Abstract
Growth hormone (GH) regulates both bone growth and remodeling, but it is unclear whether these actions are mediated directly by the GH receptor (GHR) and/or IGF-I signaling. The actions of GH are transduced by the Jak/Stat signaling pathway via Stat5, which is thought to regulate IGF-I expression. To determine the respective roles of GHR and IGF-I in bone growth and remodeling, we examined bones of wild-type, GHR knockout (GHR–/–), Stat5ab–/–, and GHR–/– mice treated with IGF-I. Reduced bone growth in GHR–/– mice, due to a premature reduction in chondrocyte proliferation and cortical bone growth, was detected after 2 weeks of age. Additionally, although trabecular bone volume was unchanged, bone turnover was significantly reduced in GHR–/– mice, indicating GH involvement in the high bone-turnover level during growth. IGF-I treatment almost completely rescued all effects of the GHR–/– on both bone growth and remodeling, supporting a direct effect of IGF-I on both osteoblasts and chondrocytes. Whereas bone length was reduced in Stat5ab–/– mice, there was no reduction in trabecular bone remodeling or growth-plate width as observed in GHR–/– mice, indicating that the effects of GH in bone may not involve Stat5 activation.
Introduction
Growth hormone (GH) regulates both increased bone size and mass during growth and is considered a major regulator of postnatal body growth (1). GH, accompanied by its binding protein (GHBP), regulates growth directly through the GH receptor (GHR) and indirectly by stimulating IGF-I expression (1). In the circulation, IGFs are bound to binding proteins (IGFBPs) that vary in their ability to prolong IGF half-life and may play a role in delivering them to target tissues. GHR activation also stimulates IGF-I expression in target cells (2). GH signals through the Jak/Stat signaling pathway via Jak2 tyrosine kinase phosphorylation of the two very homologous transcription factors, Stat5a and Stat5b (3). Jak2 activation may also lead to other signaling pathways implied in GH signaling, such as Src kinase, Ras/Raf/MAP kinase or insulin-receptor substrate 1 (IRS-1) or IRS-2 activation (4).
Within bone, a number of cell types responsible for bone growth and increased bone mass respond to both GH and IGF-I. In all, GH acts both directly and indirectly by regulating IGF or IGFBP production. Chondrocyte proliferation is stimulated directly by GH (5) and indirectly by increased local IGF-I production (6). GH stimulates osteoblast collagen production and proliferation directly (7, 8) and indirectly by increased IGF-I (9) and IGFBP production (9, 10). GH also stimulates bone resorption, although it is unclear whether this is direct or mediated by osteoblastic IGF-I (or IGFBP) production (11, 12). In short, IGF-I appears to be involved in GH effects on chondrocytes, osteoblasts, and osteoclasts, but it remains unclear whether these effects are local, dependent on direct GH, or which IGFBPs mediate these effects. The involvement of different signaling pathways in the GH and IGF-I effects on bone have not yet been analyzed, although it has been suggested that Stat5 mediates GH effects on bone growth (13).
In humans, Laron syndrome (GH insensitivity syndrome) is a hereditary dwarfism resulting from a variety of GHR mutations (14, 15). Affected patients are characterized by short stature, truncal obesity, low serum IGF-I, and elevated serum GH and GH resistance (15), and growth can be partially restored by IGF-I treatment (16, 17). Recently, Zhou et al. (18) reported a GHR/binding protein–knockout mouse model exhibiting severe postnatal growth retardation, proportionate dwarfism, absence of the GHR and GHBP, greatly decreased serum IGF-I, and elevated serum GH levels, thus representing the Laron phenotype. In addition, growth retardation has been described in the Stat5ab–/– model, suggesting that the Stat5 proteins mediate GH effects on bone growth (13).
The present study examines bone growth, structure, and turnover in normal, GHR–/–, and Stat5ab–/– mice, and GHR–/– mice treated with IGF-I, to determine the extent to which any alterations caused by GHR deficiency could be rescued by IGF-I treatment or the extent to which they are related to Stat5 activation.
Methods
Mouse generation and care.
Wild-type, GHR+/–, and GHR–/– littermates were bred from heterozygous mice with the genetic background Sv129Ola/Balb/c, and offspring were genotyped using PCR amplification as described previously (18). Wild-type and Stat5ab–/– littermates were on the background Sv129J/C57BL/6 and PCR was used to determine their genotype as described previously (13). Animal care was in accordance with institutional guidelines.
Histomorphometry and dual-energy x-ray absorptiometry.
Animals were injected with 20 mg/kg calcein and demeclocycline at 7-day intervals and sacrificed 2 days after the second injection. Bones were fixed in 3.7% formaldehyde and embedded in methylmethacrylate using a procedure modified from Baron et al. (19). Bones were dehydrated for 1 hour each in 70%, 90%, and twice in 100% acetone, then infiltrated and embedded in 85% activated methacrylate (20), 15% dibutylphthalate, and 4% benzoyl peroxide. Five-micrometer sections were stained with toluidine blue or coverslipped unstained for analysis. Histomorphometry was carried out according to standard procedures (21) in the proximal tibia using the Osteomeasure system (OsteoMetrics Inc., Atlanta, Georgia, USA). Marrow adipose volume was measured in the same region and is expressed as percentage of total marrow volume. Growth-plate widths were measured across at least 1.2 mm (using two sections for GHR–/– mice). Tibial cortical thickness and periosteal mineral apposition rate were measured along 1.02 mm, beginning 680 μm (400 μm for GHR–/–) below the chondro-osseous junction on the anterofibular side. Bone mineral density (BMD) and bone mineral content (BMC) were measured by dual-energy x-ray absorptiometry (DEXA) with a QDR 1000 instrument (Hologic Inc., Waltham, Massachusetts, USA) adapted for the mouse, as described previously (22). Coefficient of variation of three measurements on the same mouse was 1.4% for total tibia, 1.7% for proximal tibia, and 2.3% for the midshaft tibia. Statistical differences were determined by ANOVA and Fisher’s post hoc test. All values are expressed as means plus or minus SEM.
Western ligand blotting.
