Abstract
Previously, in an attempt to understand the mechanisms involved in the regulation of plasma cyclic nucleotides, we measured concentrations of adenosine 3′,5′-monophosphate (cAMP) and guanosine 3′,5′-monophosphate (cGMP) in plasma from selected blood vessels of anesthetized dogs. The observation that the renal venous plasma concentrations of both cyclic nucleotides were less than arterial concentrations suggested that the kidney might be an important site for the elimination of these compounds from plasma and prompted further investigation of the renal handling of these compounds.
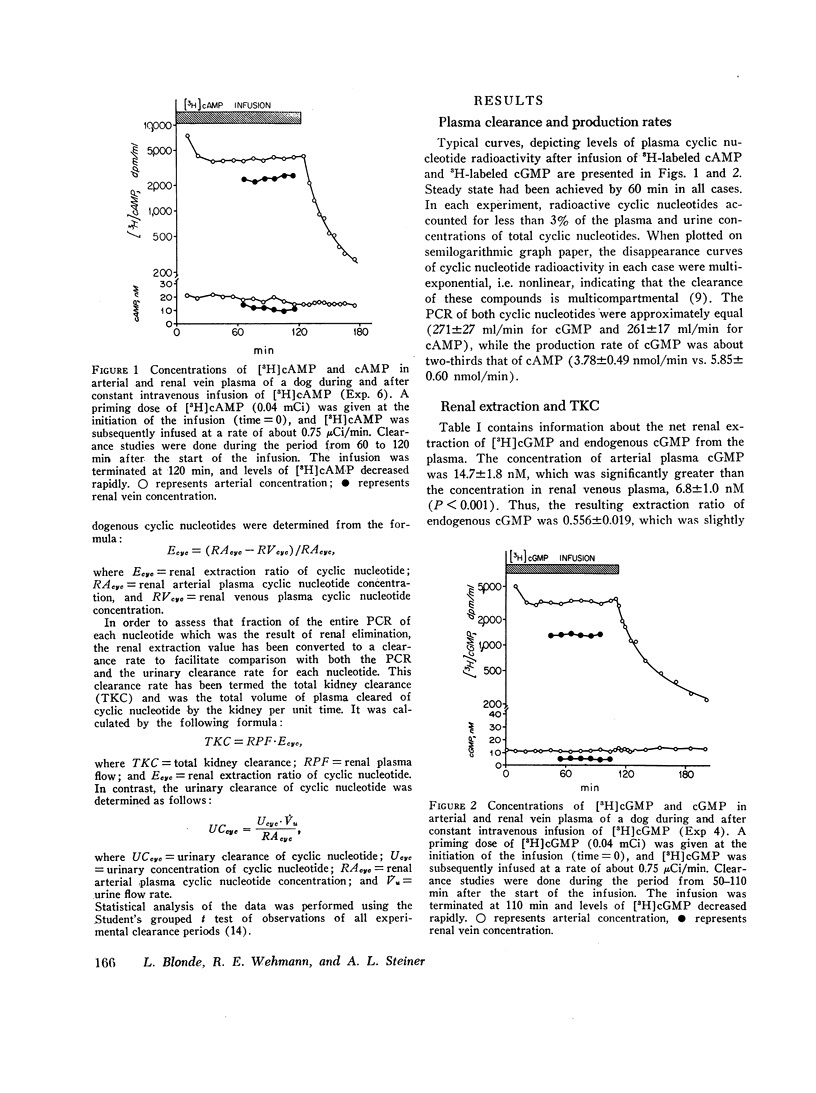
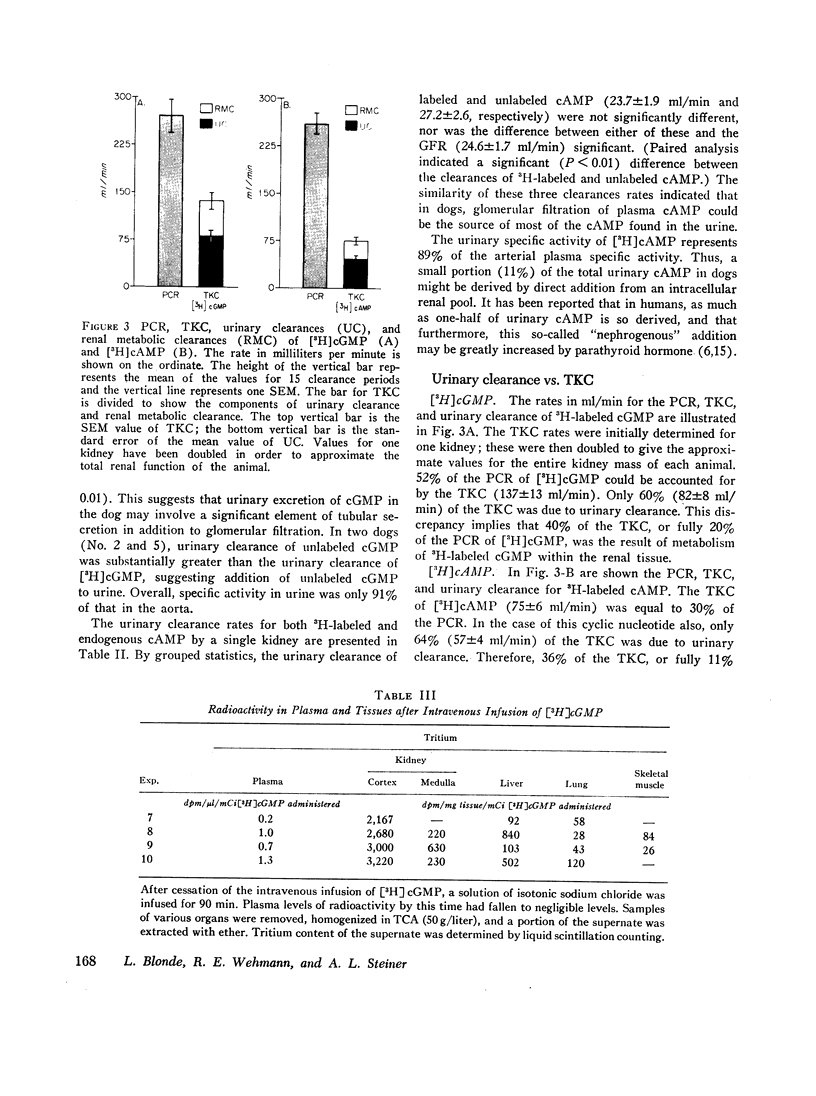
Tracer doses of either [3H]cAMP or [3H]cGMP were administered to anesthetized dogs by constant intravenous infusion, and metabolic clearance rates were determined. Concentrations of endogenous cyclic nucleotide and of cyclic nucleotide radioactivity were measured in aortic and renal venous plasma as well as in urine. Renal