specific FSP1.Cre (FSP1+ fibroblasts) or γGT.Cre (proximal tubular epithelia) and challenged all progeny with unilateral ureteral obstruction. We determined NF-κB activation by nuclear localization of phosphorylated p65 (pp65) in renal tissues after 7 days. We observed inhibition of NF-κB activation in interstitial cells and tubular epithelia in obstructed kidneys of FSP1.Cre;IκBdN and γGT.Cre;IκBdN mice, respectively, compared with IκBdN controls (P < 0.05). Deposition of extracellular matrix, however, was significantly lower in the obstructed kidneys of FSP1.Cre;IκBdN mice but not in γGT.Cre;IκBdN mice (P < 0.05). In addition, levels of mRNA encoding the profibrotic PAI-1, fibronectin-EIIIA, and type I (α1) procollagen were significantly lower in obstructed kidneys of FSP1.Cre;IκBdN mice compared with γGT.Cre;IκBdN mice (P < 0.05). Taken together, these data support a profibrotic role for fibroblasts, but not proximal tubular epithelial cells, in modulating NF-κB activation during renal fibrogenesis.
NF-κB regulates a number of downstream genes involved in a variety of cellular functions leading to tissue remodeling.1 Activation of NF-κB plays a role in various chronic kidney diseases associated with inflammation and fibrosis,2 and inhibition of NF-κB signaling effectively attenuates renal injury in experimental animals with unilateral ureteral obstruction (UUO),3 hypertension,4 subtotal nephrectomy,5 protein-overload,6 adriamycin nephropathy,7 angiotensin II infusion,8 or FK506 nephropathy.9 In these previous studies, agents such as pyrrolidine dithiocarbamate, hepatocyte growth factor, parthenolide, or renin-angiotensin system inhibitors were used to inhibit renal NF-κB activation. The specificity of these agents as inhibitors of NF-κB, however, is questionable,10 and the cells principally providing NF-κB activation in vivo are unknown. Systemic administration of inhibitors that disrupt NF-κB activation also impair host immune responses.11 Clearly, targeted renal cell-specific inhibition of NF-κB would offer a more informative probe.
Renal fibrosis is the hallmark of chronic kidney disease,12 and UUO in mice is a standard model of short-term renal fibrogenesis.13 Over a 3-week interval, UUO initiates a rapid decline of renal blood flow and glomerular filtration, which is followed by hydronephrosis, early interstitial infiltrates, and fibrosis.13 In this model there is a close association between increasing numbers of fibroblasts and tubular epithelial cell loss,13 which is the principal interest of this study.
When a truncated form of the IκBα gene (IκB dominant-negative [IκBdN]), which lacks 54 N-terminal amino acids including the phosphorylation sites essential for NF-κB activation, is transfected into tubular epithelial cells by adenovirus vector, renal tubulointerstitial injury from protein overload attenuates in rats.10 To determine which renal cells contribute functionally important NF-κB activity during renal fibrogenesis after UUO, we generated transgenic mice bearing the IκBdN gene separated from a universal CAG promoter by a floxed STOP sequence (Figure 1A) and bred these IκBdN mice with other transgenic mice carrying the gene encoding Cre recombinase under the control of cell type-specific promoters.
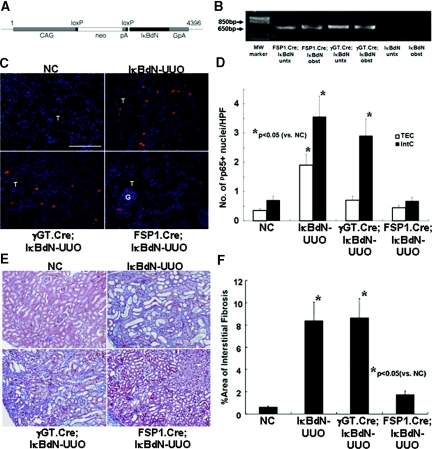
Figure 1.
