Abstract
Hemopoietic system derived progenitor cells with mesenchymal features have been identified including CD14+ monocyte-derived progenitors. However, it is unclear whether there are mesenchyme derived progenitors with hematopoietic potential. Herein, we identified a novel CD14- cell-derived population with both mesenchymal and hematopoietic features in rat peripheral blood, and this cell population is different from the CD14+ monocyte-derived progenitors but designated peripheral blood multipotential mesenchymal progenitors (PBMMPs). Phenotype analysis demonstrated expression of mesenchymal markers in PBMMPs including BMPRs, Endoglin/CD105, Fibronectin (Fn), Vimentin (Vim), Collagen (Col) I/II/III along with hematopoietic marker CD34. CD14+ cell-derived population shared the same characteristics with CFs. In mixed culture of CD14+ and CD14- cells, PBMMPs were a predominant component and expressed CD29high, CD73high, CD34high, CD45low and CD90. Except for the value of mixed T lymphocytes and CD14+ cell-derived population, hematopoietic characters of cultured PBMMPs were indicated by CD14-/CD34+/CD45-/CD90+. The mesenchymal origin was further confirmed by comparing PBMMPs with bone marrow stromal cells. Finally, we transplanted PBMMPs into a skin wound model, and results showed the specific potential of PBMMPs in not only extracellular matrix secretion but epidermal regeneration. This study provides evidence that peripheral blood contains common hematopoietic-mesenchymal progenitors from both hematopoietic and mesenchymal lineages, and CD34+ mesenchymal progenitors are a possible alternative source of epidermal cells in wound healing.
Keywords: Peripheral blood stem cells, Mesenchymal stem cells, Hemopoietic stem cells, Stem cell plasticity, Common progenitor, Wound healing
Introduction
Adult bone marrow shelters different types of stem cells, including hematopoietic and mesenchymal stem cells 1-3. If bone marrow-derived stem cells play a role in the regeneration and repair of peripheral organ and tissues, transmission of these cells through blood is to be expected. It has been proven that at least two kinds of adherent cells are isolated from the peripheral blood to date: One is circulating fibrocytes (CFs), which represent hematopoietic origin (CD11b+/CD34+/CD45+/Collgen-I+), have multi-differentiation potency and are involved in wound healing 4-6, and the other is peripheral blood-derived mesenchymal stem cells (PBMSCs), which have characteristics of mesenchymal lineage (CD34-/CD45-/CD29+/CD44+/CD73+/CD90+) and are similar to bone marrow stromal cells (BMSCs) 7,8.
Mesenchymal stem cells (MSCs) are identified as adherent fibroblast-like cells in the bone marrow that can differentiate into mesenchymal tissues, including bone, cartilage, fat, muscle, and bone marrow stroma 9. Recently, mesenchymal progenitors having similarities in morphologic and phenotypic features and differentiation potentials to MSCs have been reported at extremely low frequencies in umbilical cord blood as well as in fetal and adult peripheral blood 10-12. MSCs and MSC-like cells in peripheral blood do not express hematopoietic markers or the stem cell/endothelial cell marker CD34 9-12. However, the attempts to demonstrate PBMSCs have been unrewarding, except for a report of Fernandez et al, who identified cells with the features of MSCs in growth-factor-mobilized peripheral blood cells of breast cancer patients 13. Several studies have isolated PBMSCs using the culture conditions similar to those defined for BMSCs, which supports the existence of a small population of circulating MSCs. But the isolation of these cells is obviously difficult and these cells are subject to variation depending on the methods for isolation and sorting of peripheral blood mononuclear cells (PBMCs), and on culture conditions 12-16.
We have used the skin wound pretreatment to mobilize MSCs from the bone marrow to the peripheral blood and BMSCs' culture supernatant served as condition medium to induce PBMSCs' differentiation (unpublished data). In the absence of external influence, a population of CD14- mesenchymal cells with hematopoietic potential was harvested. However, whether this population is derived from mesenchymal or hematopoietic lineage is still unclear. In this study, we analyzed the characteristics of cells derived from CD14+ and CD14- populations, and results showed the CD14- cell derived population had both mesenchymal and hematopoietic features and was different from the CD14+ monocyte-derived progenitors. We designated this cell population as peripheral blood multipotential mesenchymal progenitors (PBMMPs). CD14+ cell-derived population shared the same characteristics with CFs and its mesenchymal origin was further confirmed by comparing PBMMPs with BMSCs. Finally, we transplanted PBMMPs into a skin wound model, and results showed the PBMMPs could not only secret extracellular matrix secretion, but also induce epidermal regeneration. Our study provides evidence that blood contains common hematopoietic-mesenchymal progenitors from both lineages, and CD34+ mesenchymal progenitors are a possible alternative source of epidermal cells in wound healing.