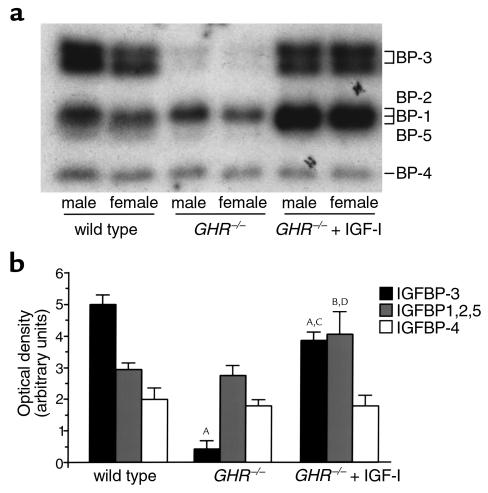
Recombinant human (h)IGF-I was iodinated using the iodogen method (Sigma-Aldrich, St. Quentin, France) and purified using Sephadex G-50 chromatography (23). Western ligand blotting was performed as described previously (24) and analyzed using densitometry (Storm; Molecular Dynamics, Rondoufle Cedex, France). Relative [125I]-IGF-I bound to IGFBPs was calculated from the integrated OD per sample. Bands at 44–45 kDa, 29–32 kDa, and 24 kDa correspond to IGFBP-3; IGFBP-2, -1, and -5; and IGFBP-4, respectively (24).
Serum and urinary biochemistry.
Serum IGF-I levels were determined after acid-ethanol extraction using a human IGF-I RIA kit (Nichols Diagnostic, Paris, France) (18). Serum osteocalcin and urinary deoxypyridinoline crosslinks were evaluated with mouse-specific kits (Biomedical Technologies, Stoughton, Massachusetts, USA, and Biometrics, Tampa, Florida, USA, respectively). Urinary deoxypyridinoline was divided by urinary creatinine concentration to correct for water excretion. Serum estradiol and testosterone were measured using human RIA kits (Immunotech, Paris, France). Serum estradiol was measured on the morning of estrus, which was confirmed by vaginal smears. Serum parathyroid hormone (PTH) was measured using a rat PTH RIA kit (Nichols Institute Diagnostic, Avon, France).
IGF-I treatment.
GHR–/– mice were treated with recombinant hIGF-I (Genentech Inc., South San Francisco, California, USA) using micropumps (ALZET, Palo Alto, California, USA) releasing 6 mg/kg/d for 14 days. Micropumps were inserted dorsally at 2 weeks of age and removed at 4 weeks of age (2–4 weeks) or inserted at 4 weeks of age (4–6 weeks) or at both 2 weeks and at 4 weeks of age (2–6 weeks). Serum was collected for IGF-I and IGFBP determination from 2- to 6-week-old mice when the implant was replaced at 4 weeks.
Results
Body and longitudinal bone growth in GHR–/– mice.
No radiographic evidence of skeletal malformation other than reduced size was seen in adult GHR–/– mice. No difference in the effect of GHR deficiency was detected between males and females, except where indicated, therefore data are combined for both genders.
Whereas body or femoral length were not altered until 2 weeks of age, GHR–/– mice exhibited reduced size compared with wild-type animals from this time onward (Figure 1a). Growth rate from 2 to 6 weeks of age was reduced in GHR–/– mice compared with wild-type mice (Figure 1a, left graph), and subsequent growth continued at equal rates in GHR–/– and wild-type mice.
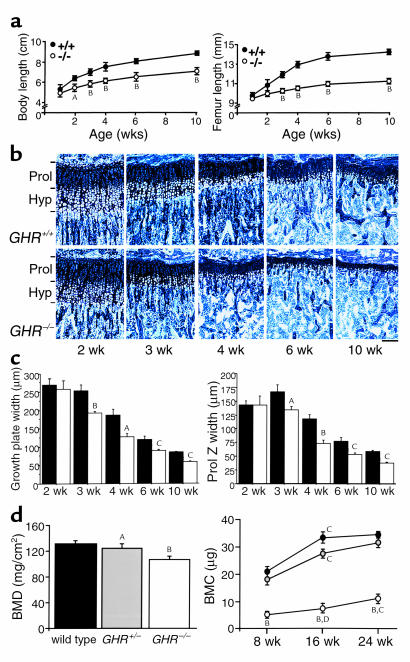
Figure 1.
Impaired growth of GHR–/– mice. (a) Growth curves of total body length (left) and femoral length (right) in wild-type (+/+) and GHR–/– (–/–) mice (n = 8 per group). AP < 0.01, and BP < 0.001 vs. wild-type. (b) Toluidine blue–stained proximal tibial sections from wild-type and GHR–/– mice at 2, 3, 4, 6, and 10 weeks. Horizontal bars indicate limits of the proliferative (Prol) and hypertrophic (Hyp) zones. Scale bar, 100 μm. (c) Growth-plate and proliferative-zone width (Prol Z width) were reduced in GHR–/– mice (open) versus wild-type littermates (filled); n = 8 per group. AP < 0.05, BP < 0.01, and CP < 0.001 vs. wild-type. (d) Total femoral BMD (left) in 16-week-old littermates in wild-type (filled), heterozygote (gray, GHR+/–), and knockout (open, GHR–/–) mice. Proximal femoral BMC (right) at 8, 16, and 24 weeks of age in wild-type (filled), GHR+/– (gray), and GHR–/– (open) mice (n = 8 per group). AP < 0.05 and BP < 0.0001 vs. wild-type; CP < 0.001 and DP < 0.01 vs. same transgene group at previous time point.
To further analyze the delayed growth in GHR–/– mice, growth-plate dynamics were studied (Figure 1b). Growth-plate organization remained normal; chondrocytes were arranged in columnar stacks and appeared to progress normally through hypertrophy, because there was no change in the ratio of hypertrophic to proliferative zones at any age (data not shown). After 2 weeks of age, however, the growth plate narrows earlier in GHR–/– mice compared with wild-type animals (Figure 1, b and c). The altered growth-plate width is characterized by reduced proliferative-zone width (Figure 1c).
Cortical bone growth in GHR–/– mice.