The IκBdN gene product blocks activation of the NF-κB pathway in obstructed kidneys. (A) A map of the IκBdN transgene. (B) The IκBdN gene transcripts were amplified from the total RNA extracts from the kidneys of FSP1.Cre;IκBdN and γGT.Cre;IκBdN mice but not from those of IκBdN mice. (C) In the untreated kidneys of IκBdN mice, p65 Ser-276 phosphorylation (pp65 in red) was not found. Numerous nuclei of tubular epithelial cells and fibroblasts were positive for pp65 in the obstructed kidneys of IκBdN mice. The number of pp65 + nuclei was lower, especially in the tubular epithelial cells, in the obstructed kidneys of γGT.Cre;IκBdN mice than those of IκBdN mice. In the obstructed kidney of FSP1.Cre;IκBdN mice, induction of pp65 was significantly suppressed compared with that of IκBdN mice (objective lens, ×40; bar, 100 μm). (D) Quantification of the number of pp65+ nuclei in the obstructed kidneys by the point-counting method described under Concise Methods. (E) Compared with the untreated kidney, significant ECM deposition was observed in the renal interstitium of IκBdN mice with UUO. Significant ECM deposition was also observed in the obstructed kidney of γGT.Cre;IκBdN mice. ECM deposition was significantly decreased in the obstructed kidney of FSP1.Cre;IκBdN mice compared with that of IκBdN mice (Masson's trichrome stain; objective lens, ×10). (F) ECM deposition in the kidneys was quantitatively measured. The data shown in D and F were obtained from six independent mice. T, tubules; G, glomerulus; untx, untreated; obst, obstructed; NC, negative control; TEC, tubular epithelial cells; IntC, interstitial cells; MW, molecular weight.
We previously described a gene encoding fibroblast-specific protein 1 (FSP1),14 also known as S100A4, that is present in fibroblasts producing type I collagen,15,16 in bone marrow fibrocytes,17 and in tubular epithelial cells14,18–21 or endothelial cells22,23 undergoing transition to fibroblasts.24 The FSP1 promoter contains a proximal cis-acting enhancer element, FTS-1, which broadly functions to engage the larger fibrosis-related transcriptome.20 FSP1.Cre mice have been successfully used to create null alleles in fibroblasts for TGFβ type II receptors,25 EP4 receptors,26 and Pten.27 In our preliminary experiments, crossing transgenic mice expressing Cre recombinase under the control of the FSP1 promoter (FSP1.Cre mice)25 with mice in which an EGFP reporter gene is separated from the universal CAG promoter by a floxed STOP sequence (EGFP mice)28 produced conditional progeny whose FSP1 promoter is active in fibroblasts that costain for enhanced green fluorescent protein (EGFP) and heat shock protein 47, the collagen type I chaperone protein29 (Supplemental Figure S1, A and B). The number of EGFP+ interstitial cells by point counting is significantly increased in obstructed kidneys compared with untreated kidneys from FSP1.Cre;EGFP mice (9.2 ± 8.3 and 0.4 ± 0.1 cells/high power field; P < 0.05).
We also reported previously that the promoter for γ-glutamyltranspeptidase (γGT) driving Cre recombinase only expresses in the cortical tubular epithelium in the kidney of γGT.Cre mice,17 confirmed by others.30 As expected, γGT promoter-driven EGFP+ cortical tubular cells were found in the kidneys of γGT.Cre;EGFP mice (Supplemental Figure S1A). In those mice stressed by UUO, in confirmation of the previous findings,17,21 not only cortical tubular epithelium but also solitary cells in the interstitium were positive for EGFP (Supplemental Figure S1A), some of which were also positive for heat shock protein 47 (Supplemental Figure S1B), and some of these latter cells are likely derived by epithelial-mesenchymal transition after ureteral obstruction.
We next crossed γGT.Cre or FSP1.Cre mice with IκBdN mice to generate progeny in which inhibition of NF-κB activation by IκBdN is predicted in cortical tubular epithelial cells or fibroblasts, respectively. This prediction was confirmed by the following results. As shown in Figure 1B, the 712-bp fragment within the IκBdN gene transcript10 could be amplified from untreated and obstructed kidneys of FSP1.Cre;IκBdN and γGT.Cre;IκBdN mice but not from IκBdN controls. These findings suggest that recombination by each cell-specific promoter floxed the IκBdN gene driven by the CAG promoter. The numbers of nuclei positive for phosphorylated p65 Ser-276 (pp65; activated NF-κB)1 within cortical tubular epithelial cells and fibroblasts in the obstructed kidneys of γGT.Cre;IκBdN and FSP1.Cre;IκBdN mice, respectively, were significantly (P < 0.05) lower than those of IκBdN control mice (Figure 1, C and D). The finding that NF-κB activation was attenuated also in tubular epithelial cells from FSP1.Cre;IκBdN mice (Figure 1, C and D) suggests that some intermediate epithelia are undergoing epithelial-mesenchymal transition, activating IκBdN by early expression of FSP1,17, or perhaps FSP1+ fibroblasts transactivate the NF-κB pathway in tubular epithelial cells by paracrine effects in the obstructed kidney.