Materials and methods
Cell Culture
All animal procedures are approved by the responsible Animal Care and Use Committee of the Fourth Military Medical University. Blood samples were obtained from the jugular vein of SD rats aged 8 weeks and weighing about 220 g and bone marrow was aspirated from the tibia and femur of 2-week-old SD rats. About 5-ml blood and bone marrow were anti-coagulated by heparin in tubes. Following being mixed with same volume of phosphate-buffered saline (PBS), the samples were added onto 7-ml Percoll-Paque (1.083 g/ml; GE Healthcare, UK) in a 15-ml tube. Centrifugation was performed at 2,100 rpm for 20 min. The cells in the interface layer were collected into another 15-ml tube, washed twice with PBS, and counted afterwards under a light microscope. After an additional wash with PBS, PBMCs were re-suspended in Dulbecco's modified Eagles medium (DMEM) containing 10% FCS (Gibco, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma, USA), seeded at a density of 2×106 cells/ml in a T25 flask and grown in the absence of additional growth factors at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium was replaced every 3 days. When the cell confluence reached 80%, cells were trypsinized, re-seeded in new T75 flasks and maintained under the same culture condition. Cells at the 3rd passage were collected and used for the following assays.
CFs were isolated from human Leukopaks (Blood Center of Fourth Military Medical University, Xi'an) by Percoll-Paque density gradient centrifugation (1.073 g/ml) and grown under the same condition. When the fibroblast-like cells were observed, macrophage colony-stimulating factor (M-CSF) was added at a final concentration of 50 ng/ml.
Immunomagnetic Selection and Cell Interaction
Initially isolated cells were a hybrid system and the origin of PBMMPs was not clear. Therefore, the total PBMCs prepared from each animal were fractionated by immunomagnetic selection of monocytes using anti-rat CD14 antibody attached to Dynabeads followed by in vitro culture of each fraction. Enriched preparations were verified to be above 98% in purity by CD14 staining as determined by flow cytometry. Because CD14+ cells accounted for about one tenth of CD14- cells and the isolation was performed with a very small number of seeded cells. We set 105 cells and 106 cells as one unit, respectively, which is a condition more close to normal composition and helps to study the interaction between two kinds of cells and count the clone numbers instead of percentage. Using the Millicell co-culture system (0.4 μm; Millipore, USA), one unit of CD14+ or CD14- cells was maintained in either the upper or the lower chamber of 24-well plate. After 7 days of culture, clones (cells which relatively centralized and had a clear margin were also considered as one clone) growing in the lower chamber were counted. In the mixed culture, CD14+ and CD14- cells with different units were seeded in 6-well plate at the ratio of 1:1, 1:2, 1:3, 1:4 and 1:5 to observe the clone formation.
In order to preliminarily investigate the requirement of T lymphocytes in PBMMPs' differentiation, CD14- cells were seeded in 6-well plate and the non-adherent cells (presumably T lymphocytes) were removed by washing with PBS 3~5 times 3 days later. The adherent cells were further maintained for 7 days and observed under an inverted light microscope.
RT-PCR
Total RNA was extracted from PBMMPs and BMSCs using the RNeasy extraction kit (Invitrogen, USA) and genomic DNA was removed by DNase I, according to the manufacturer's instrucctions. First-strand cDNA synthesis and PCR were performed as described previously 6. The primers were as follows: β-actin: 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3' (Forward); 5'-TAA AAC GCA GCT CAG TAA CAG TCC G-3' (Reverse); Collagen (Col)-I: 5'-GGA GAG TAC TGG ATC GAC CCT AAC-3' (Forward); 5'-CTG ACC TGT CTC CAT GTT GCA-3' (Reverse); Col-III: 5'-GAA AAA ACC CTG CTC GGA ATT-3' (Forward); 5'-GGA TCA ACC CAG TAT TCT CCA CTCT-3' (Reverse). The PCR conditions included predenaturation at 94℃ for 2 min, and 35 cycles of denaturation at 94℃ for 45 sec, annealing at 57℃ for 45 sec and extention at 72℃ for 60 sec followed by a final extention at 72℃ for 10 min. The products were subjected to 1% agarose gel electrophoresis.