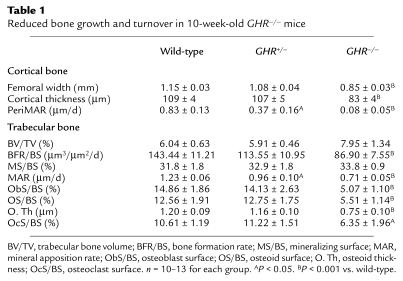
Cortical thickness (CoTh) was not altered at 2 or 3 weeks of age (mean CoTh in micrometers at 2 weeks ± SEM: wild-type, 53.4 ± 10.7; GHR–/–, 45.7 ± 5.0); reduced CoTh was first detected at 4 weeks (mean CoTh in micrometers at 4 weeks ± SEM: wild-type, 83.5 ± 8.0; GHR–/–, 57.9 ± 2.9; P < 0.01). Femoral width and tibial CoTh were reduced in 10-week-old GHR–/– mice (Table 1) and associated with reduced periosteal bone growth, indicated by periosteal mineral apposition rate (PeriMAR) (Table 1). In many GHR–/– mice, no periosteal growth was detected at 10 weeks of age; fluorochrome labels appeared in patches deposited at different intervals, rather than two labeled fronts as in control animals. In heterozygous mice, neither CoTh nor femoral width were altered, but PeriMAR was reduced at 10 weeks of age (Table 1).
Table 1.
Reduced bone growth and turnover in 10-week-old GHR–/– mice
Reduced BMD and BMC confirmed the reduced CoTh in GHR–/– mice (Figure 1d). In addition, the normal increase in BMC between 8 and 16 weeks was almost attenuated in GHR–/– mice (Figure 1d). To account for differences in trabecular and cortical bone distribution, the tibia was divided into three equal regions (22). In the proximal tibia, an area comprised of both cortical and trabecular bone, BMD was reduced by 16.1%, whereas in the diaphysis, which is almost exclusively cortical bone, BMD was reduced by 28.3%. Since bone mass is reduced mainly in the diaphysis, and CoTh is also reduced, the reduced BMD and BMC in GHR–/– mice is probably due to reduced cortical bone mineral.
Trabecular bone and bone turnover in GHR–/– mice.
Trabecular bone volume, number, and thickness were not altered in GHR–/– mice (Table 1 and data not shown); however, bone turnover was significantly reduced (Table 1). Trabecular bone-formation rate was dramatically reduced in GHR–/– and GHR+/– mice compared with wild-type littermates. No other bone-turnover parameters were significantly altered in heterozygote mice. Most histomorphometric markers of bone formation and resorption were reduced in GHR–/– mice (Table 1), but despite reduced osteoblast and osteoid surfaces, mineralizing surface was not altered in GHR–/– mice. Since osteoclast surface was reduced, fluorochrome labels remaining in the matrix longer than expected due to reduced bone resorption may have masked an altered mineralizing surface (25). Neither serum osteocalcin nor urinary deoxypyridinoline crosslinks were significantly altered (data not shown).
Circulating PTH was normal in GHR–/– mice (34.3 ± 9.5 pg/ml) versus wild-type mice (28.4 ± 7.8 pg/ml), indicating that bone turnover was not altered secondary to changed PTH levels. Estradiol and testosterone levels in GHR–/– and wild-type mice were normal (31.1 ± 9.3 pg/ml vs. 25.7 ± 6.1 pg/ml for estradiol and 2.37 ± 0.9 ng/ml vs. 2.84 ± 0.3 ng/ml for testosterone, respectively).
Marrow adipocyte volume was increased in male GHR–/– mice (mean percentage of marrow volume ± SEM: wild-type, 0.27 ± 0.25; GHR+/–, 0.53 ± 0.32; GHR–/–, 1.3 ± 0.54; GHR–/– significant at P < 0.05 vs. wild-type). This was not observed in female mice (data not shown).
Histomorphometric analysis of Stat5ab–/– mice.
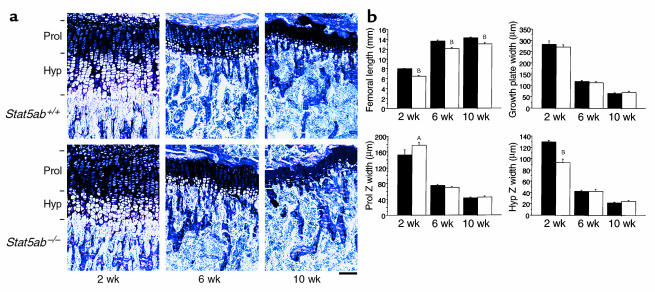
Stat5ab–/– mice were significantly smaller than wild-type littermates, as described previously (13). In contrast to GHR–/– mice, the difference in femur length was detected at 2 weeks, but was less severe in mature mice (Figure 2). In 10-week-old Stat5ab–/– mice, both femoral length and width were reduced to 90% of control, whereas in GHR–/– mice, femoral length and width were reduced to 75% of their control. The difference in femur length and width between Stat5ab–/– mice and GHR–/– mice, compared with their relative controls, was statistically significant (P < 0.0001 for both).
Figure 2.
Longitudinal bone-growth and growth-plate dynamics are altered in 2-week-old, but not mature, Stat5ab–/– mice. (a) Representative proximal tibial sections stained with toluidine blue from 2-, 6-, and 10-week-old wild-type and Stat5ab–/– mice. Horizontal bars indicate the limits of the proliferative (Prol) and hypertrophic (Hyp) zones. Scale bar, 100 μm. (b) Femoral length, femoral width, and widths of the proliferative and hypertrophic zones of the proximal tibial growth plate in wild-type and Stat5ab–/– mice at 2, 6, and 10 weeks of age; n = 6–12 per group. AP < 0.05, BP < 0.01 vs. control mice.
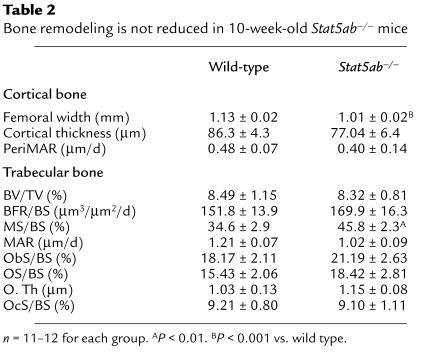
Reduced femoral length in Stat5ab–/– mice appeared to relate to a development defect, since irregularities in the growth plate were observed at 2 weeks, but not thereafter. At 2 weeks, while growth-plate width was unchanged in Stat5ab–/– mice, the proliferative-zone width was reduced while the hypertrophic-zone width was increased, suggesting reduced chondrocyte proliferation and delayed chondrocyte hypertrophy or apoptosis in the absence of Stat5 signaling (Figure 2b). Whereas tibial cortical width was reduced in both GHR–/– and Stat5ab–/– mice, periosteal bone growth was not reduced in Stat5ab–/– mice (Table 2).