It is also of particular interest that deposition of interstitial extracellular matrix (ECM) is significantly (P < 0.05) decreased in the FSP1.Cre;IκBdN kidneys after UUO but not in the γGT.Cre;IκBdN kidneys compared with IκBdN controls (Figure 1, E and F). This is because the NF-κB pathway in FSP1+ fibroblasts derived from sources other than cortical tubular epithelium that constitute 64% of whole fibroblasts17 would still be active in the obstructed kidneys of γGT.Cre;IκBdN mice but not in those of FSP1.Cre;IκBdN mice, suggesting that the results are consistent with an important role for NF-κB activity in FSP1+ fibroblasts. Additionally, the numbers of F4/80+ macrophages and FSP1+ fibroblasts in the renal interstitium of the FSP1.Cre;IκBdN mice after UUO are significantly lower (P < 0.05) than those in the γGT.Cre;IκBdN and IκBdN mice (Figure 2, A through C). All of these findings suggested that NF-κB activation in FSP1+ fibroblasts plays a pivotal role in expanding renal fibrogenesis.
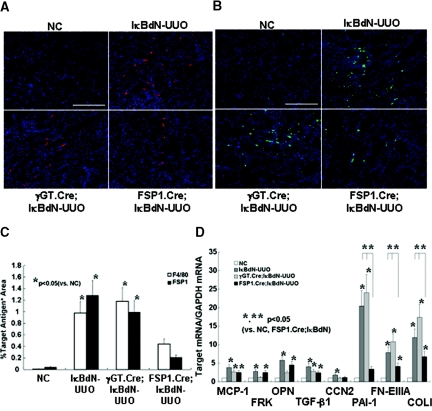
Figure 2.
Infiltration of monocytes/fibroblasts and levels of fibrosis-related mRNAs are decreased in the obstructed kidneys of FSP1.Cre;IκBdN mice. (A and B) Immunostaining for F4/80+ monocytes (in red) (A) and FSP1+ fibroblasts (in green) (B) in the obstructed kidneys (objective lens, ×40; bar, 100 μm). (C) Quantitative analysis revealed that both the F4/80+ and FSP1+ areas were significantly narrowed in the obstructed kidney of FSP1Cre.IκBdN mice, compared with those of IκBdN and γGT.Cre;IκBdN mice. (D) The mRNA levels of representative inflammatory-related gene, MCP-1, and fibrosis-related gene TGF-β1 were significantly increased in the obstructed kidneys of IκBdN, γGT.Cre;IκBdN, and FSP1.Cre;IκBdN mice. Other inflammatory-related mRNAs such as FRK and OPN were significantly increased in the obstructed kidneys of IκBdN mice, which were suppressed in the γGT.Cre;IκBdN mice but not in the FSP1.Cre;IκBdN mice. In contrast, other fibrosis-related mRNAs such as PAI-1, FN-EIIIA, and COLI were significantly increased in the obstructed kidneys of IκBdN and γGT.Cre;IκBdN mice, whereas increases in those genes were suppressed in the obstructed kidneys of FSP1.Cre;IκBdN mice. In the case of the CCN2 gene, its mRNA level was increased in the obstructed kidneys of IκBdN mice, whereas such increases were suppressed in those of γGT.Cre;IκBdN and FSP1.Cre;IκBdN mice. The data shown in C and D were obtained from six independent mice. NC, negative control.
Although expression of chemoattractant genes such as fractalkine (FRK) and osteopontin (OPN) are expectedly decreased in obstructed kidneys from γGT.Cre;IκBdN mice, expression of profibrotic transcripts such as plasminogen activator-1 (PAI-1), fibronectin EIIIA (FN-EIIIA), and type I (α1) procollagen (COLI) are increased to the same degrees as those in IκBdN controls (Figure 2D). In contrast, in the obstructed kidneys of FSP1.Cre.IκBdN mice, expression of all of these profibrotic transcripts was significantly decreased (P < 0.05), whereas expression of chemoattractant genes was unchanged compared with those of IκBdN mice (Figure 2D). The reason that F4/80+ monocyte infiltration is lower in the obstructed kidney of FSP1.Cre;IκBdN mice in spite of a slight increase in transcripts encoding FRK and OPN is unclear. Because F4/80+ macrophages do not express FSP1,31 FSP1 promoter-driven expression of IκBdN is unlikely in these cells (Figure S1B). An explanation may be that PAI-1 recruits inflammatory cells into the obstructed kidney,12 or other pivotal chemoattractant signals13,32 not tested in this study were suppressed in FSP1.Cre;IκBdN mice.