Phenotype Analysis
PBMMP and BMSCs were sub-cultured on sterile cover-slips overnight for adherence. Following washing with PBS, a fraction of cells were fixed in cool acetone for 10 min at room temperature and incubated with one of the following rabbit pAbs: anti-BMPR IA, anti-BMPR II, anti-Endoglin/CD105 (1:100; Santa cruz, USA), mouse mAbs: anti-Col I (1:400; abcam, UK), anti-Col II (1:1000; Thermo, USA) and goat mAb: anti-CD34 (1:100; RnD, USA) overnight at 4℃. Then, these cells were incubated for 60 min with FITC-conjugated goat anti-rabbit, goat anti-mouse IgG and rabbit anti-goat IgG (1:100; Santa cruz, USA), independently. The remaining cells were fixed in formaldehyde for 10 min at room temperature, and the endogeneous peroxidase activity was quenched with 0.3% hydrogen peroxide for 15 min. Cells were then incubated with one of the following mouse mAbs: anti-Fibronectin (Fn) (0.1 µg/ml), anti-Vimentin (Vim) (5 µg/ml; Chemicon, USA) and α-smooth muscle actin (SMA) (1:100; Santa cruz) overnight at 4℃ followed by treatment with second antibody. Visualization was performed using REAL™ EnVision™ Detection System (DAKO, Denmark). The primary antibody was replaced with PBS serving negative control.
For flow cytometry analysis, following two washes, aliquots containing 105 cells of mixture and BMSCs were incubated at 4℃ for 1 h with 100 μl of saturating concentration of mouse mAb against CD4, CD8b (Biolegend, USA), CD73 (BD Pharmingen, USA), rabbit pAb against CD14 (Santa cruz, USA), goat mAb against CD34 (RnD, USA), FITC-conjugated mouse mAb against CD29, CD45, CD90 and PE-conjugated mouse mAb against CD26 (Biolegend, USA) and isotype-matched control, independently. Following two washes, these cells were incubated at 4℃ for another hour with 100 μl FITC-conjugated F(ab')2 fragments of mAb against mouse IgG and PE-conjugated rabbit pAb against goat IgG (1:50) in 2% BSA/PBS followed by washing twice, and re-suspended in PBS. Quantitative fluorescence analysis was performed using an FACS Calibur cytometer and CellQuest software program (Becton Dickinson, USA). The cell number (at least 10,000 cells) versus fluorescence intensity was recorded in each sample. CFs was incubated with FITC-conjugated mouse mAb against CD11b, CD14, CD34, CD44, CD45 and CD90 (eBioscience, USA) and analyzed with the same procedures.
Cell Cycle Analysis
PBMMPs and BMSCs were trypsinized, fixed in 70% alcohol and analyzed for cell cycle by flow cytometry (Beckman Coulter, USA).
Osteogenic and Adipogenic Induction
PBMMPs and BMSCs were seeded in a 6-well plate at a density of 5×105 cells/well in basic medium for 24 h to allow adherence. Then, the medium was replaced with osteogenic induction medium containing DMEM, 10% FCS, 0.1 μM dexamethasone, 10 mM β-glycerolphosphate and 50 mg/L ascorbate-2-phosphate, and adipogenic induction medium consisting DMEM, 10% FCS, 0.25 μM dexamethason, 100 μM indomethacin, 0.5 mM 3-isobutyl methylxanthine and 10 mg/L insulin. Cells were maintained for 2 weeks and media were replaced twice weekly. The mineralized nodules were stained with Alizarin Red S and neutral lipid vacuoles with Oil-red O (Sigma, USA).
