Abstract
Cell-based therapies against HIV/AIDS have been gaining increased interest. Natural killer (NK) cells are a key component of the innate immune system with the ability to kill diverse tumor cells and virus-infected cells. While NK cells have been shown to play an important role in the control of HIV-1 replication, their functional activities are often compromised in HIV-1-infected individuals. We have previously demonstrated the derivation of NK cells from human embryonic stem cells (hESCs) with the ability to potently kill multiple types of tumor cells both in vitro and in vivo. We now demonstrate the derivation of functional NK cells from human induced pluripotent stem cells (iPSCs). More importantly, both hESC- and iPSC-derived NK cells are able to inhibit HIV-1 NL4-3 infection of CEM-GFP cells. Additional studies using HIV-1-infected human primary CD4+ T cells illustrated that hESC- and iPSC-derived NK cells suppress HIV-1 infection by at least three distinct cellular mechanisms: killing of infected targets through direct lysis, antibody-dependent cellular cytotoxicity, and production of chemokines and cytokines. Our results establish the potential to utilize hESC- and iPSC-derived NK cells to better understand anti-HIV-1 immunity and provide a novel cellular immunotherapeutic approach to treat HIV/AIDS.
Natural killer (NK) cells are a key component of the innate immune system with the ability to respond to diverse tumor cells and virus-infected cells without the need for prior antigen sensitization (9, 15, 26). The activation of NK cells is associated with protection against HIV-1 infection and inhibition of HIV-1 replication both in vivo and in vitro (2, 15, 26, 34). However, NK cell function is often compromised in HIV-1-infected individuals (14, 15). Additionally, while the treatment of HIV-1-infected individuals with antiretroviral medications effectively reduces viral loads, the restoration of cellular immunity in treated patients proceeds slowly, and they may never return to their preinfection status (15, 25).
Cell-based immunotherapy using either T or NK effector cells has been used to treat malignancies such as leukemia, melanoma, and renal cell carcinoma (35, 39, 40). Similar strategies have also been postulated as a novel therapeutic approach for HIV-1 treatment in clinical practice (1, 24). One notable case demonstrated an apparent cure of HIV-1 infection by hematopoietic cell transplantation using a donor deficient for the expression of the HIV-1 coreceptor CCR5 (24). However, this patient was transplanted primarily to treat acute myelogenous leukemia, and a wider application of this strategy is severely limited due to high risk associated with transplantation and low availability of suitable HLA-matched CCR5-deficient donors. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) provide potential alternative approaches to generating hematopoietic cells for therapy against HIV. Our group and others have reported that hESCs and iPSCs can give rise to diverse lymphoid and myeloid lineages (3, 11, 18, 42, 46, 50, 51). Additional studies have demonstrated that hESC-derived macrophages and dendritic cells are susceptible to HIV-1 infection, indicating the potential of hESCs for HIV cell/gene therapy (3, 5).
Previously, our group demonstrated the derivation of NK cells from hESCs with the ability to potently kill multiple types of tumor cells both in vitro and in vivo (50, 51). We now demonstrate the successful derivation of NK cells from human induced pluripotent stem cells (iPSCs). Additionally, we find that these hESC- and iPSC-derived NK cells have potent anti-HIV-1 activity. Therefore, these studies establish the use of hESC- and iPSC-derived NK cells as a novel system to better understand anti-HIV immunity and suggest the potential to establish a readily available cell-based strategy to treat HIV/AIDS.
MATERIALS AND METHODS
NK cell differentiation from hESCs and iPSCs.
The hESC line H9 (Wicell, Madison, WI) and iPSC line BJ1-iPS12 (36) (kindly provided by George Daley, Boston Children's Hospital) were maintained as undifferentiated cells as described previously (29). A two-step stromal cell coculture system was used for NK cell differentiation from hESCs and iPSCs as described previously (50, 51). Briefly, hESCs or iPSCs were cocultured with the murine bone marrow stromal cell line M210-B4 (American Type Culture Collection, Manassas, VA) for 19 to 21 days to allow hematopoietic differentiation. CD34+CD45+ hematopoietic progenitors were then enriched by using EasySep selection kits (Stem Cell Technologies) and cocultured with a confluent monolayer of irradiated murine AFT024 cells (fetal liver-derived stromal cell line; kindly provided by K. Moore and I. Lemischka) under NK cell culture conditions for 4 to 5 weeks. Cells were harvested and analyzed for phenotype and function. UCB-NK cells derived under the same conditions were used as controls in all assays.
Phenotyping of hESC- and iPSC-derived NK cells.
Single-cell suspensions were stained with allophycocyanin (APC)-, phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, and peridinin chlorophyll protein (PerCP)-cy5.5-coupled IgG or specific antibodies against human blood surface antigens, including CD45-PE, CD56-APC, CD16-PerCP-cy5.5, NKG2D-PE, NKp44-PE, NKp46-PE, TRAIL-PE, CD158b-FITC, CD158e1/2-FITC (all from BD Pharmingen), FasL-PE (R&D), CD158a/h-PE, and CD158i-PE (Beckman Coulter), as shown in Fig. 1. All analyses were performed with a FACSCalibur instrument (BD Biosciences) and analyzed with FlowJo software (Tree Star). NK cells isolated from peripheral blood (PB-NK) using an NK cell-negative selection kit (Miltenyi Biotech) were used as positive controls.
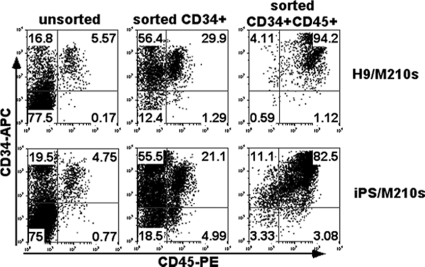
FIG. 1.
Hematopoietic development from hESCs and iPSCs cocultured with the stromal cell line M210-B4. hESCs and iPSCs were first allowed to differentiate on M210-B4 stromal cells for 19 to 21 days to develop hematopoietic progenitor cells. These hematopoietic progenitors were enriched based on CD34 and CD45 surface expression using magnetic sorting methods and then cultured on AFT024 cells with defined cytokines for NK cell derivation. Shown are data from flow cytometric analyses of unsorted, CD34+-sorted, and then CD34+ CD45+-sorted hESCs (top row) or iPSCs (bottom row).