Table 2.
Bone remodeling is not reduced in 10-week-old Stat5ab–/– mice
In direct contrast to GHR–/– mice, bone turnover was not reduced in Stat5ab–/– mice (Table 2). In fact, bone turnover was slightly higher than in wild-type mice, suggesting that the effect of GH on bone turnover is not mediated by Stat5 activation and compensatory signaling pathways exist in Stat5-deficient mice.
GH/IGF-I axis and IGFBPs in GHR–/– mice.
Serum IGF-I levels in GHR–/– mice were decreased by approximately 90% (mean ng/ml ± SEM: wild-type, 287 ± 21; GHR–/–, 37 ± 6; P < 0.0001). Western ligand blot analysis suggested reduced IGFBP-3 levels in GHR–/– sera (Figure 3). Wild-type serum exhibited a doublet at 44–45 kDa (IGFBP-3), poorly defined bands at 29, 30, and 32 kDa (IGFBP-5, IGFBP-1, and IGFBP-2, respectively), and one band at 24 kDa (IGFBP-4), as described previously (24) (Figure 3a). In contrast, GHR–/– sera demonstrated very little IGFBP-3 binding to IGF-I, suggesting reduced IGFBP-3 levels (Figure 3a). There were no differences in the binding of other IGFBPs between GHR–/– and wild-type mice (Figure 3b).
Figure 3.
Reduced IGFBP-3 profile in GHR–/– mouse serum and partial restoration by IGF-I treatment. (a) Western ligand blot of serum from 6-week-old wild-type, GHR–/–, and GHR–/– mice treated with IGF-I for 2 weeks (GHR–/– + IGF-I). (b) Densitometric analysis of IGF-binding activity; male and female results combined. AP < 0.001, BP < 0.01 vs. wild-type, and CP < 0.001, DP < 0.01 vs. GHR–/–.
Effects of IGF-I treatment in GHR–/– mice: IGF/GH axis and IGFBP levels.
IGF-I treatment restored serum IGF-I to 207.2 ± 19.0 ng/ml, compared with 37 ng/ml in GHR–/– mice, but still lower than levels in wild-type mice (approximately 287 ng/ml). Therefore, treatment was sufficient to increase circulating IGF-I by 5.6-fold compared with GHR–/– mice and only 25% less than in wild-type mice.
Reduced IGF-I binding by IGFBP-3 in GHR–/– mice was partially restored by IGF-I treatment (Figure 3, a and b). IGF-I binding to the IGFBP-1, -2, or -5 was also increased, but it is not possible to determine which IGFBP is elevated by this technique.
IGF-I treatment restores longitudinal growth in GHR–/– mice.
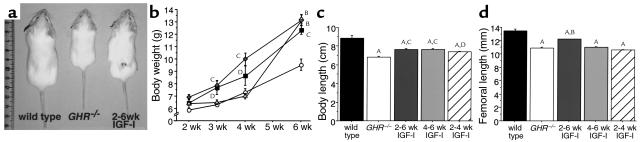
All IGF-I–treated mice were larger than untreated GHR–/– mice, but not fully restored to normal size (Figure 4, a and b). Body weight and length at 6 weeks was increased in all IGF-I–treated animals (Figures 4, b and c). Femoral length was significantly increased only in mice treated with IGF-I from 2 to 6 weeks (Figure 4b).
Figure 4.
Body size and bone length are increased in GHR–/– mice after IGF-I treatment. (a) Representative photograph of 6-week-old wild-type (left), GHR–/– (center), and GHR–/– treated with IGF-I (6 mg/kg/d) from age 2 to 6 weeks (right). (b) Body weight in untreated (open circles) and IGF-I–treated knockout mice. Mice were treated with IGF-I from weeks 2–6 (dark gray square), 4–6 (light gray triangle), or 2–4 (gray diamond). (c) Body length at 6 weeks of age was increased by all IGF-I treatment regimens. (d) Femoral length was significantly increased only in mice treated for 4 weeks. n = 5–10 per group. AP < 0.001 vs. wild-type, BP < 0.001, CP < 0.01, DP < 0.05 vs. GHR–/–.
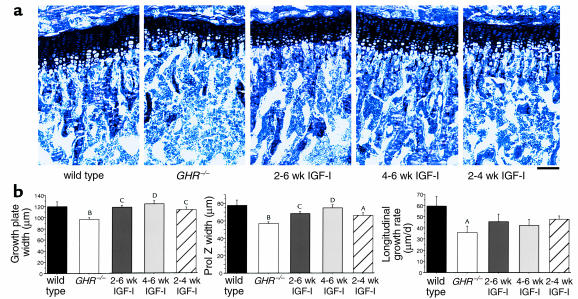
Both growth-plate and proliferative-zone width were fully restored in GHR–/– mice treated with IGF-I until 6 weeks of age, but not when treatment was discontinued at 4 weeks (Figure 5, a and b). Even when IGF-I treatment was commenced at 4 weeks (when chondrocyte proliferation is reduced by 60% in GHR–/– mice) growth-plate and proliferative-zone width were increased. While striking, this was insufficient to increase femoral length relative to GHR–/– mice, probably because growth rate is at a low level for both genotypes between 4 and 6 weeks.
Figure 5.
Chondrocyte proliferation is restored in GHR–/– mice treated with IGF-I. (a) Representative proximal tibial sections stained with toluidine blue from 6-week-old wild-type, GHR–/–, and GHR–/– mice treated with IGF-I from weeks 2–6, 4–6, and 2–4. Scale bar, 100 μm. (b) Tibial growth-plate width, proliferative-zone width, and longitudinal growth rate were increased in IGF-I–treated mice. n = 4–10 per group. AP < 0.01, BP < 0.001 vs. wild-type, CP < 0.05, DP < 0.01 vs. GHR–/–.
IGF-I treatment restores cortical growth and trabecular bone turnover in GHR–/– mice.