Additionally, it is of interest that the expression of transcripts encoding TGF-β1, a master gene of organ fibrogenesis,12 is similar in the obstructed kidneys of IκBdN, γGT.Cre;IκBdN, and FSP1.Cre;IκBdN mice despite less fibrogenesis in the obstructed kidneys of FSP1.Cre;IκBdN mice (Figure 1, E and F, and Figure 2D). This suggests that FSP1+ fibroblasts are effector cells of TGF-β1 activity in the obstructed kidneys, and the profibrotic actions of TGF-β1 are mediated, at least partially, through the NF-κB pathway in those cells.
We also found consistency in a parallel experiment where treatment with TGF-β1 significantly increased the mRNA levels encoding PAI-1 and FN-EIIIA in cultured renal fibroblasts, which are suppressed by pretreatment with an Iκ kinase inhibitor (Supplemental Figure S2). Although detailed mechanistic links between TGF-β1 signaling to NF-κB activation in renal fibroblasts remain unknown at present, in chondrosarcoma cells, TGF-β1 activates the NF-κB pathway through phosphatidylinositol 3-kinase and Akt.33 TGF-β1 also synergistically induces NF-κB with TNF-α through protein kinase A-dependent p65 acetylation in human lung epithelial cells.34 The NF-κB pathway likely plays a profibrotic role not only in renal but also in lung, skin, and intestinal fibroblasts.35–37
The fibroblasts involved in renal fibrogenesis in obstructed kidneys likely originate from several sources including interstitial resident fibroblasts,17,38 bone marrow-derived fibrocytes,31,17 tubular epithelia,17 and endothelial cells.22 FSP1 promoter-dependent recombination is likely active in all of these sources of fibroblasts. Taking the data from γGT.Cre;IκBdN and FSP1.Cre;IκBdN mice together suggests that FSP1+ fibroblasts derive from multiple sources and play a profibrotic role in NF-κB-mediated fibrosis in the kidneys. Consistent with this study, we also reported previously that suicide deletion of FSP1+ fibroblasts with a nucleoside analog attenuates renal fibrosis after ureter obstruction.39 We suggest that a FSP1 promoter-driven approach will target cells critical for fibrosis-specific therapeutics, and further studies will refine its application to clinical nephrology.
CONCISE METHODS
Transgenic Mice
Three sets of transgenic mice were used, and another was constructed for this study. The FSP1.Cre and γGT.Cre mice express Cre recombinase under the control of FSP1 promoter mainly in fibroblasts25 and that of γGT promoter mainly in tubular epithelia,17 respectively. The EGFP reporter mice were a generous gift from Prof. J. Miyazaki (Osaka University).28 In the IκBdN mice, the IκBdN gene encoding a nondegraded IκBα that lacks 54 amino acids of the NH2 terminus of wild-type human IκBα was removed from pBKCMV-IκBdN (a generous gift from Prof. A. Takayanagi, Keio University School of Medicine) by digestion with HindIII, and the overhang ends were blunted with Klenow fragment. The blunted IκBdN transgene was then ligated to the SmaI site of the pCALNL5 plasmid (a generous gift from Prof. I. Saito, University of Tokyo),40 which was separated from the universal CAG promoter by a floxed STOP (neo + pA) sequence (Figure 1A). The construct selected for proper orientation was then linearized using SalI and HindIII, and the purified DNA was injected into B6xSJL zygotes. The resulting progeny were crossed to C57B6 mice and selected by Southern blot and PCR. All of these mice were backcrossed to SJL mice more than six times before experimentation. The Institutional Animal Care and Use Committee at the Saitama Medical University and Keio University School of Medicine approved the transgenic protocols.