HIV-1 replication inhibition assay.
CEM-GFP cells with green fluorescent protein (GFP) expression driven by the HIV-1 long terminal repeat (LTR) promoter were used as targets. CEM-GFP cells were infected with HIV-1 NL4-3 (multiplicity of infection [MOI] of 0.5) for 4 h at 37°C and were then washed twice with fresh medium. A total of 1 × 105 cells were plated with hESC-NK cells (or iPSC-NK cells) and CEM-GFP cells at the indicated effector-to-target cell (E:T) ratios or alone for 14 days in the presence of 100 IU/ml interleukin 2 (IL-2). Cells were collected on days 4, 7, 11, and 14 for determinations of GFP expression by flow cytometry. The loss of GFP expression in the CEM-GFP cells correlates with the loss of HIV replication. The supernatant from CEM-GFP cell cocultures was used for the quantification of p24 Gag production to measure viral production by an enzyme-linked immunosorbent assay (ELISA). PB-NK cells were used as positive controls.
NK cell activation against HIV-1-infected primary CD4+ T cells.
Primary NK cells and CD4+ T cells were enriched from peripheral blood mononuclear cells (PBMCs) (Memorial Blood Bank, Minneapolis, MN) using either an NK cell-negative selection kit (Miltenyi Biotech) or a CD4-PE positive selection kit (Stem Cell Technologies Inc.). Cell purity was determined by flow cytometry to be >95%. NK cells were maintained in RMPI 1640 medium plus 10% fetal bovine serum (FBS) and 100 IU/ml IL-2 before being used in the assays. CD4+ T cells were stimulated with phytohemagglutinin (PHA; Sigma-Aldrich) in RMPI 1640 medium with 10% FBS and 2 mM l-glutamine supplemented with 100 IU/ml IL-2 for 48 to 72 h. At day 3, expanded CD4+ T cells were infected with a laboratory-adapted strain, HIV-1 SF2 (X4R5) (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) (32). The anti-HIV activities of hESC-NK and iPSC-NK cells were evaluated by different assays, including direct lysis, antibody (Ab)-dependent cell-mediated cytotoxicity (ADCC), and chemokine and cytokine production.
Assay for NK cell-mediated direct lysis.
The expression of CD107a was used as a marker of the cytotoxic activity of NK cells, as previously described (2). To detect NK cell killing through direct lysis, either hESC-NK, iPSC-NK, UCB-NK, or PB-NK cells (1 × 105 cells) were incubated with HIV-1-infected CD4+ T cells at an E:T ratio of 5:1. After a 1-h incubation, monensin (Sigma-Aldrich) was added to cocultures in the presence of CD107a-PE (BD Pharmingen) for four more hours at 37°C. Cells were stained with CD56-APC for 20 min. Cells were then fixed in 1% paraformaldehyde for flow cytometric analysis.
Analysis of ADCC-mediated NK cell cytotoxicity.
To assess NK cell killing through ADCC, HIV-1-infected CD4+ T cells were treated with 3.3 μg/ml anti-gp41 Ab (246-D; NIH AIDS Research and Reference Reagent Program) (38) at 37°C for 30 min. Cells were washed with medium twice and then incubated with either hESC-NK cells or iPSC-NK cells at a 5:1 ratio. As negative controls, cells preincubated with 3.3 μg/ml IgG1 isotype Abs (BD Pharmingen) were used. Again, CD107a expression was evaluated to indicate NK cell activity through ADCC.
Measurement of chemokine production.
To determine NK cell inhibition of HIV-1 replication through chemokines and cytokines, intracellular staining was performed to measure the chemokines CCL3, CCL4, CCL5, and gamma interferon (IFN-γ). After a 4-h coincubation with HIV-1-infected CD4+ T cells in the presence of brefeldin A (Sigma-Aldrich), hESC-NK, UCB-NK, or PB-NK cells were stained with CCL3-PE, CCL4-PE, CCL5-PE, and IFN-γ (BD Pharmingen) using a fixation/permeabilization kit (BD Biosciences) and analyzed by flow cytometry.
Statistical analysis.
A paired Student's t test was used to assess differences the percentages of GFP-positive (GFP+) cells between HIV-1-infected CEM-GFP cells cocultured with or without NK cells, the levels of p24 between time points, and the levels of CD107a or CCL4 in NK cells upon stimulation with or without HIV-infected targets. All results are presented as means ± standard deviations (SD). All tests were two tailed, and P values of <0.05 were considered statistically significant.
RESULTS
Functional NK cells derived from both hESCs and iPSCs.