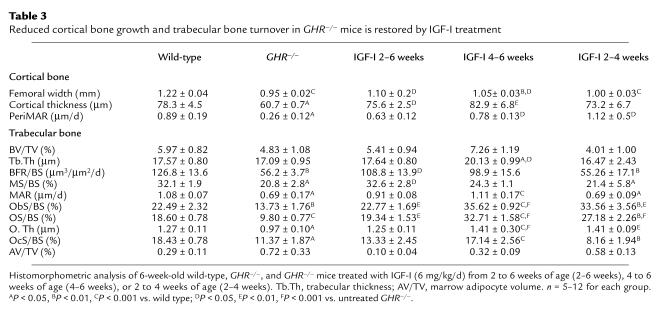
As at 10 weeks, femoral and cortical width and PeriMAR were all reduced in 6-week-old GHR–/– mice (Table 3). In all IGF-I–treated groups, PeriMAR and CoTh were no longer lower than in wild-type mice, but femoral width was restored only in mice treated for 4 weeks (Table 3).
Table 3.
Reduced cortical bone growth and trabecular bone turnover in GHR–/– mice is restored by IGF-I treatment
Bone turnover was reduced in 6-week-old GHR–/– mice (Table 3). IGF-I treatment restored bone turnover in GHR–/– mice, and in the 4- to 6-week-old and 2- to 4-week-old treated mice osteoblast and osteoid surface was increased above that of wild-type mice (Table 3). The 4- to 6-week-old treated mice demonstrated a dramatic increase in bone formation, such that trabecular bone volume and trabecular thickness were slightly elevated. Fluorochrome markers of bone formation (bone formation rate and mineralizing surface) were restored only in mice treated for 4 weeks, probably because these are measured over a 7-day period. The slight increase in trabecular bone volume and trabecular thickness may indicate a more potent effect on bone formation (Table 2), given that IGF-I–induced increases in osteoclast surface were quite modest (Table 3). When treatment was suspended at 4 weeks, most histomorphometric indicators of bone turnover were equivalent to levels in GHR–/– mice by 6 weeks of age, except for osteoblast or osteoid surface (Table 3).
Adipocyte volume was slightly, but not significantly, increased in 6-week-old male GHR–/– mice. While GHR status did not affect adipocyte volume in females, adipocyte volume was slightly reduced by IGF-I treatment in all GHR–/– mice (Table 3). Similarly, the ratio of body fat to total body weight was elevated in 6-week-old GHR–/– mice and reduced by IGF-I treatment. (Mean ratio ± SEM: wild-type, 0.77 ± 0.09; GHR–/–, 1.89 ± 0.11A; 2–6 weeks, 0.80 ± 0.12B; 4–6 weeks: 0.32 ± 0.08A,B; 2–4 weeks: 1.36 ± 0.12A. AP < 0.05 vs. wild-type; BP < 0.05 vs. GHR–/–.)
Discussion
It is well known that GH plays an important role in longitudinal bone growth, because bone growth is impaired both in GH-deficient humans (14, 15) and in the GHR–/– mouse (18, 26). Although the growth plate does not fully close in rodents as it does in humans, reduced bone length in GHR–/– mice is associated with premature growth-plate contraction and reduced chondrocyte proliferation. This is consistent with GH-stimulated chondrocyte-colony formation in vitro (27) and growth-plate narrowing with age, associated with reduced circulating GH, IGF-I, and IGFBP3 (28) and reduced local IGF-I (29). Reduced chondrocyte proliferation in the GHR–/– mouse is not detectable until 3 weeks of age; before this, bone growth proceeded normally, confirming that GH is not required for normal murine prenatal development or early postnatal growth (30). This is consistent with low serum IGF-I and GH levels in fetal and early postnatal development (2) and low IGF-I expression in fetal growth plates (29). Maternal IGFs and IGFBPs may play a role in developmental and postnatal growth before weaning (3 weeks of age) in normal and GHR–/– mice (30).
While hIGF-I treatment of GHR–/– mice did not completely prevent growth retardation, femoral length was greater in treated GHR–/– mice. Similarly, growth-plate and proliferative-zone widths in treated GHR–/– mice were equivalent to wild-type mice at 6 weeks. This is consistent with IGF-I–stimulated chondrocyte proliferation in vitro (27) and IGF-I–stimulated growth in rodents and humans (16, 17, 31). When treatment was suspended at 4 weeks, increased body length, but not proliferative-zone width, was maintained until 6 weeks of age, suggesting that continued IGF-I treatment is needed to maintain normal chondrocyte proliferation in GHR–/– mice. Thus, IGF-I can prevent the early reduction in chondrocyte proliferation in GHR–/– mice and restore longitudinal bone growth if treatment is continued, supporting a direct effect of IGF-I on chondrocyte proliferation. Circulating IGF-I levels in our studies remained at about 75% of normal at 4 weeks of postnatal growth. This suggests that early recombinant IGF-I treatment can compensate for the absence of GHR and that administration to human patients should begin before atrophy of the growth-plate starts. Thus, earlier treatment and stabilized circulating IGF-I levels could be useful in the treatment of retarded growth due to malfunction of the GH system in humans.
While longitudinal growth was impaired in Stat5ab–/– mice, this occurred earlier and was less severe in mature mice than in GHR–/– animals. Similarly, there was no difference in growth-plate or proliferative-zone width in mature Stat5ab–/– mice. Rather, the reduced growth in Stat5ab–/– mice appears to relate to a developmental or early postnatal defect in endochondral ossification since altered growth-plate organization was observed at 2 weeks, but not thereafter. Since IGF-I levels in Stat5ab–/– mice are reduced to only 50% of control (13) and the growth-plate defect occurs while maternal IGF-I is still available, IGF-I treatment alone may be insufficient to counteract the growth defect in Stat5ab–/– mice. The altered ratio of proliferative- to hypertrophic-zone width suggests reduced chondrocyte proliferation and delayed chondrocyte hypertrophy/apoptosis in the absence of Stat5 signaling. It also indicates that regulation of chondrocyte proliferation by GH may not be mediated through Stat5 only or that redundant pathways exist, probably emanating from Jak2 activation, which phosphorylates multiple substrates.
