Abstract
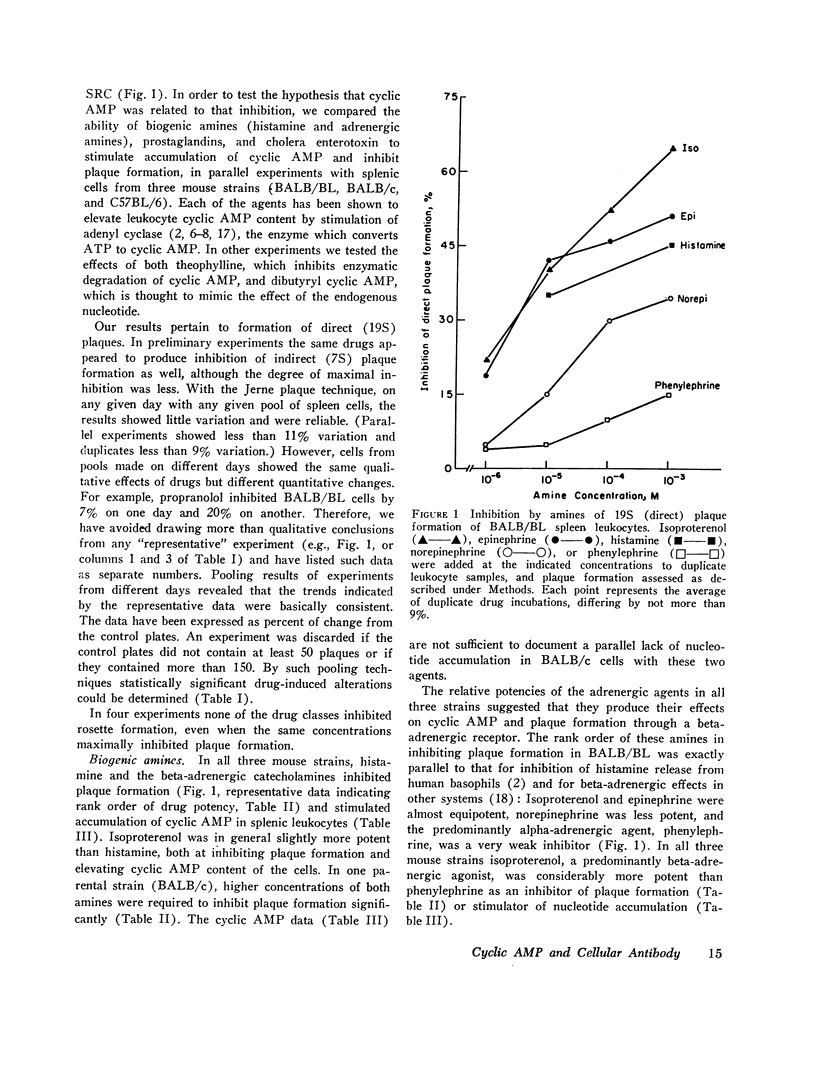
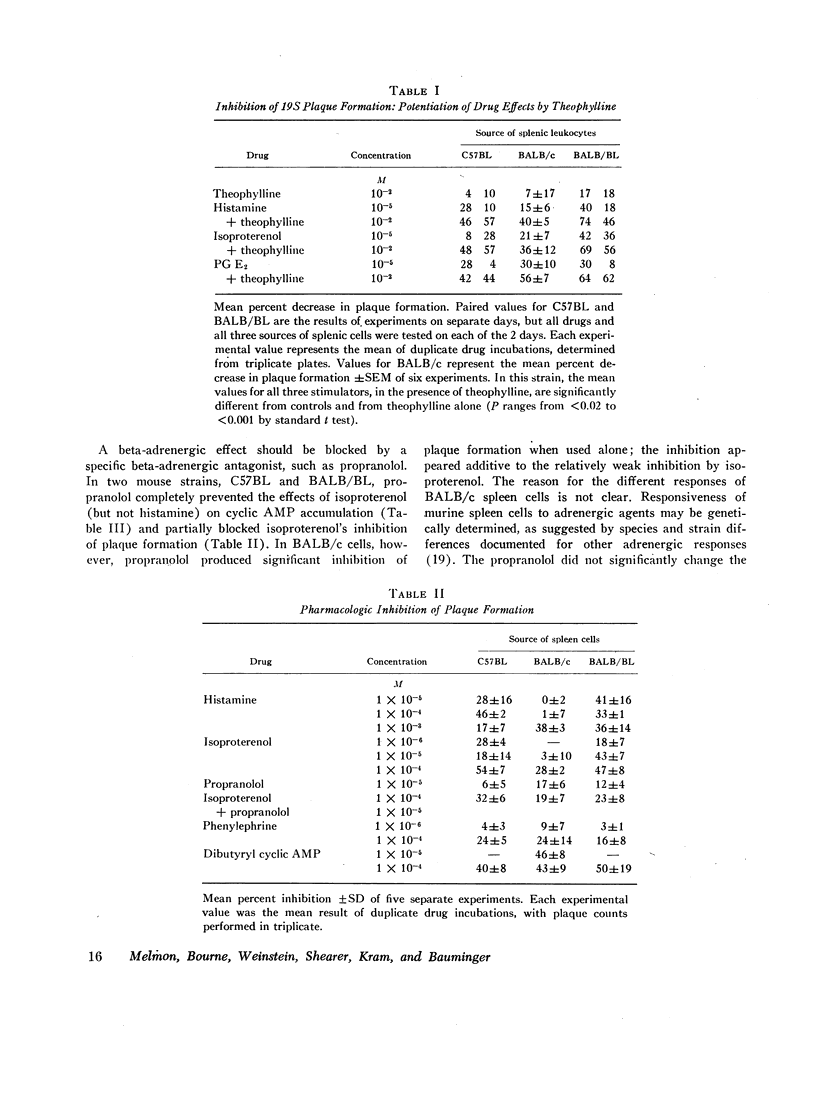
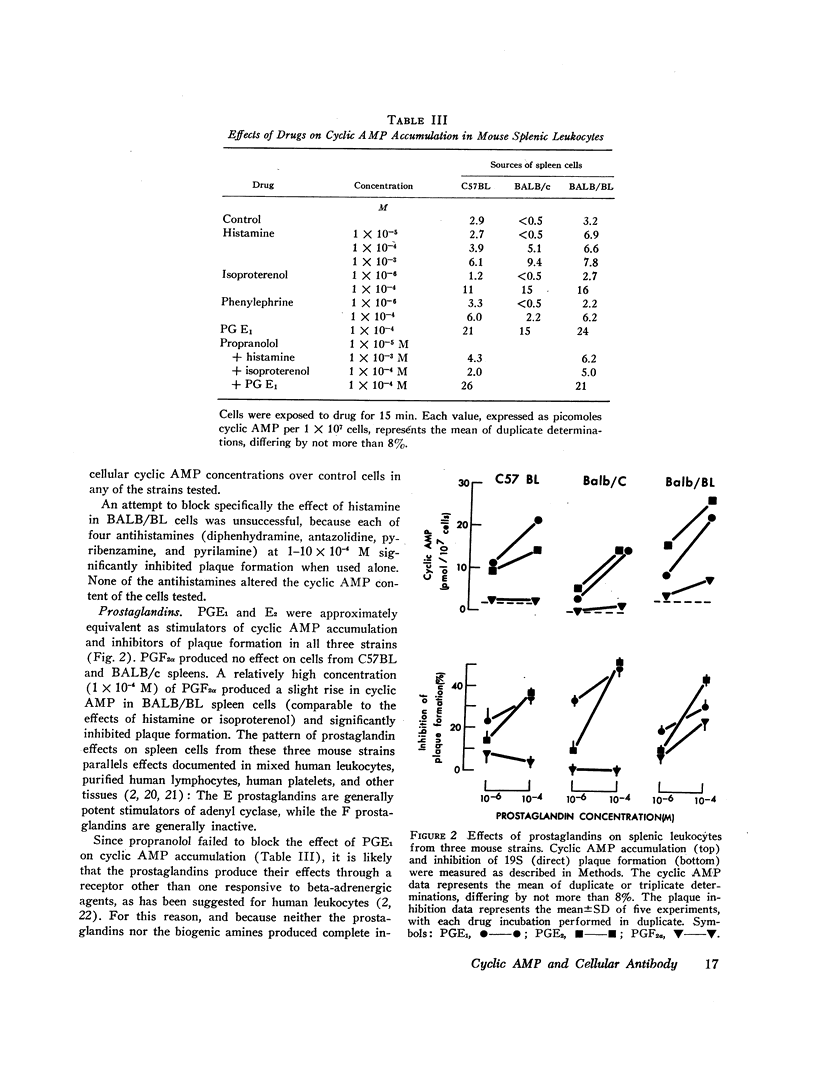
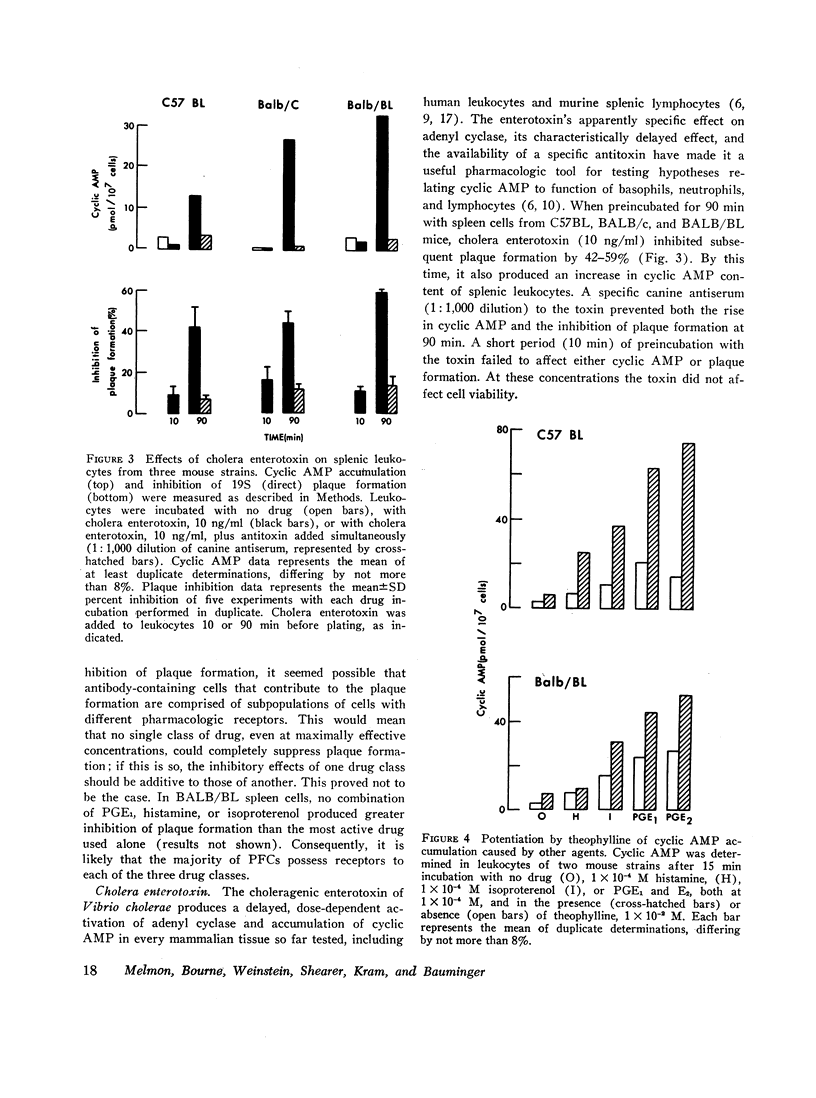
Histamine, beta-adrenergic amines, and prostaglandins inhibited hemolytic plaque formation by splenic leukocytes from immunized mice. The same agents had previously been shown to prevent both the IgE-mediated release of histamine from human basophils and the immunologically specific cytolytic activity of murine lymphocytes, through stimulation of the production of cyclic AMP in leukocytes. We therefore tested the hypothesis that cyclic AMP might mediate an inhibitory effect of these drugs by comparing the ability of these agents to inhibit plaque formation with their effects on cyclic AMP accumulation in leukocytes. In splenic cells from three mouse strains, the dose-dependent effects of these agents of cyclic AMP correlated with their inhibition of plaque formation. Beta- but not alpha-adrenergic agonists were effective in both systems, and the effects of isoproterenol were inhibited by propranolol. Histamine was approximately equipotent with isoproterenol in both systems. Two prostaglandins (E1 and E2) were effective in both systems, but prostaglandin F2α was not. Dibutyryl cyclic AMP, a lipid-soluble analog of the endogenous nucleotide, inhibited plaque formation by cells of all three strains. Theophylline, an inhibitor of cyclic AMP degradation, inhibited plaque formation slightly, but potentiated the