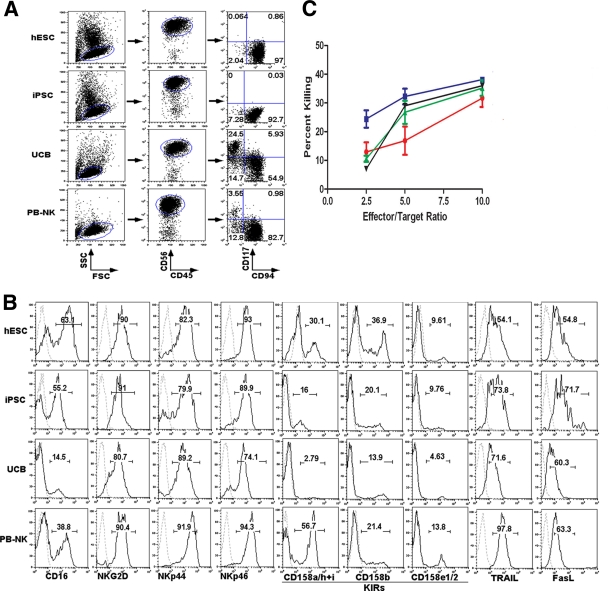
NK cells can be routinely produced from hESCs, as previously described (50, 51). Briefly, undifferentiated hESCs were induced to hematopoietic differentiation by coculture with the mouse bone marrow-derived stromal cell line M210-B4. After 19 to 21 days, CD34+CD45+ hematopoietic progenitor cells were isolated from the coculture (Fig. 1) and transferred to a second support cell line, AFT024, with defined NK cell-promoting cytokines, leading to the development of proliferating cells that display the typical NK cell phenotype and functionality (Fig. 2) (50, 51). We have now used these methodologies to induce hematopoietic progenitors from human iPSCs (from reprogrammed fibroblasts) (Fig. 1) and have successfully produced CD45+CD56+ NK cells (Fig. 2A, second row). Interestingly, similar to the hESC-derived NK cells, the iPSC-derived CD45+CD56+ cells are a uniform CD117−CD94+ population that was demonstrated previously to be more-mature NK cells with greater cytotoxic activity (17, 20, 50). This is also similar to NK cells isolated from peripheral blood (PB-NK cells) (Fig. 2A, bottom row) although distinct from NK cells produced from CD34+ umbilical cord blood cells (UCB-NK cells), which are a mixture of CD117−CD94+ and CD117+CD94− NK cell populations (Fig. 2A, third row). Additional phenotypic analyses confirmed that hESC-NK, iPSC-NK, and PB-NK cells have similar expression levels of key NK cell surface receptors, including killer immunoglobulin-like receptors (KIRs) (CD158a/h, CD158i, CD158b, and CD158e1/2); the FcγRIIIa receptor (CD16); the NK cell-activating receptors NKG2D, NKp44, and NKp46; and the apoptosis-inducing ligands FasL and TRAIL (Fig. 2B), whereas UCB-NK cells have relatively lower levels of these markers.
FIG. 2.
Generation of NK cells from hESCs and iPSCs. (A) Flow cytometric analysis of CD56+CD45+ NK cells derived from hESCs, iPSCs, and UCB progenitor cells cultured under NK cell conditions for 5 weeks. These cells were compared to NK cells isolated from peripheral blood (PB-NK). Both hESC- and iPSC-derived CD56+CD45+ cells are a uniform population of CD117−CD94+ cells, similar to PB-NK cells, whereas UCB-derived CD56+CD45+ cells are a mixture of CD117−CD94+ and CD117+CD94− populations. (B) Expression of activating and inhibitory receptors on hESC-NK, iPSC-NK, UCB-NK, and PB-NK cells, as indicated. (C) Four-hour 51Cr release NK cell-mediated cytotoxicity assay. Both hESC-NK (blue) and iPSC-NK (green) cells, similar to PB-NK cells (black), show higher cytolytic activities against K562 cells than do UCB-NK cells (red) at the indicated effector-to-target cell (E:T) ratios (n = 3) (P < 0.05).
Next, we evaluated the NK cell-mediated cytotoxicity of these NK cell populations against K562 erythroleukemia cells using a standard 51Cr release assay (Fig. 2C). We have previously shown that hESC-NK cells are capable of killing various tumor cells more efficiently than UCB-NK cells both in vitro and in vivo (50). Here, we demonstrate that hESC-NK cells, iPSC-NK cells, and PB-NK cells all kill these targets with similar efficiencies (and more effectively than UCB-NK cells) (Fig. 2C). Based on these phenotypical and functional characterizations, we hypothesized that hESC- and iPSC-derived NK cells would be functional against HIV-1 infection.
hESC- and iPSC-derived NK cells suppress HIV-1 replication in CEM cells.
As noted previously, several studies have demonstrated the anti-HIV-1 activity of PB-NK cells (2, 15, 37). To examine the anti-HIV activity of hESC-NK and iPSC-NK cells, the CEM-GFP reporter T-cell line was infected with HIV-1 NL4-3 and then cocultured with hESC-NK or UCB-NK cells for 2 weeks. Since CEM-GFP cells express GFP driven by the HIV long terminal repeat (19), the expression of GFP was used as an indicator of the percentage of infected cells (see Fig. 3A as a representative). We found that the hESC-NK cells inhibited HIV-1 activity by over 90% after 11 days at an effector-to-target cell (E:T) ratio of 10:1 relative to HIV-1-infected CEM-GFP cultures without NK cells (average of three experiments) (Fig. 3B). Table 1 shows the ability of hESC-NK cells to inhibit HIV-1 replication during the 2-week period when hESC-NK and infected CEM-GFP cells were cocultured.
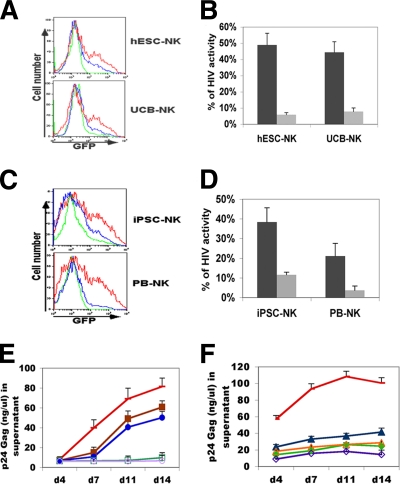
FIG. 3.