venous plasma [3H]cGMP was 39% and [3H]cAMP was 65% of the concentration in arterial plasma. Endogenous cyclic nucleotide levels showed a similar relationship. The plasma clearance rates (PCR) were 271±27 ml/min (mean±SE) for cGMP and 261±17 for cAMP. The total kidney clearance (calculated as the renal plasma flow × renal cyclic nucleotide extraction ratio) accounted for 52±4% and 30±2% of the PCR for cGMP and cAMP, respectively. Only about two-thirds of the total kidney clearance of each cyclic nucleotide could be accounted for by urinary excretion, the remainder presumably being the result of renal metabolism.
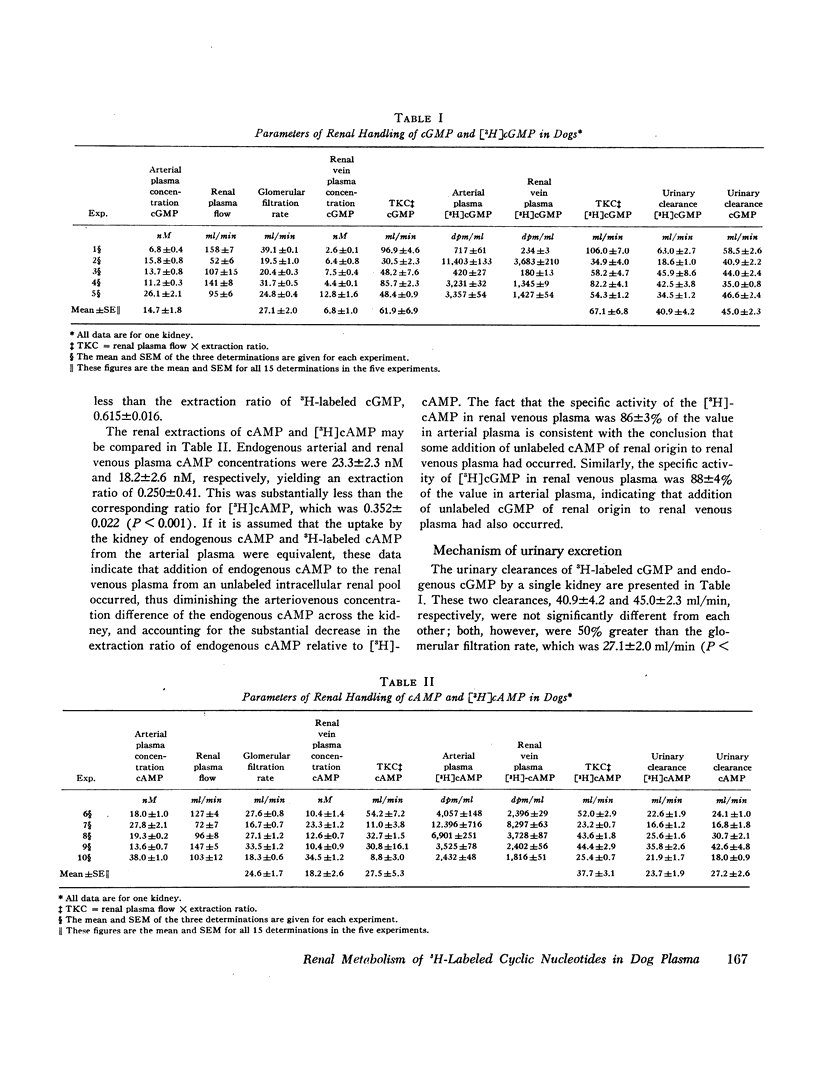
The urinary clearances of 3H-labeled cGMP (40.9±4.2 ml/min) and endogenous cGMP (45.0±2.3 ml/min) were not significantly different from each other. Both were approximately 50% greater than the glomerular filtration rate, which was 27.1±2.0 ml/min, indicating that a significant amount of urinary cGMP is derived from plasma by tubular secretion.