In Vivo Transplantation
Immunotolerance of 4~6-week old C57 female mice weighing about 20 g was induced with a single abdominal subcutaneous injection of cyclophosphamide (cyclo) (200 mg/kg; Hengrui, China). Seven days later, intraperitoneal injection (i.p.) of cyclo (20 mg/kg) in normal saline was performed thrice (once every other day). This method developed by our department renders the transplanted cells to maintain their most functions and remain for at least one month without being rejected (unpublished data). Animals were anesthetized with 0.1% pentobarbital sodium i.p. and the full-thickness dorsal skin (1.5 cm in diameter) including the panniculus carnosus muscle was excised. PBMMPs labeled with fluorescent dye PKH-26 (106 cells/ 0.5 ml physiological saline per mouse) were injected into the wound bed and marginal area which were covered with a sterile semi-permeable membrane (Tegaderm, 3M) followed by wound suturing to preserve humidity. The control mice were injected only with an equal volume of normal saline.
Histological and Immunofluorescence analysis
Paraffin embedded sections (5 μm in thickness) were de-paraffined, hydrated, submitted to different antigen retrievals, and quenched for endogenous peroxidase (3% H2O2 in PBS). Nonspecific staining was blocked with 10% goat serum. Immunoreactivity was detected using REAL™ EnVision™ Detection System. The sections were treated with mouse anti-Col I mAb overnight at 4°C, and with appropriate secondary for 1 h at 37°C. Sections were counterstained with hematoxylin, dehydrated, and mounted. Cryosections (6 μm thick) were fixed with cool acetone and blocked with serum. The primary antibody was mouse anti-CK14 or CK19 mAbs (1:100; abcam, UK). After incubation overnight, TRITC-conjugated goat anti-mouse IgG and FITC-conjugated goat anti-mouse IgG were added followed by incubation at 37℃ for 1 h. Finally, Hoechst33342 was used for counterstaining. The sections of negative control were incubated with PBS as primary antibody. Sections were then visualized under fluorescent microscope.
Statistical analysis
Comparisons between two groups were tested for statistical significance with the independent sample t test with SPSS version 13.0 statistic software package. A value of P<0.05 was considered statistically significant.
RESULTS
PBMMPs are derived from CD14- but not CD14+ Cells, and CD14+ Cells Partly Inhibit PBMMPs' Growth and Proliferation
To determine the origin of PBMMPs, we analyzed the growth of adherent CD14+ and CD14- PBMCs cultured on plastic. In CD14- wells, when the fibroblast-like cells initially appeared after 2~3 days of culture, cell body became large and had branches, showing morphology of MSCs (Fig.1A-a). In CD14+ wells, small clusters of round cells developed and cell processes were found. After 4~5 days of culture, spindle-shaped adherent cells made their appearance (Fig.1A-b). In the mixed culture, fibroblast and stromal-like adherent cells became the predominant cell type in this system (Fig.1A-c). These isolated cells had adherence to various extents, CD14+ cell-derived population closely adhered to plastic and was difficult to trypsinize, PBMMPs adhered loosely and those in the mixed culture had moderate adherence. In addition, lymphocytes could not be completely removed by washing gently. Therefore, the percentage of adherent cells was not used as an index.
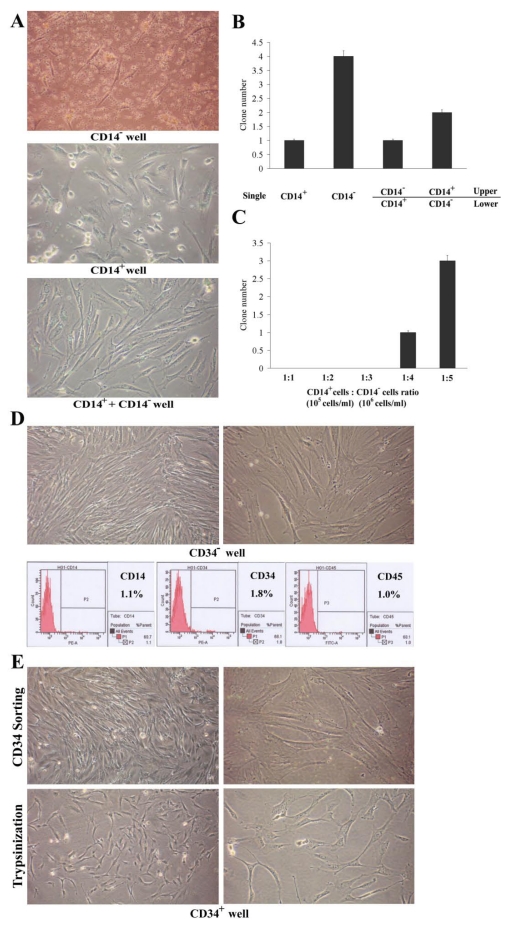
Fig 1.