hESC- and iPSC-derived NK cells inhibit the replication of HIV-1 in the CEM-GFP T-cell line. CEM-GFP cells were incubated with HIV-1 NL4-3 for 4 h. Cells were then cocultured with hESC-NK, iPSC-NK, UCB-NK, or PB-NK cells for 14 days. In these studies, HIV-1 infection was assessed by flow cytometry for GFP expression. (A) Histogram representing GFP expression in CEM cells cocultured with hESC-NK or UCB-NK cells at E:T ratios of 10:1 (green line), 1:1 (blue line), or 0:1 (red line) at day 11. (B) Activity of HIV-1 measured by the inhibition of GFP+ cells in cocultures of hESC-NK or UCB-NK cells with CEM-GFP cells at day 11 with E:T ratios of 1:1 (dark bars) and 10:1 (light bars). (C) Histogram of GFP expression in CEM cells cocultured with iPSC-NK or PB-NK cells at an E:T ratio of 5:1 (green line), 1:1 (blue line), or 0:1 (red line) at day 11. (D) Activity of HIV-1 in cocultures of iPSC-NK or PB-NK cells with CEM-GFP cells at day 11 at E:T ratios of 1:1 (dark bars) and 5:1 (light bars). All data in B and D show statistically decreased HIV-1 activity (P < 0.01). (E and F) Quantification of supernatant for p24 Gag protein in cocultures of CEM-GFP cells with NK cells. (E) HIV-infected CEM-GFP cells with no NK cell treatment (red), UCB-NK cells (maroon), and hESC-NK cells (blue) at 1:1 E:T ratios and UCB-NK cells (dark green) and hESC-NK cells (lilac) at 10:1 E:T ratios. (F) iPSC-NK cells (dark blue) and PB-NK cells (light green) at a 1:1 E:T ratio and iPSC-NK cells (orange) and PB-NK cells (purple) at a 5:1 E:T ratio. For statistical analysis, shown are data for UCB-NK cells and hESC-NK cells at 1:1 E:T ratios versus HIV-infected CEM-GFP cells (P < 0.05 for each) and UCB-NK cells and hESC-NK cells at 10:1 E:T ratios versus HIV-infected CEM-GFP cells (P < 0.01 for each) (E) and iPSC-NK cells at a 1:1 E:T ratio, iPSC-NK cells at a 5:1 E:T ratio, PB-NK cells at a 1:1 E:T ratio, and PB-NK cells at a 5:1 E:T ratio versus HIV-infected CEM-GFP cells (all P < 0.05) (F). Data in B, D, and E represent the means of data from three experiments and standard deviations, and data in F represent the means of data from two experiments and standard deviations. These results demonstrate that all hESC-NK, UCB-NK, iPSC-NK, and PB-NK cells significantly inhibit HIV replication over this 14-day time course.
TABLE 1.
NL4-3-infected CEM-GFP cells cocultured with hESC-NK and UCB-NK cellsa
| Day | % of GFP+ NL4-3-infected CEM-GFP cells |
||
|---|---|---|---|
| Without coculture | Cocultured with hESC-NK cells at a 10:1 ratio | Cocultured with UCB-NK cells at a 10:1 ratio | |
| 4 | 1.12 | 0.24 | 0.18 |
| 7 | 3.32 | 0.68 | 0.84 |
| 11 | 26.4 | 0.74 | 2.16 |
| 14 | 41.6 | 13.9 | 17.9 |
Fourteen-day time course of HIV-1 NL4-3 replication in CEM-GFP cells cocultured with or without hESC-derived NK cells or UCB-derived NK cells (E:T ratio of 10:1). Virus replication is represented as a percentage of GFP+ CEM cells in each culture. Samples were measured on days 7, 11, and 14, as indicated.
As iPSCs are a potential resource for HIV-1 treatment, we also investigated the iPSC-NK cell-mediated inhibition of HIV-1 replication using the same assay described above. An E:T ratio of 5:1 decreased HIV replication by 80% when iPSC-NK cells were cultured with HIV-infected CEM-GFP cells (average of three experiments) (Fig. 3C and D and Table 2). As noted by studies reported previously by other groups, PB-NK cells significantly decreased HIV-1 replication in CEM-GFP cells (2, 37, 49) (Fig. 3C and D and Table 2). Indeed, we find that hESC-NK cells and iPSC-NK cells have anti-HIV activities similar to those of PB-NK cells (Fig. 3 and Tables 1 and 2). The findings that both hESC- and iPSC-derived NK cells dramatically decreased HIV-1 infectivity and spreading in CEM target cells in a dose-dependent manner further suggest that NK cells are directly responsible for the inhibition of HIV-1 replication (Fig. 3A to D and Tables 1 and 2). The anti-HIV activity of NK cells was further supported by measurements of p24 Gag release, which was significantly decreased in both hESC-NK and iPSC-NK cell cocultures (Fig. 3E and F).
TABLE 2.
NL4-3-infected CEM-GFP cells cocultured with iPSC-NK and PB-NK cellsa
| Day | % of GFP+ NL4-3-infected CEM-GFP cells |
||
|---|---|---|---|
| Without coculture | Cocultured with iPSC-NK cells at a 5:1 ratio | Cocultured with PB-NK cells at a 5:1 ratio | |
| 4 | 3.45 | 1.06 | 0.11 |
| 7 | 12 | 4.95 | 0.74 |
| 11 | 36.4 | 10.6 | 2.6 |
| 14 | 26.2 | 8.7 | 1.9 |
Fourteen-day time course of HIV-1 NL4-3 replication in CEM-GFP cells cocultured with or without iPSC-derived NK cells or PB-derived NK cells (E:T ratio of 5:1). Virus replication is represented as a percentage of GFP+ CEM cells in each culture. Samples were measured on days 7, 11, and 14, as indicated.
hESC- and iPSC-derived NK cells are functional against HIV-1-infected primary CD4+ T cells.
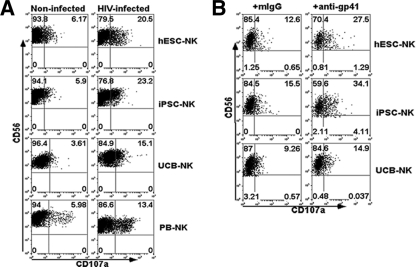
To better assess the cytotoxic activity of hESC-NK and iPSC-NK cells against HIV-1-infected cell targets, we next evaluated the expression of CD107a on NK cells stimulated by HIV-1-infected CD4+ T cells. CD107a is a membrane protein localized to cytolytic granules in NK cells that becomes expressed on the cell surface upon degranulation and provides a well-established measure of NK cell cytotoxic activity (2, 10). Here, primary CD4+ T cells isolated from the peripheral blood of healthy donors were incubated with HIV-1 SF2 and monitored for HIV-1 infection via intracellular staining for p24. At days 7 to 10 of HIV-1 SF2 infection, CD4+ T cells were coincubated with either hESC-NK, iPSC-NK, UCB-NK, or PB-NK cells for 4 h. Surface CD107a expression on NK cells was assessed by flow cytometry. The results shown in Fig. 4A demonstrate that the percentage of CD107a+CD56+ cells was significantly increased in both hESC-NK and iPSC-NK cell populations upon stimulation with HIV-1-infected CD4+ T cells compared with NK cells coincubated with noninfected CD4+ T cells: 18.7% (HIV-1-infected cells) versus 4.7% (noninfected cells) for hESC-NK cells and 21.5% (HIV-1-infected cells) versus 6.8% (noninfected cells) for iPSC-NK cells (average of three experiments) (representative data are shown in Fig. 4A). The increase of surface CD107a expression on hESC- and iPSC-NK cells upon exposure to HIV-infected T cells was even greater than those observed for autologous PB-NK cells (14.1% versus 6.5%) and UCB-NK cells (14.4% versus 5.7%). These results indicate that both hESC-NK and iPSC-NK cells provide cytotoxic activity against HIV-1-infected cell targets through a direct interaction.