DNA was extracted from tail biopsies from FSP1.Cre, γGT.Cre, EGFP, IκBdN, FSP1.Cre;IκBdN, γGT.Cre;EGFP, and γGT. Cre;IκBdN mice, and genotyping PCR was performed with an Extract-N-Amp Tissue PCR Kit (XNAT2; Sigma, St. Louis, MO) according to the manufacturer's instructions. The following PCR primers and annealing temperatures were used: γGT.Cre and FSP1.Cre: 5′-AGGTGTAGAGAAGGCACTTAGC and 3′-CTAATCGCCATCTTCCCAGCAGG, 63°C; EGFP: 5′-AGCAAAGGGCGAGGAGCTGTT and 3′-GTAGGTCAGGGTGGTCACGA, 55°C; and IκBdN: 5′-GAGGATCGTTTCGCATGATT and 3′-TATTCGGCAAGCAGGCATCG, 58°C. Cycle programs were performed in a thermocycler (Icycler; Bio-Rad Japan, Tokyo, Japan). All of the primer reactions were started for 4 minutes at 95°C, followed by 35 cycles of 1 minute at 95°C, 1 minute at the primer's appropriate annealing temperature, and 1 minute at 72°C, finishing with 5 minutes at 72°C. The products were analyzed by electrophoresis in 1% agarose using TAE buffer.
In Vivo Experiments
Six male, 5- to 6-week-old mice from each transgenic line, γGT.Cre;IκBdN, FSP1.Cre;IκBdN, and IκBdN, were used to generate fibrosis in the UUO model. Manipulation for ureter ligation was reported in detail previously.17 The mice were sacrificed 7 days after ureteral ligation, and obstructed kidney tissues were sampled for RNA extraction and paraformaldehyde fixation for paraffin blocks. IκBdN mice with ureteral ligation and sham manipulation (n = 6) were used as positive and the negative controls, respectively.
Microscopic Immunohistochemistry
Sections (4 μm) cut from paraffin-embedded kidneys were processed for Masson's trichrome staining. Interstitial fibrosis was quantitatively determined with Mac SCOPE software (version 2.5; Mitani Corp., Fukui, Japan) in 10 high-power (×200) cortical fields, and the interstitial fibrosis indices were expressed as the mean percentage area in blue per one cortical field in Masson's trichrome-stained sections.41 For immunohistochemistry, the antibodies against EGFP (ab13970; Abcam, Cambridge, UK), pp65 (3037; Cell Signaling Technology, Boston, MA), mouse monocytes (F4/80; AbD Serotec, Oxford, UK), and fibroblasts (FSP1)14 were diluted (1:400) into 1% BSA in PBS as the blocking buffer and then applied on the sections for 8 hours. Except for EGFP, the signals were amplified using TSATM kit (Molecular Probe/Invitrogen, Carlsbad, CA) and labeled by Alexa Fluor 555 (Molecular Probe). Alexa Fluor 488-labeled secondary antibody was used after anti-EGFP antibody according to a conventional indirect method. Negative control sections were treated as described above, but the primary antibody was omitted. YO-PRO-1 (Molecular Probe) or TO-PRO-3 (Molecular Probe) was used to detect the nuclei. A confocal laser scanning microscopy (FV1000; Olympus, Tokyo, Japan) was used for data acquisition. The number of pp65+ nuclei were counted in 10 microscopic fields using a 40× objective lens, and its indices were expressed as the mean number per one field. The numbers of stained cells for anti-F4/80 and anti-FSP1 were quantitatively assessed as described above.
Reverse Transcriptase (RT)-PCR
Total RNA was extracted from the homogenates of kidney tissues with TRIzolTM (Life Technologies BRL, Grand Island, NY) according to the manufacturer's instructions. All of the RNA samples were pretreated with the RNase-free DNase I (Qiagen, Basel, Switzerland). cDNA was synthesized with a kit (Ready-To-Go T-Primed First-Strand Kit; GE Healthcare, Buckinghamshire, UK). The cDNA was amplified by PCR with the use of primers for IκBdN (forward, 5′-CTCCAGCAGACTCCACTCCACT-3′, and reverse, 5′-ACACCAGCCACCACCTTCTGAT-3′), yielding a 712-bp fragment. Cycle programs were started with 4 minutes at 95°C, followed by 35 cycles of 1 minute at 95°C, 1.5 minutes at 60°C, and 1.5 minutes at 72°C, finishing with 5 minutes at 72°C.