Cortical bone is widened by osteoblastic bone formation on the outer bone surface (the periosteum) during growth until late adulthood in humans and rodents. Consistent with effects of GHR deficiency on longitudinal growth, impaired cortical growth was not detected before 4 weeks of age in GHR–/– mice, indicating an important role of GH in postdevelopmental bone modeling through mesenchymal-derived periosteal osteoblasts. In most 10-week-old GHR–/– mice, no mineral apposition could be detected at all, whereas this growth continues until 40 weeks in wild-type mice of this strain (unpublished observations), suggesting that periosteal growth has prematurely ceased in GHR–/– mice. In contrast, while cortical width was reduced in Stat5ab–/– mice, periosteal bone growth was not, suggesting that this GHR effect is also not mediated through Stat5.
Recombinant hIGF-I treatment restored periosteal growth in GHR–/– mice, although femoral width was significantly elevated only after 4 weeks of treatment, demonstrating that IGF-I stimulates bone formation by mesenchymal-derived osteoblasts.
Reduced cortical growth in GHR–/– mice indicates a strong anabolic effect of GH on cortical bone mass, consistent with reduced midshaft BMD and BMC reported here. This confirms previous reports in humans and rodents (26, 32), but also demonstrates that the low cortical bone mass in GHR–/– mice relates to an early cessation of periosteal bone growth. Previous densitometry studies suggested reduced trabecular bone mass in GH deficiency (26, 32), while others suggest this relates to reduced cortical width, since most mouse-bone densitometry techniques do not adequately account for altered bone size (33). Here we specifically measure trabecular bone volume histomorphometrically and observe that while cortical bone mass is reduced, trabecular bone volume is not altered in GHR–/– mice.
Trabecular bone remodeling is markedly reduced in GHR–/– mice, again reflecting effects of aging (34). While reduced bone turnover with age occurs simultaneously with reduced circulating GH, IGF-I, and IGFBP-3 levels (28), it has been difficult to determine a direct relationship between these variables.
Reduced osteoblast surface in GHR–/– mice suggests impaired osteoblast proliferation or life span and is consistent with direct GH stimulation of osteoblast proliferation in vitro and in vivo (8, 35). Reduced mineral appositional rate and osteoid thickness are also consistent with GH-stimulated collagen production by mature osteoblasts (7). Few studies have measured bone turnover in patients with Laron syndrome, but biochemical markers of bone formation are lowered in adults and children with Laron syndrome (36). Osteoclast function in GH deficiency has not been reported in either rodent models or humans; we note here a significant reduction in osteoclast surface. Recent studies demonstrate that while osteoblasts mediate GH stimulation of osteoclast proliferation, osteoclastic bone resorption can also be stimulated directly by GH (11, 12). It remains unclear, however, whether the reduced bone resorption in GHR–/– mice reflects a loss of direct GH stimulation or relates to coupling of osteoclast proliferation to the low level of bone formation in these mice.
As with other skeletal effects, reduced bone formation in GHR–/– mice was rescued by hIGF-I treatment. This is consistent with IGF-I stimulation of osteoblast proliferation and function in vitro and in vivo (37, 38) and increased markers of bone formation in IGF-I–treated women (39) and patients with Laron syndrome (36). These observations suggest that reduced bone formation in GHR–/– mice may reflect not only the loss of effective GH, but also reduced IGF-I levels. IGF-I treatment affects osteoclast function less dramatically: as in humans, where only the highest dose increased bone resorption (40), osteoclast surface was increased only in the 4 to 6–week treatment group and did not compensate for the increased bone formation, such that trabecular bone volume increased. While local IGF-I perfusion increases bone formation in rats, but either does not change (38) or reduces osteoclast surface (41), the present study suggests IGF-I may increase osteoclast number by an endocrine or paracrine mechanism rather than by a direct effect on osteoclasts.
Bone turnover was not reduced in Stat5ab–/– mice, but was slightly increased, suggesting that Stat5 is not the only mediator of GH in osteoblasts and osteoclasts. Trabecular bone formation is also reduced in the prolactin-receptor knockout (PrlR–/–) mouse (22). The PrlR is structurally similar to the GHR, and PrlR signaling is disturbed in mice lacking Stat5, indicated by mammary gland and ovarian defects in Stat5ab–/– mice (13) which are also observed in the PrlR–/– mouse (42). Altered expression of liver enzymes in Stat5ab–/– mice also demonstrates Stat5 involvement in GH signaling in the liver (13). Taken together, these results suggest that while Stat5 is essential for prolactin and GH signaling in other tissues, the effects of prolactin and GH in bone are not mediated by Stat5 only, but may involve other signaling cascades such Ras/Raf/MAP kinase or the adaptor proteins IRS-1 and IRS-2 (4).
Increased marrow adiposity in adult male GHR–/– mice is consistent with reduced adiposity after short-term GH treatment in a male patient (43), and GH inhibited adipogenesis in vitro (44). Surprisingly, marrow adiposity was not altered in female GHR–/– mice, suggesting that testosterone, while unchanged in GHR–/– mice, may be involved in this effect. Recombinant hIGF-I treatment reduced marrow adiposity and the ratio of body fat to total body weight in GHR–/– mice, indicating IGF-I mediation of this GH effect also.
In GHR–/– mice, serum IGF-I decreased to approximately 10% of wild-type levels, whereas Stat5ab–/– IGF-I levels are approximately 50% of wild-type (13). Since partial restoration of IGF-I levels in GHR–/– mice almost completely rescues the effects of the knockout on bone growth and remodeling, the difference in bone phenotype between the GHR- and Stat5-deficient mice may relate solely to the higher circulating IGF-I level in the Stat5ab–/– mice. This further supports a major role for IGF-I in mediating GH effects in bone and suggests that pathways other than Stat5 may be important for GH stimulation of IGF-I production.
In GHR–/– mice, IGFBP-3 is virtually absent, resulting in an altered ratio between the different binding proteins, as in Laron syndrome (45). IGF-I treatment increased both serum IGFBP-3 and one of the IGFBPs, IGFBP-1, -2, or -5, probably IGFBP-2. This is consistent with GH and IGF-I treatment of Laron syndrome (45, 46) and suggests that IGF-I alone can partially restore the IGFBP profile, though more detailed studies remain to be done.