effects of histamine, isoproterenol, and the prostaglandins on both cyclic AMP accumulation and plaque formation. Finally, cholera enterotoxin, a potent activator of adenyl cyclase, produced a delayed inhibition of plaque formation and a parallel increase in leukocyte cyclic AMP content; both effects of the toxin were blocked by canine antitoxin. These results suggest that leukocyte cyclic AMP may act as a “second messenger” to suppress plaque formation in vitro. The inhibitory effects of hormones and cyclic AMP on plaque formation are strikingly similar to their effects on in vitro models of immediate and cell-mediated hypersensitivity. The physiologic significance of these findings is not yet known.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assem E. S., Schild H. O. Inhibition by sympathomimetic amines of histamine release by antigen in passively sensitized human lung. Nature. 1969 Dec 6;224(5223):1028–1029. doi: 10.1038/2241028a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L. Pharmacologic control of allergic histamine release in vitro: evidence for an inhibitory role of 3',5'-adenosine monophosphate in human leukocytes. J Immunol. 1972 Mar;108(3):695–705. [PubMed] [Google Scholar]
- Bourne H. R., Melmon K. L. Adenyl cyclase in human leukocytes: evidence for activation by separate beta adrenergic and prostaglandin receptors. J Pharmacol Exp Ther. 1971 Jul;178(1):1–7. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Production of highly purified choleragen and choleragenoid. J Infect Dis. 1970 May;121(Suppl):63+–63+. doi: 10.1093/infdis/121.supplement.s63. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Orange R. P., Austen K. F. Pharmacologic inhibition of the antigen-induced release of histamine and slow reacting substance of anaphylaxis (SRS-A) from monkey lung tissues mediated by human IgE. J Immunol. 1971 May;106(5):1267–1273. [PubMed] [Google Scholar]
- Kooman W. J., Orange R. P., Austen K. F. Immunochemical and biologic properties of rat IgE. 3. Modulation of the IgE-mediated release of slow-reacting substance of anaphylaxis by agents influencing the level of cyclic 3',5'-adenosine monoposphate. J Immunol. 1970 Nov;105(5):1096–1102. [PubMed] [Google Scholar]
- Lichtenstein L. M., Henney C. S., Bourne H. R., Greenough W. B., 3rd Effects of cholera toxin on in vitro models of immediate and delayed hypersensitivity. Further evidence for the role of cyclic adenosine 3',5'-monophosphate. J Clin Invest. 1973 Mar;52(3):691–697. doi: 10.1172/JCI107230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- McKinney G. R., Lish P. M. Interaction of beta adrenergic blockade and certain vasodilators in dextran-induced rat paw edema. Proc Soc Exp Biol Med. 1966 Feb;121(2):494–496. doi: 10.3181/00379727-121-30813. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein J., Sela M. Receptors for histamine can be detected on the surface of selected leukocytes. Science. 1972 Aug 25;177(4050):707–709. doi: 10.1126/science.177.4050.707. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Weinstein Y., Shearer G. M., Bourne H. R., Bauminger S. Separation of specific antibody-forming mouse cells by their adherence to insolubilized endogenous hormones. J Clin Invest. 1974 Jan;53(1):22–30. doi: 10.1172/JCI107542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G. Cluster formation in vitro by mouse spleen cells and sheep erythrocytes. J Immunol. 1968 Dec;101(6):1264–1270. [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G., Connell M. S., Priore R. L. Cellular differentiation of the immune system of mice. I. Separate splenic antigen-sensitive units for different types of anti-sheep antibody-forming cells. J Exp Med. 1968 Sep 1;128(3):437–457. doi: 10.1084/jem.128.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Melmon K. L., Weinstein Y., Sela M. Regulation of antibody response by cells expressing histamine receptors. J Exp Med. 1972 Nov 1;136(5):1302–1307. doi: 10.1084/jem.136.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Melmon K. L., Bourne H. R., Sela M. Specific leukocyte receptors for small endogenous hormones. Detection by cell binding to insolubilized hormone preparations. J Clin Invest. 1973 Jun;52(6):1349–1361. doi: 10.1172/JCI107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Adenyl cyclase activity in human platelets. Biochem Biophys Res Commun. 1969 Apr 29;35(2):265–272. doi: 10.1016/0006-291x(69)90277-0. [DOI] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]