FIG. 4.
hESC-NK and iPSC-NK cell activation against HIV-1-infected human CD4+ primary T cells. Surface expression of CD107a provides a measure of NK cell cytolytic activation. Shown are data from flow cytometric analyses of CD107a+ NK cells following stimulation with HIV-1-infected CD4+ T cells. Uninfected CD4+ T cells were used as controls. (A) Representative analysis of surface CD107a expression on hESC-NK, iPSC-NK, UCB-NK, and PB-NK cells stimulated with HIV-1-infected cells (right) versus uninfected control cells (left). All NK cell populations stimulated by HIV-1-infected CD4+ T cells show significantly increased CD107a expression compared to CD107a expression after stimulation by uninfected T cells (P < 0.01 for both hESC-NK and iPSC-NK cell groups and P < 0.05 for UCB-NK and PB-NK cell groups [n = 4]). (B) Expression of CD107a on hESC-NK and iPSC-NK cells upon stimulation with HIV-1-infected CD4+ T cells treated with either anti-gp41 Ab or IgG isotype to evaluate for increased anti-HIV activity via ADCC. Again, all three NK cell populations demonstrated increased CD107a surface expression after the addition of anti-gp41 compared to the isotype control (P < 0.01 for hESC-NK or iPSC-NK cells treated with HIV-1-infected CD4+ T cells plus anti-gp41 versus isotype IgG-treated HIV-1-infected CD4+ T cells and P < 0.05 for UCB-NK cells treated with HIV-1-infected CD4+ T cells plus anti-gp41 versus isotype IgG-treated HIV-1-infected CD4+ T cells [n = 3 for all studies]).
Not only can NK cells kill virus-infected cells directly, but they can also lyse targets via antibody-dependent cell-mediated cytotoxicity (ADCC) through the Fc receptor (CD16) (13, 21). We therefore treated HIV-1-infected CD4+ T cells with an anti-gp41 antibody known to mediate ADCC (38) prior to coculture with either hESC-NK or iPSC-NK cells. These studies demonstrated that both hESC-NK and iPSC-NK cells expressed significantly higher levels of CD107a when stimulated with HIV-1-infected CD4+ T-cell targets plus anti-HIV-1-specific antibodies (Fig. 4B), suggesting that these NK cells were capable of killing HIV-1-infected cell targets through ADCC. Again, the increase in CD107a expression on both hESC-NK and iPSC-NK cells was greater than that on UCB-NK cells, which corresponded to the lower level of CD16 expression on UCB-NK cells (Fig. 2B).
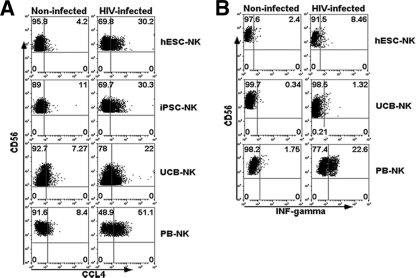
NK cells are also known to control HIV-1 infection through the production of β-chemokines, such as CCL3 (macrophage inflammatory protein 1α [MIP-1α]), CCL4 (MIP-1β), and CCL5 (RANTES), which can competitively inhibit R5-tropic HIV-1 entry into CD4+ T-cell targets (1, 6, 15). To examine the effect of hESC-NK and/or iPSC-NK cells on chemokine production, NK cells were cocultured with HIV-1-infected CD4+ T cells, and the levels of chemokines were examined by intracellular staining and flow cytometry. These studies demonstrate a significant upregulation of all three chemokines in both hESC-NK and iPSC-NK cells upon stimulation with HIV-1-infected CD4+ T cells (Fig. 5A; as a representative of CCL4, others are not shown). In addition, NK cell-produced cytokines, such as IFN-γ, can mediate anti-HIV activity via noncytolytic mechanisms (27). The results shown in Fig. 5B demonstrate that the level of IFN-γ production was increased when hESC-NK cells were stimulated with HIV-infected CD4+ T cells although to a lesser degree than PB-NK cells. These studies illustrate that human pluripotent stem cell-derived NK cells could potentially inhibit HIV-1 activity through the production of β-chemokines and cytokines.
FIG. 5.
Anti-HIV chemokine and cytokine production by hESC-NK and iPSC-NK cells. Shown are data for intracellular staining measured by flow cytometry analysis for the production of CCL4 in hESC-NK, iPSC-NK, UCB-NK, and PB-NK cells upon stimulation with HIV-1-infected CD4+ T cells or control CD4+ T cells (P < 0.01 for both hESC-NK and iPSC-NK cells treated with HIV-1-infected CD4+ T cells versus noninfected CD4+ T cells and P < 0.05 for UCB-NK cells treated with HIV-1-infected CD4+ T cells versus noninfected CD4+ T cells [n = 4]) (A) and IFN-γ production in hESC-NK, UCB-NK, and PB-NK cells upon stimulation with HIV-1-infected CD4+ T cells or control CD4+ T cells (n = 3) (B).