Origination of PBMMP and interaction with monocytes. (A) In CD14- wells, cell body became large and extended branches, showing morphology of MSCs. In CD14+ wells, adherent cells with a spindle-shaped morphology made their appearance. In mixed culture, adherent cells appearing fibroblast and stromal-like morphology became the predominant cell type. (B) CD14- cell fraction gives rise to more clones in single culture, its growth and proliferation are partly inhibited by CD14+ cell fraction, but CD14- cell fraction doesn't definitely support the amplification of CD14+ cell fraction in co-culture system (0.4 μm pore diameter). (C) When the ratio of CD14+ cell fraction to CD14- cell fraction was equal to 1:4, cell clones were observed in mixed culture, and the clone number is in direct proportion to the ratio. (Magnification 100×)
Using single culture and Millicell co-culture system, we examined the in vitro cellular requirements in PBMMPs clone formation from CD14- cells and their interaction with CD14+ cells (Fig.1B). When CD14+ and CD14- cells were maintained independently for 1 week, 1 and 4 clones in average were found in the wells. When the CD14+ cells were grown in the lower chamber and CD14- cells in the upper chamber for 1 week, no significant increase of clone numbers appeared in the lower chamber. Controversially, about half of the clone numbers appeared in the lower chamber when the CD14- cells were grown in the lower chamber and CD14+ cells in the upper chamber for 1 week. These results indicated the growth and proliferation of CD14- cells were partly inhibited by CD14+ cells, but CD14- cells did not definitely promote the amplification of CD14+ cells. Similar results were obtained in the mixed culture experiment (Fig.1C), in which when the ratio of CD14+ cells to CD14- cells was 1:1 (normal condition), 1:2 and 1:3 (a slight increase in CD14- cells), there were no clone observed, while when the ratio was above 1:4 (a significant increase in CD14- cells), clones were significantly increased. Culture was performed for about 2 weeks, the adherent PBMMPs were trypsinized for flow cytometry when the cell confluence reached 80% (data not shown).
PBMMPs Need Lymphocytes' Support, and Express both Mesenchymal and Hematopoietic Features
After 7 days of culture, the stromal-like cells were the predominant component of adherent cells (Fig.2A-a,b) and the adherent cells could proliferate. Five to ten days, there was clone formation and the cells gradually conjugated (Fig.2A-c). When the suspended cells (presumably T lymphocytes) were removed as possible, cell proliferation terminated and karyopyknosis and fragmentation occurred (Fig.2A-d).
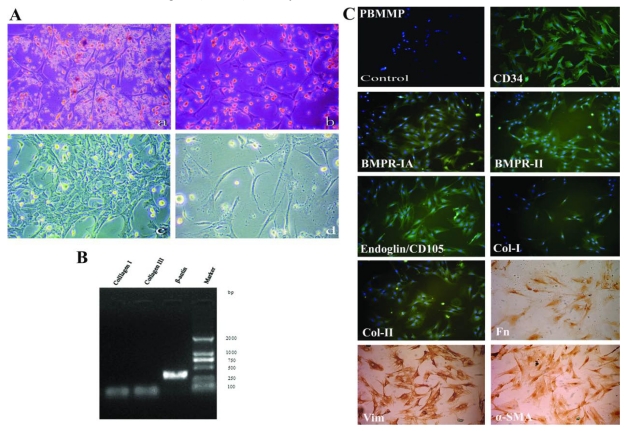
Fig 2.
PBMMPs needs lymphocytes' support, and expresses both mesenchymal andhematopoietic features. (A) After 7 days, stromal-like cells took the most parts of adherent cells (a,b). Clone formation in the ensuing 5∼10 days cells (c). When floating cells (presumably T lymphocytes) were washed away as possible, cell proliferation ceased and karyopyknosis and fragmentation occurred (d). (B) Col-I/III genes expression under the control of β-actin was shown by RT-PCR. (C) PBMMP was positive for mesenchymal stem cell markers BMPR-IA, BMPR-II and Endoglin (CD105), the cytoskeletal component Vim, the fibroblast products Col-I, Col-II and Fn, the myofibroblast marker α-SMA and hematopoietic stem cell marker CD34 by immunofluorescence and immunohistochemistry. (Magnification 100×)
Based on the mesenchymal morphology, Col-I/III expressions were detected aiming to investigate the role of PBMMPs in the wound healing, and an affirmative result was acquired under the control of β-actin (Fig.2B). Then, a systematic phenotypic characterization by immunofluorescence and immunohistochemistry was performed. PBMMPs were positive for mesenchymal stem cell markers BMPR-IA, BMPR-II and Endoglin (CD105), the cytoskeletal component Vim, the fibroblast products Col-I, Col-II and Fn, the myofibroblast marker α-SMA and most importantly, hematopoietic stem cell marker CD34 (Fig.2C).