Real-Time Quantitative RT-PCR (qPCR)
We performed qPCR as described previously.41 In brief, a real-time quantitative one-step RT-PCR assay was performed to quantify mRNA levels using QuantiTect SYBR Green RT-PCR (Qiagen) and an Mx3000P QPCR system (Stratagene, La Jolla, CA). The primers used for qPCR were as follows: MCP-1 primer: forward, 5′-CTCTCTTCCTCCACCACCAT-3′, and reverse, 5′-ACTGCATCTGGCTGAGCCA-3′; FRK primer: forward, 5′-CGCGTTCTTCCATTTGTGTA-3′, and reverse, 5′-CATGATTTCGCATTTCGTCA-3′; OPN primer: forward, 5′-CCCTTTCCGTTGTTGTCCTG-3′, and reverse, 5′-CCCTCGATGTCATCCCTGTT-3′; TGF-β1 primer: forward, 5′-CAGTGGCTGAACCAAGGAGAC-3′, and reverse, 5′-ATCCCGTTGATTTCCACGTG-3′; CCN2 primer: forward, 5′-GTGGAATATTGCCGGTGCA-3′, and reverse, 5′-CCATTGAAGCATCTTGGTTCG-3′; PAI-1 primer: forward, 5′-AGGATCGAGGTAAACGAGAGC-3′, and reverse, 5′-GCGGGCTGAGATGACAAA-3′; FN-EIIIA primer: forward, 5′-ATCCGGGAGCTTTTCCCTG-3′, and reverse, 5′-TGCAAGGCAACCACACTGAC-3′; COLI primer: forward 5′-TGTAAACTCCCTCCACCCCA-3′, and reverse, 5′-TCGTCTGTTTCCAGGGTTGG-3′; and glyceraldehyde-3-phosphate dehydrogenase primer: forward, 5′-TGCAGTGGCAAAGTGGAGATT-3′, and reverse, 5′-TTGAATTTGCCGTGAGTGGA-3′. All of these oligodeoxynucleotides were designed by using Primer Express software (Perkin Elmer, Foster City, CA). Preliminary RT-PCR experiments in which these primer sets were used yielded appropriately sized, single products.
Statistical Analysis
The values are presented as the means ± SEM. The statistical differences between groups were evaluated by ANOVA, followed by a Bonferroni/Dunnett's test; significant P values were ≤0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The authors thank M. Funabashi for her technical assistance. This research was supported in part by grants from Asubio Pharma Co. Ltd., the Ministry of Education, Culture, Sports, Science and Technology (Japan), and the Ministry of Health, Labour and Welfare (Japan). EGN was supported by National Institutes of Health Grant DK-46282.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Hayden MS, Ghosh S: Shared principles in NF-kB signaling. Cell 132: 344–362, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Guijarro C, Egido J: Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 59: 415–424, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Giannopoulou M, Dai C, Tan X, Wen X, Michalopoulos GK, Liu Y: Hepatocyte growth factor exerts its anti-infammatory action by disrupting nuclear factor-kB signaling. Am J Pathol 173: 30–41, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano SA, Vaziri ND: Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther 315: 51–57, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Fujihara CK, Antunes GR, Mattar AL, Malheiros DMAC, Vieira JM, Zatz R: Chronic inhibition of nuclear factor-kB attenutaes renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol 292: F92–F99, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Gomez-Garre D, Largo R, Tejera N, Fortes J, Manzarbeitia F, Egido J: Activation of NF-kB in tubular epithelial cells of rats with intense proteinuria. Hypertension 37: 1171–1178, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Rangan GK, Wang Y, Tay YC, Harris DC: Inhibition of nuclear factor-kB activation reduces cortical tubulointerstitial injury in proteinuric rats. Kidney Int 56: 118–134, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Ozawa Y, Kobori H: Crucial role of Rho-nuclear factor-kB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol 293: F100–F109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamada S, Nakatani T, Asai T, Tashiro K, Komiya T, Sumi T, Okamura M, Kim S, Iwao H, Kishimoto T, Yamanaka S, Miura K: Inhibition of nuclear factor-kB activation by pyrrolidine dithiocarbamate prevents chronic FK506 nephropathy. Kidney Int 63: 306–314, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Takase O, Hirahashi J, Takayanagi A, Chkaraishi A, Marumo T, Ozawa Y, Hayashi M, Shimizu N, Saruta T: Gene transfer of truncated IkBa prevents tubulointerstitial injury. Kidney Int 63: 501–513, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Ouaaz F, Li M, Beg AA: A critical role for the RelA subunit of nuclear factor kB in regulation of multiple immune-response genes and in Fas-induced cell death. J Exp Med 189: 999–1004, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okada H, Kalluri R: Cellular and molecular pathways that lead to progression and regression of renal fibrogenesis. Curr Mol Med 5: 467–474, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Chevalier RL, Forbes MS, Thronhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Strutz F, Okada H, Lo CW, Danoff TM, Carone RL, Tomaszewski JE, Neilson EG: Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okada