DISCUSSION
These studies demonstrate the successful derivation of NK cells from iPSC-derived hematopoietic progenitors using a culture system previously demonstrated to be suitable to drive NK cell differentiation from hESC-derived hematopoietic progenitor cells and UCB CD34+ progenitors (50, 51). To date, this provides the most functional and definitive evidence of human iPSC-derived blood cells. Indeed, we have now generated NK cells with similar activities from several other iPSC lines. The phenotypic profiles of hESC-NK and iPSC-NK cells are similar to those of their PB-NK cell counterparts. Also, both hESC-NK and iPSC-NK cells have a tumor-killing capacity comparable to that of PB-NK cells, indicating that both hESC-NK and iPSC-NK cells are fully functional (Fig. 2). Interestingly, the UCB-derived NK cells are a mixture of phenotypically mature and immature populations. UCB-derived NK cells typically express lower levels of KIRs and CD94 than either pluripotent stem cell-derived NK cells or peripheral blood NK cells (50, 51). Both in vivo and in vitro models of NK cell development support a direct interaction with stromal cells, which are required for the acquisition of receptors, including KIRs (reviewed in reference 52). Since the AFT024 stromal cells used in this study are murine derived and do not express human class I major histocompatibility complex (MHC) proteins, the low levels of KIRs on the in vitro-derived NK cells fit a model where the interaction between KIR receptors and MHC class I proteins help regulate KIR acquisition during NK cell maturation. However, hESC- and iPSC-derived NK cells routinely have higher levels of KIR expression than do UCB-derived NK cells. This may be due to other signaling pathways activated in the hESCs/iPSCs during hematopoietic development under the initial M2-10B4 coculture conditions that were used to support the differentiation of hESCs and iPSCs into hematopoietic progenitor cells. For example, we have previously demonstrated that these conditions result in the upregulation of some lineage-specific transcription factors, including ID proteins that are known to promote NK cell development (33). Therefore, these studies suggest that NK cell development from hESC- or iPSC-derived hematopoietic progenitors does not depend on stromal cell interactions in the same fashion as that of UCB progenitors.
NK cells have been associated with the control of HIV-1 infection both in vivo and in vitro (2, 34). In this study, our results also clearly illustrate that hESC-NK and iPSC-NK cells exercise their anti-HIV-1 activity potentially through at least three distinct cellular mechanisms: direct lysis, ADCC, and the production of soluble mediators (β-chemokines and IFN-γ). Interestingly, we noticed that a higher percentage of hESC-NK and iPSC-NK cells showed degranulation (assessed by CD107a expression) than PB-NK and UCB-NK cells upon activation by HIV-1-infected CD4+ T cells, whereas PB-NK cells produced more CCL4 and IFN-γ than did hESC-NK and/or iPSC-NK cells. It is possible that these cellular mechanisms may be utilized differently by NK cells derived from human pluripotent stem cells (both hESCs and iPSCs) compared to NK cells isolated from peripheral blood. Several studies have shown that NK cell receptors, such as KIR3DS1, NKG2D, and NKp44, are associated with anti-HIV-1 activity (2, 7, 8, 34, 37, 41, 48). Indeed, we demonstrate that all these receptors are expressed on hESC/iPSC-derived NK cells (Fig. 2). Additionally, NK cells kill targets through the recognition of the receptors of FasL and TRAIL (4, 53). We find that both FasL and TRAIL are robustly expressed on the surface of hESC-NK and iPSC-NK cells (Fig. 2B), suggesting that these two (and possibly other) members of the TNFR (tumor necrosis factor receptor) gene superfamily of death receptors may also be involved in the NK cell-mediated killing of HIV-1-infected targets.
Overall, these results have at least two key implications. First, both hESCs and iPSCs provide an excellent model to study human lymphocyte development and function utilizing a homogeneous cell population that can be cultured and genetically modified under well-controlled conditions. Second, human pluripotent stem cells (hESCs and iPSCs) can serve as a novel “universal” source for a cellular immunotherapeutic approach for the treatment of HIV/AIDS and malignancies. Indeed, iPSC technology would potentially enable patient-specific anti-HIV-1 cellular treatment. It should also be possible to engineer hESCs and iPSCs to express anti-HIV-1 receptors, as was shown previously to be effective with anti-HIV cytotoxicity for CD8+ T cells isolated from peripheral blood or derived from hematopoietic stem cells (HSCs) (30, 45).
Notably, some recent studies have suggested that iPSCs are significantly more difficult to differentiate into specific cell lineages and therefore are potentially less useful for therapeutic applications than hESCs (16, 23). While our studies (and those of others) do find variation in the efficiency for hematopoietic differentiation between different iPSC lines (12), we also demonstrate that once we obtain differentiated hematopoietic progenitor (CD34+CD45+) cells from iPSCs, these populations do have similar potentials for NK cell development.
Clearly, we are entering into a new era of stem cell-based therapies to treat and cure a wider range of diseases (28). Based on these studies, HIV/AIDS can be added to the list of conditions to be treated and potentially cured by hESC- and iPSC-derived cells. CCR5-deficient HSCs could also potentially be derived from hESCs/iPSCs (for example, creating iPSCs from a CCR5-deficient individual). However, to date, despite intense efforts by multiple groups, HSCs capable of long-term multilineage hematopoietic engraftment have not been efficiently derived from hESCs or iPSCs (31, 43, 44, 47). Therefore, at this time, a clinical scenario utilizing CCR5-deficient hESC/iPSC-derived HSCs as a starting cell population to transplant and potentially cure HIV/AIDS (24) is not yet feasible. As described here, the use of hESC/iPSC-derived NK cells as a source of novel anti-HIV and anticancer (50, 51) immunotherapy currently provides a safer and more viable approach. This strategy of anti-HIV immunotherapy could be applied to successfully treat malignancies, as has been done previously (35, 39). Here, there are two possible scenarios. For patients who are refractory to standard highly active antiretroviral therapy (HAART), this anti-HIV immunotherapy could be used to better manage or possibly eliminate the viral burden. Alternatively, patients controlling viral load on HAART could receive adoptively transferred hESC/iPSC-derived NK cells to eliminate remaining disease reservoirs (22). Additionally, as hESCs and iPSCs can be routinely genetically modified, it will also be possible to test the antiviral activities of hESC-NK/iPSC-NK cells modified to express receptors that specifically recognize HIV-1 targets, such as what has been defined for certain T-cell receptors (TCRs) (30, 45). In this manner, hESC/iPSC-derived NK cells can potentially serve as a universal cell source for targeted anti-HIV immunotherapies rather than having to modify immune effector cells on a patient-specific basis. While the translation of these approaches to clinical therapies still has significant hurdles to overcome (28), clearly, the use of human pluripotent-derived NK cells opens up promising new approaches to treat lethal malignancies and infectious diseases.