This study clearly demonstrates that while cortical and longitudinal bone growth and bone turnover are all reduced in GHR deficiency, many of these effects can be substantially reversed by IGF-I treatment, suggesting that the main defect may relate to reduced IGF-I levels in the absence of GHR. Since longitudinal bone growth was not affected in the liver-specific IGF-I–knockout mouse (47, 48), locally produced IGF-I and/or direct effects of GH may substitute for deficient systemic IGF-I. Although the actions of GH on a range of cell types may be mediated by Stat5 signaling, the bone phenotypes in GHR- and Stat5-knockout animals are surprisingly different, suggesting that the effects of GH on bone, whether mediated directly or through IGF-I, are not mediated by the Stat5 transcription factors but by other cytokine or signaling cascades.
Acknowledgments
We thank Jennifer Juel and Nancy Troiano for histological sectioning and staining, James Ihle for helpful discussions, and Genentech Inc. for providing recombinant hIGF-I. The excellent secretarial assistance of Claudine Coridun is greatly appreciated. This work was partially supported by grants from INSERM and Hoechst Marion Roussel (to P.A. Kelly) and NIH grant DE-04724 (to R. Baron).
Footnotes
Natalie A. Sims and Philippe Clément-Lacroix contributed equally to this work.
Roland Baron and Paul A. Kelly are co-senior authors.
Philippe Clément-Lacroix’s present address is: Hoechst Marion Roussel France, Romainville, France.
References
- 1.Ohlsson C, Bengtsson BA, Isaksson OG, Adreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 2.Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 3.Wood TJ, et al. Mediation of growth hormone-dependent transcriptional activation by mammary gland factor/Stat5. J Biol Chem. 1995;270:9448–9453. doi: 10.1074/jbc.270.16.9448. [DOI] [PubMed] [Google Scholar]
- 4.Carter-Su C, Smit LS. Signaling via JAK tyrosine kinases: growth hormone receptor as a model system. Recent Prog Horm Res. 1998;53:61–82. [PubMed] [Google Scholar]
- 5.Isaksson OG, Jansson JO, Gause IA. Growth hormone stimulates longitudinal bone growth directly. Science. 1982;216:1459–1464. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- 6.Isgaard J, Moller C, Isaksson OG, Nilsson A. Regulation of insulin-like growth factor messenger ribonucleic acid in rat growth plate by growth hormone. Endocrinology. 1988;122:1515–1520. doi: 10.1210/endo-122-4-1515. [DOI] [PubMed] [Google Scholar]
- 7.Morel G, et al. Evidence for a direct effect of growth hormone on osteoblasts. Cell Tissue Res. 1993;273:279–286. doi: 10.1007/BF00312829. [DOI] [PubMed] [Google Scholar]
- 8.Kassem M, Mosekilde L, Eriksen EF. Growth hormone stimulates proliferation of normal human bone marrow stromal osteoblast precursor cells in vitro. Growth Regul. 1994;4:131–135. [PubMed] [Google Scholar]
- 9.Ernst M, Rodan GA. Increased activity of insulin-like growth factor (IGF) in osteoblastic cells in the presence of growth hormone (GH): positive correlation with the presence of the GH-induced IGF-binding protein BP-3. Endocrinology. 1990;127:807–814. doi: 10.1210/endo-127-2-807. [DOI] [PubMed] [Google Scholar]
- 10.Mohan S, et al. Studies on regulation of insulin-like growth factor binding protein (IGFBP)-3 and IGFBP-4 production in human bone cells. Acta Endocrinol. 1992;127:555–564. doi: 10.1530/acta.0.1270555. [DOI] [PubMed] [Google Scholar]
- 11.Guicheux J, et al. Growth hormone stimulatory effects on osteoclastic resorption are partly mediated by insulin-like growth factor I: an in vitro study. Bone. 1998;22:25–31. doi: 10.1016/s8756-3282(97)00224-x. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama K, et al. Stimulatory effect of growth hormone on bone resorption and osteoclast differentiation. Endocrinology. 1996;137:35–41. doi: 10.1210/endo.137.1.8536635. [DOI] [PubMed] [Google Scholar]
- 13.Teglund S, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 14.Laron Z, Pertzelan A, Mannheimer S. Genetic pituitary dwarfism with high serum concentration of growth hormone: a new inborn error of metabolism? Isr J Med Sci. 1966;2:152–155. [PubMed] [Google Scholar]
- 15.Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev. 1994;15:369–390. doi: 10.1210/edrv-15-3-369. [DOI] [PubMed] [Google Scholar]
- 16.Ranke MB, et al. Insulin-like growth factor I improves height in growth hormone insensitivity: two years’ results. Horm Res. 1993;44:253–264. doi: 10.1159/000184637. [DOI] [PubMed] [Google Scholar]
- 17.Guevara-Aguirre J, et al. Two-year treatment of growth hormone (GH) receptor deficiency with recombinant insulin-like growth factor I in 22 children: comparison of two dosage levels and to GH-treated GH deficiency. J Clin Endocrinol Metab. 1997;82:629–633. doi: 10.1210/jcem.82.2.3743. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci USA. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baron, R., Vignery, A., Neff, L., Silverglate, A., and Maria, A.S. 1983. Processing of undecalcified bone specimens for bone histomorphometry. In Bone histomorphometry: technique and interpretation. R.R. Recker, editor. CRC Press. Boca Raton, Florida, USA. 13–35.
- 20.Jowsey J, et al. Quantitative microradiographic studies of normal and osteoporotic bone. J Bone Joint Surg Am. 1965;47A:785–806. [PubMed] [Google Scholar]
- 21.Parfitt AM, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 22.Clement-Lacroix P, et al. Osteoblasts are a new target for prolactin: analysis of bone. Endocrinology. 1999;140:96–105. doi: 10.1210/endo.140.1.6436. [DOI] [PubMed] [Google Scholar]
- 23.Kelly, P.A., et al. 1993. Binding and signal transduction of prolactin and growth hormone receptor. In Molecular and clinical advances in pituitary disorders. S. Melmed, editor. Endocrine Research and Education Inc. Los Angeles, California, USA. 255–259.