H, Danoff TM, Kalluri R, Neilson EG: Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol 273: F563–F574, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS: Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 171: 899–907, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kie JH, Kapturczak MH, Traylor A, Agarwal A, Hill-Kapturczak N: Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim J, Seok YM, Jung KJ, Park KM: Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol 297: F461–F470, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, Xu C, Dimitrova YN, Rauscher FJ, III, Neilson EG: A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest 117: 482–491, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH: Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R: Fibroblasts in kidney fibrosis emerge via endothelial-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R: Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y: New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL: TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303: 848–851, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Tsutsumi R, Xie C, Wei X, Zhang M, Zhang X, Flick LM, Schwarz EM, O'Keefe RJ: PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J Bone Miner Res 24: 1753–1762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, Naidu S, Wei G, Sharma SM, Stephens JA, Fernandez SA, Gurcan MN, Weinstein MB, Barsky SH, Yee L, Rosol TJ, Stromberg PC, Robinson ML, Pepin F, Hallett M, Park M, Ostrowski MC, Leone G: Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461: 1084–1091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawamoto S, Niwa H, Tashiro F, Sano S, Kondoh G, Takeda J, Tabayashi K, Miyazaki J: A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett 470: 263–268, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Okada H, Ban S, Nagao S, Takahashi H, Suzuki H, Neilson EG: Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney Int 58: 587–597, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Starremans PG, Li X, Finnerty PE, Guo L, Takakura A, Neilson EG, Zhou J: A mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5′ end of Pkd1. Kidney Int 73: 1394–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Inoue T, Plieth D, Venkov CD, Xu C, Neilson EG: Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int 67: 2488–2493, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Vielhauer V, Anders HJ, Mack M, Cihak J, Strutz F, Stangassinger M, Luckow B, Groene HJ, Schlondorff D: Obstructive nephropathy in the mouse: Progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol 12: 1173–1187, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Yeh YY, Chiao CC, Kuo WY, Hsiao YC, Chen YJ, Wei YY, Lai TH, Fong YC, Tang CH: TGF-b1 increases motililty and aVb3 integrin up-regulation via PI3K, Akt and NF-kB-dependent pathway in human chondrosarcoma cells. Biochem Pharmocol 75: 1292–1301, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Ishinaga H, Jono H, Lim JH, Komatsu K, Xu X, Lee J, Woo CH, Xu H, Feng XH, Chen LF, Yan C, Li JD: Synergistic induction of nuclear factor-kB by transforming growth factor-b and tumour necrosis factor-a is mediated by protein kinase A-dependent RelA acetylation. Biochem J 417: 583–591, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Chetty A, Cao GJ, Nielsen HC: Insulin-like growth factor-I signaling mechanisms, type I collagen and alpha smooth muscle actin in human fetal lung fibroblasts. Pediatr Res 60: 389–394, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Messadi DV, Doung HS, Zhang Q, Kelly AP, Tuan TL, Reichenberger E, Le AD: Activation of NFkB signal pathways in keloid fibroblasts. Arch Dermatol Res 296: 125–133, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Theiss AL, Simmons JG, Jobin C, Lund PK: Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem 280: 36099–36109, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iwano M, Fischer A, Okada H, Pleith D, Xue C, Danoff T, Neilson E: Conditional abatement of tissue fibrosis using nucleotide analogs to corrupt DNA replication selectively in transgenic fibroblasts. Mol Ther 3: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Kanegae Y, Takamori K, Sato Y, Lee G, Nakai M, Saito I: Efficient gene activation on mammalian cell chromosomes using recombinant adenovirus producing Cre recombinase. Gene 181: 207–212, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Okada H, Inoue T, Kikuta T, Watabe T, Kanno Y, Ban S, Sugaya T, Horiuchi M, Suzuki H: A possible anti-inflammatory role of angiotensin II type 2 recerptor in immune-mediated glomerulonephritis during type 1 receptor blockade. Am J Pathol 169: 1577–1589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.