In contrast, the urinary clearances of 3H-labeled cAMP (23.7±1.9 ml/min) and endogenous cAMP (27.2±2.6 ml/min) were nearly equal both to each other and to the glomerular filtration rate, which was 24.6±1.7 ml/min. Thus, in the dog, glomerular filtration of plasma cAMP appears to be responsible for most of the cAMP found in urine. Renla production of cAMP, which in humans contributes from a third to a half of the urinary cAMP, was quantitatively of minor importance in the dog.
Thus, under the conditions of these experiments in dogs, renal elimination appears to be responsible for half of the PCR of cGMP and about a third of the PCR of cAMP. About a third of the renal elimination of both cyclic nucleotides appears to be due to metabolic degradation within the kidney, and the balance is due to excretion in the urine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHMAN D. F., LIPTON R., MELICOW M. M., PRICE T. D. Isolation of adenosine 3', 5'-monophosphate and guanosine 3', 5'-monophosphate from rat urine. Biochem Biophys Res Commun. 1963 May 22;11:330–334. doi: 10.1016/0006-291x(63)90566-7. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- Broadus A. E., Hardman J. G., Kaminsky N. I., Ball J. H., Sutherland E. W., Liddle G. W. Extracellular cyclic nucleotides. Ann N Y Acad Sci. 1971 Dec 30;185:50–66. doi: 10.1111/j.1749-6632.1971.tb45235.x. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Kaminsky N. I., Hardman J. G., Sutherland E. W., Liddle G. W. Kinetic parameters and renal clearances of plasma adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2222–2236. doi: 10.1172/JCI106441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadus A. E., Kaminsky N. I., Northcutt R. C., Hardman J. G., Sutherland E. W., Liddle G. W. Effects of glucagon on adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in human plasma and urine. J Clin Invest. 1970 Dec;49(12):2237–2245. doi: 10.1172/JCI106442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Parathyroid function and the renal excretion of 3'5'-adenylic acid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman J. G., Davis J. W., Sutherland E. W. Measurement of guanosine 3',5'-monophosphate and other cyclic nucleotides. Variations in urinary excretion with hormonal state of the rat. J Biol Chem. 1966 Oct 25;241(20):4812–4815. [PubMed] [Google Scholar]
- Hardman J. G., Robison G. A., Sutherland E. W. Cyclic nucleotides. Annu Rev Physiol. 1971;33:311–336. doi: 10.1146/annurev.ph.33.030171.001523. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Ishikawa S., Davis J. W., Sutherland E. W. Determination of guanosine 3',5'-monophosphate in tissues and of guanyl cyclase in rat intestine. J Biol Chem. 1969 Dec 10;244(23):6371–6376. [PubMed] [Google Scholar]
- Kaminsky N. I., Broadus A. E., Hardman J. G., Jones D. J., Jr, Ball J. H., Sutherland E. W., Liddle G. W. Effects of parathyroid hormone on plasma and urinary adenosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2387–2395. doi: 10.1172/JCI106458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- Price T. D., Ashman D. F., Melicow M. M. Organophosphates of urine, including adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate. Biochim Biophys Acta. 1967 May 30;138(3):452–465. doi: 10.1016/0005-2787(67)90542-4. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- TAIT J. F. REVIEW: THE USE OF ISOTOPIC STEROIDS FOR THE MEASUREMENT OF PRODUCTION RATES IN VIVO. J Clin Endocrinol Metab. 1963 Dec;23:1285–1297. doi: 10.1210/jcem-23-12-1285. [DOI] [PubMed] [Google Scholar]