Acknowledgments
We thank Xinghui Tian, Katherine Hill, and Jeremy Allred for helpful advice and assistance with these studies.
This work was supported by NIH grant HL77923 (D.S.K.), a Grand Challenges Exploration grant from the Bill & Melinda Gates Foundation (D.S.K.), and a fellowship grant from amfAR—the Foundation for AIDS Research (Z.N.).
Z.N. designed and performed experiments and wrote the manuscript; D.A.K., C.L.C., and M.K.H. designed and performed experiments and edited the manuscript; P.S. and L.M.M. helped with experimental design, provided reagents, and edited the manuscript; I.-H.P. provided essential reagents; and D.S.K. designed experiments and wrote and edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Alter, G., and M. Altfeld. 2009. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J. Intern. Med. 265:29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter, G., M. P. Martin, N. Teigen, W. H. Carr, T. J. Suscovich, A. Schneidewind, H. Streeck, M. Waring, A. Meier, C. Brander, J. D. Lifson, T. M. Allen, M. Carrington, and M. Altfeld. 2007. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 204:3027-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, J. S., S. Bandi, D. S. Kaufman, and R. Akkina. 2006. Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy. Retrovirology 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase, H., N. Arase, and T. Saito. 1995. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J. Exp. Med. 181:1235-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandi, S., and R. Akkina. 2008. Human embryonic stem cell (hES) derived dendritic cells are functionally normal and are susceptible to HIV-1 infection. AIDS Res. Ther. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 7.Bonaparte, M. I., and E. Barker. 2004. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104:2087-2094. [DOI] [PubMed] [Google Scholar]
- 8.Boulet, S., R. Song, P. Kamya, J. Bruneau, N. H. Shoukry, C. M. Tsoukas, and N. F. Bernard. 2010. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J. Immunol. 184:2057-2064. [DOI] [PubMed] [Google Scholar]
- 9.Caligiuri, M. A. 2008. Human natural killer cells. Blood 112:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlsten, M., N. K. Bjorkstrom, H. Norell, Y. Bryceson, T. van Hall, B. C. Baumann, M. Hanson, K. Schedvins, R. Kiessling, H. G. Ljunggren, and K. J. Malmberg. 2007. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 67:1317-1325. [DOI] [PubMed] [Google Scholar]
- 11.Choi, K. D., M. A. Vodyanik, and I. I. Slukvin. 2009. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin−CD34+CD43+CD45+ progenitors. J. Clin. Invest. 119:2818-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi, K. D., J. Yu, K. Smuga-Otto, G. Salvagiotto, W. Rehrauer, M. Vodyanik, J. Thomson, and I. Slukvin. 2009. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27:559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung, A. W., E. Rollman, R. J. Center, S. J. Kent, and I. Stratov. 2009. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J. Immunol. 182:1202-1210. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 15.Fauci, A. S., D. Mavilio, and S. Kottilil. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5:835-843. [DOI] [PubMed] [Google Scholar]
- 16.Feng, Q., S. J. Lu, I. Klimanskaya, I. Gomes, D. Kim, Y. Chung, G. R. Honig, K. S. Kim, and R. Lanza. 2010. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 28:704-712. [DOI] [PubMed] [Google Scholar]
- 17.Freud, A. G., A. Yokohama, B. Becknell, M. T. Lee, H. C. Mao, A. K. Ferketich, and M. A. Caligiuri. 2006. Evidence for discrete stages of human natural killer cell differentiation in vivo. J. Exp. Med. 203:1033-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galic, Z., S. G. Kitchen, A. Kacena, A. Subramanian, B. Burke, R. Cortado, and J. A. Zack. 2006. T lineage differentiation from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 103:11742-11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gervaix, A., D. West, L. M. Leoni, D. D. Richman, F. Wong-Staal, and J. Corbeil. 1997. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc. Natl. Acad. Sci. U. S. A. 94:4653-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzywacz, B., N. Kataria, M. Sikora, R. A. Oostendorp, E. A. Dzierzak, B. R. Blazar, J. S. Miller, and M. R. Verneris. 2006. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood 108:3824-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta, N., J. Arthos, P. Khazanie, T. D. Steenbeke, N. M. Censoplano, E. A. Chung, C. C. Cruz, M. A. Chaikin, M. Daucher, S. Kottilil, D. Mavilio, P. Schuck, P. D. Sun, R. L. Rabin, S. Radaev, D. Van Ryk, C. Cicala, and A. S. Fauci. 2005. Targeted lysis of HIV-infected cells by natural killer cells armed and triggered by a recombinant immunoglobulin fusion protein: implications for immunotherapy. Virology 332:491-497. [DOI] [PubMed] [Google Scholar]
- 22.Haase, A. T. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464:217-223. [DOI] [PubMed] [Google Scholar]
- 23.Hu, B. Y., J. P. Weick, J. Yu, L. X. Ma, X. Q. Zhang, J. A. Thomson, and S. C. Zhang. 2010. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. U. S. A. 107:4335-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutter, G., D. Nowak, M. Mossner, S. Ganepola, A. Mussig, K. Allers, T. Schneider, J. Hofmann, C. Kucherer, O. Blau, I. W. Blau, W. K. Hofmann, and E. Thiel. 2009. Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N. Engl. J. Med. 360:692-698. [DOI] [PubMed] [Google Scholar]
- 25.Iannello, A., M. R. Boulassel, S. Samarani, O. Debbeche, C. Tremblay, E. Toma, J. P. Routy, and A. Ahmad. 2010. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 184:114-126. [DOI] [PubMed] [Google Scholar]
- 26.Iannello, A., O. Debbeche, S. Samarani, and A. Ahmad. 2008. Antiviral NK cell responses in HIV infection. I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J. Leukoc. Biol. 84:1-26. [DOI] [PubMed] [Google Scholar]