- 24.Monget P, Monniaux D, Pisselet C, Durand P. Changes in insulin-like growth factor-I (IGF-I), IGF-II, and their binding proteins during growth and atresia of ovine ovarian follicles. Endocrinology. 1993;132:1438–1446. doi: 10.1210/endo.132.4.7681760. [DOI] [PubMed] [Google Scholar]
- 25.Turner RT, Evans GL, Wakley GK. Mechanism of action of estrogen on cancellous bone balance in tibiae of ovariectomized growing rats: inhibition of indices of formation and resorption. J Bone Miner Res. 1993;8:359–366. doi: 10.1002/jbmr.5650080313. [DOI] [PubMed] [Google Scholar]
- 26.Sjogren K, et al. Disproportional skeletal growth and markedly decreased bone mineral content in growth hormone receptor –/– mice. Biochem Biophys Res Commun. 2000;267:603–608. doi: 10.1006/bbrc.1999.1986. [DOI] [PubMed] [Google Scholar]
- 27.Lindahl A, Isgaard J, Carlsson L, Isaksson OGP. Differential effects of growth hormone and insulin-like growth factor I on colony formation of epiphyseal chondrocytes in suspension culture in rats of different ages. Endocrinology. 1987;121:1061–1069. doi: 10.1210/endo-121-3-1061. [DOI] [PubMed] [Google Scholar]
- 28.Kelijman M. Age-related alterations of the growth hormon/insulin-like-growth-factor I axis. J Am Geriatr Soc. 1991;39:295–307. doi: 10.1111/j.1532-5415.1991.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 29.Shinar DM, Endo N, Haperin D, Rodan GA, Weinreb M. Differential expression of insulin-like growth factor-I (IGF-I) and IGF-II messenger ribonucleic acid in growing rat bone. Endocrinology. 1993;132:1158–1167. doi: 10.1210/endo.132.3.8440176. [DOI] [PubMed] [Google Scholar]
- 30.Evain-Brion D. Hormonal regulation of fetal growth. Horm Res. 1994;42:207–214. doi: 10.1159/000184195. [DOI] [PubMed] [Google Scholar]
- 31.Guler HP, Zapf J, Scheiwiller E, Froesch ER. Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci USA. 1988;85:4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreassen TT, Jorgensen PH, Flyvbjerg A, Orskov H, Oxlund H. Growth hormone stimulates bone formation and strength of cortical bone in aged rats. J Bone Miner Res. 1995;10:1057–1067. doi: 10.1002/jbmr.5650100710. [DOI] [PubMed] [Google Scholar]
- 33.Bachrach LK, et al. Bone mineral, histomorphometry, and body composition in adults with growth hormone receptor deficiency. J Bone Miner Res. 1998;13:415–421. doi: 10.1359/jbmr.1998.13.3.415. [DOI] [PubMed] [Google Scholar]
- 34.Liang CT, et al. Impaired bone activity in aged rats: alterations at the cellular and molecular levels. Bone. 1992;13:435–441. doi: 10.1016/8756-3282(92)90087-d. [DOI] [PubMed] [Google Scholar]
- 35.Brixen K, et al. Short-term treatment with growth hormone stimulates osteoblastic and osteoclastic activity in osteopenic postmenopausal women: a dose response study. J Bone Miner Res. 1995;10:1865–1874. doi: 10.1002/jbmr.5650101205. [DOI] [PubMed] [Google Scholar]
- 36.Laron Z, Klinger B. IGF-I treatment of adult patients with Laron syndrome: preliminary results. Clin Endocrinol Metab. 1994;41:631–638. doi: 10.1111/j.1365-2265.1994.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 37.Langdahl BL, Kassem M, Moller MK, Eriksen EF. The effects of IGF-I and IGF-II on proliferation and differentiation of human osteoblasts and interactions with growth hormone. Eur J Clin Invest. 1998;28:176–183. doi: 10.1046/j.1365-2362.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 38.Wakisaka A, Tanaka H, Barnes J, Liang CT. Effect of locally infused IGF-I on femoral gene expression and bone turnover activity in old rats. J Bone Miner Res. 1998;13:13–19. doi: 10.1359/jbmr.1998.13.1.13. [DOI] [PubMed] [Google Scholar]
- 39.Ghiron LJ, et al. Effects of recombinant insulin-like growth factor-I and growth hormone on bone turnover in elderly women. J Bone Miner Res. 1995;10:1844–1852. doi: 10.1002/jbmr.5650101203. [DOI] [PubMed] [Google Scholar]
- 40.Ebeling PR, Jones JD, O’Fallon WM, Janes CH, Riggs BL. Short-term effects of recombinant human insulin-like growth factor I on bone turnover in normal women. J Clin Endocrinol Metab. 1993;777:1384–1387. doi: 10.1210/jcem.77.5.8077337. [DOI] [PubMed] [Google Scholar]
- 41.Spencer EM, Liu CC, Si EC, Howard GA. In vivo actions of insulin-like growth factor (IGF-I) on bone formation and resorption in rats. Bone. 1991;12:21–26. doi: 10.1016/8756-3282(91)90050-s. [DOI] [PubMed] [Google Scholar]
- 42.Ormandy CJ, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 43.Kroger H, Soppi E, Loveridge N. Growth hormone, osteoblasts, and marrow adipocytes: a case report. Calcif Tissue Int. 1997;61:33–35. doi: 10.1007/s002239900289. [DOI] [PubMed] [Google Scholar]
- 44.Hansen LH, Madsen B, Teisner B, Nielsen JH, Billestrup N. Characterization of the inhibitory effect of growth hormone on primary preadipocyte differentiation. Mol Endocrinol. 1998;12:1140–1149. doi: 10.1210/mend.12.8.0154. [DOI] [PubMed] [Google Scholar]
- 45.Ono T, Kanzaki S, Seino Y, Baylink DJ, Mohan S. Growth hormone (GH) treatment of GH-deficient children increases serum levels of insulin-like growth factors (IGFs), IGF-binding protein-3 and -5, and bone alkaline phosphatase isoenzyme. J Clin Endocrinol Metab. 1996;81:2111–2116. doi: 10.1210/jcem.81.6.8964836. [DOI] [PubMed] [Google Scholar]
- 46.Kanety H, et al. Long-term effects of insulin-like growth factor (IGF)-I on serum IGF-I, IGF-binding protein-3 and acid labile subunit in Laron syndrome patients with normal growth hormone binding protein. Eur J Endocrinol. 1997;137:626–630. doi: 10.1530/eje.0.1370626. [DOI] [PubMed] [Google Scholar]
- 47.Yakar S, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjogren K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]