- 27.Iannello, A., O. Debbeche, S. Samarani, and A. Ahmad. 2008. Antiviral NK cell responses in HIV infection. II. Viral strategies for evasion and lessons for immunotherapy and vaccination. J. Leukoc. Biol. 84:27-49. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman, D. S. 2009. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood 114:3513-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman, D. S., E. T. Hanson, R. L. Lewis, R. Auerbach, and J. A. Thomson. 2001. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 98:10716-10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitchen, S. G., M. Bennett, Z. Galic, J. Kim, Q. Xu, A. Young, A. Lieberman, A. Joseph, H. Goldstein, H. Ng, O. Yang, and J. A. Zack. 2009. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PLoS One 4:e8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledran, M. H., A. Krassowska, L. Armstrong, I. Dimmick, J. Renström, R. Lang, S. Yung, M. Santibanez-Coref, E. Dzierzak, M. Stojkovic, R. A. J. Oostendorp, L. Forrester, and M. Lako. 2008. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell 3:85. [DOI] [PubMed] [Google Scholar]
- 32.Levy, J. A., A. D. Hoffman, S. M. Kramer, J. A. Landis, J. M. Shimabukuro, and L. S. Oshiro. 1984. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science 225:840-842. [DOI] [PubMed] [Google Scholar]
- 33.Martin, C. H., P. S. Woll, Z. Ni, J. C. Zuniga-Pflucker, and D. S. Kaufman. 2008. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood 112:2730-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin, M. P., X. Gao, J. H. Lee, G. W. Nelson, R. Detels, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, J. Trowsdale, M. Wilson, S. J. O'Brien, and M. Carrington. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31:429-434. [DOI] [PubMed] [Google Scholar]
- 35.Miller, J. S., Y. Soignier, A. Panoskaltsis-Mortari, S. A. McNearney, G. H. Yun, S. K. Fautsch, D. McKenna, C. Le, T. E. Defor, L. J. Burns, P. J. Orchard, B. R. Blazar, J. E. Wagner, A. Slungaard, D. J. Weisdorf, I. J. Okazaki, and P. B. McGlave. 2005. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105:3051-3057. [DOI] [PubMed] [Google Scholar]
- 36.Park, I. H., R. Zhao, J. A. West, A. Yabuuchi, H. Huo, T. A. Ince, P. H. Lerou, M. W. Lensch, and G. Q. Daley. 2008. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141-146. [DOI] [PubMed] [Google Scholar]
- 37.Richard, J., S. Sindhu, T. N. Pham, J. P. Belzile, and E. A. Cohen. 2010. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood 115:1354-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson, W. E., Jr., M. K. Gorny, J. Y. Xu, W. M. Mitchell, and S. Zolla-Pazner. 1991. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J. Virol. 65:4169-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg, S. A., N. P. Restifo, J. C. Yang, R. A. Morgan, and M. E. Dudley. 2008. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8:299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruggeri, L., M. Capanni, E. Urbani, K. Perruccio, W. D. Shlomchik, A. Tosti, S. Posati, D. Rogaia, F. Frassoni, F. Aversa, M. F. Martelli, and A. Velardi. 2002. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295:2097-2100. [DOI] [PubMed] [Google Scholar]
- 41.Scott-Algara, D., V. Arnold, C. Didier, T. Kattan, G. Pirozzi, F. Barre-Sinoussi, and G. Pancino. 2008. The CD85j+ NK cell subset potently controls HIV-1 replication in autologous dendritic cells. PLoS One 3:e1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slukvin, I. I., M. A. Vodyanik, J. A. Thomson, M. E. Gumenyuk, and K. D. Choi. 2006. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J. Immunol. 176:2924-2932. [DOI] [PubMed] [Google Scholar]
- 43.Tian, X., M. K. Hexum, V. R. Penchev, R. J. Taylor, L. D. Shultz, and D. S. Kaufman. 2009. Bioluminescent imaging demonstrates transplanted human embryonic stem cell-derived CD34+ cells preferentially develop into endothelial cells. Stem Cells 27:2675-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian, X., P. S. Woll, J. K. Morris, J. L. Linehan, and D. S. Kaufman. 2006. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells 24:1370-1380. [DOI] [PubMed] [Google Scholar]
- 45.Varela-Rohena, A., P. E. Molloy, S. M. Dunn, Y. Li, M. M. Suhoski, R. G. Carroll, A. Milicic, T. Mahon, D. H. Sutton, B. Laugel, R. Moysey, B. J. Cameron, A. Vuidepot, M. A. Purbhoo, D. K. Cole, R. E. Phillips, C. H. June, B. K. Jakobsen, A. K. Sewell, and J. L. Riley. 2008. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat. Med. 14:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vodyanik, M. A., J. A. Bork, J. A. Thomson, and I. I. Slukvin. 2005. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood 105:617-626. [DOI] [PubMed] [Google Scholar]
- 47.Wang, L., P. Menendez, F. Shojaei, L. Li, F. Mazurier, J. E. Dick, C. Cerdan, K. Levac, and M. Bhatia. 2005. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J. Exp. Med. 201:1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward, J., M. Bonaparte, J. Sacks, J. Guterman, M. Fogli, D. Mavilio, and E. Barker. 2007. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood 110:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward, J. P., M. I. Bonaparte, and E. Barker. 2004. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. AIDS 18:1769-1779. [DOI] [PubMed] [Google Scholar]
- 50.Woll, P. S., B. Grzywacz, X. Tian, R. K. Marcus, D. A. Knorr, M. R. Verneris, and D. S. Kaufman. 2009. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 113:6094-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woll, P. S., C. H. Martin, J. S. Miller, and D. S. Kaufman. 2005. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J. Immunol. 175:5095-5103. [DOI] [PubMed] [Google Scholar]
- 52.Yokoyama, W. M., S. Kim, and A. R. French. 2004. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22:405-429. [DOI] [PubMed] [Google Scholar]
- 53.Zamai, L., M. Ahmad, I. M. Bennett, L. Azzoni, E. S. Alnemri, and B. Perussia. 1998. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 188:2375-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]