CD14+ Cell-derived Population Shares the Same Characters with CFs
In CD14+ cell culture, adherent cells appeared spindle-shaped, and some of them transformed to macrophage-like cells during culture (Fig.3A). These cells were difficult to passage, and the large and round cells became predominant in the late period. These features are similar to those of CFs, a well recognized hematopoietic derived progenitor cells with mesenchymal features (Fig.3B-a,b). Under the influence of M-CSF, CFs could maintain and proliferate for a long time (Fig.3B-c). CFs were difficult to proliferate and passage under normal condition, and macrophage-like cells spread in the disk for more than one month (Fig.3B-d).
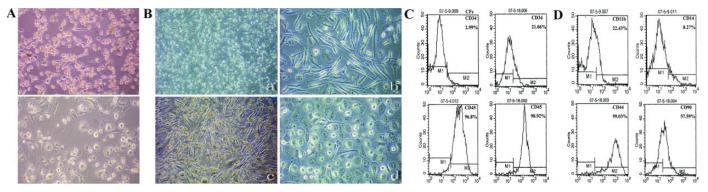
Fig 3.
CD14+ cell-derived population shares the same characters with CFs. (A) Adherent CD14+ cells appeared a spindle-shaped morphology, and some of them changed tomacrophage-like cells during culture. (B) CFs also appeared a spindle-shaped morphology, it was maintained and proliferated by the incubation of G-CSF, and they transformed to macrophage-like cells without external stimulation for more than one month. (C) Enhanced expression of CD34 from 2.99% to 21.06% and CD45 from 96.8% to 98.92% in the early and late period of primary culture. (D) CFs also displayed hematopoietic markers CD11b (22.43%), CD14 (8.27%) and mesenchymal markers CD44 (99.03%), CD90 (57.59%). (Magnification 40×, 100×)
We then investigated the phenotypes of CFs, which displayed hematopoietic markers CD11b/CD14low/CD34/CD45high/CD90 (Fig.3C,D) and mesenchymal markers Col-I/Col-III/Fn/Vim (data not shown). Because of difficulty in passaging, the expressions of hematopoietic markers CD34 and CD45 in the early period were compared with those in the late period of primary culture (Fig.3C). Results showed an enhanced expressions of CD34 (2.99% to 21.06%) and CD45 (96.8% to 98.92%) (Fig.3C). These findings indicated the maturation of CFs, and the high expression of CD45 confirmed its hematopoietic origin. Similar to this, CD14+ cell-derived population had a decreased CD14 expression, increased CD34 expression and high expression of CD45 (data not shown). These results support CD14+ cell-derived population is the same cell type of CFs in rat blood, they are hematopoiesis-originated and share different characters in morphology, proliferation, differentiation and phenotypes with PBMMPs.
PBMMPs Are the Predominant Components in Mixed Culture, Shows an Enhanced Expression of CD34 but Maintained the Expressions of Mesenchymal Markers during Culture
In the late phase of primary mixed culture, CD14- cell-derived fibroblast and stromal-like cells occupied most of areas (Fig.4A) and CD14+ cell-derived round or spindle-shaped cells were sparsely distributed, the cells at the 3rd passage were universally fibroblast-like, and the latter ones were scarcely found. These cells were passaged every 5~7 days, up to a total of thirteen passages over 2 months. We analyzed the phenotypes of cells at 3rd passage in mixed culture by flow cytometry. Less than 20% of cells were positive for T lymphocyte markers CD4 (11.5%), CD8b (19.8%) and CD26 (12.7%) (also expressed in macrophages) and about 70% were positive for hematopoietic stem cell markers CD34 (73.1%) and CD90 (69.5%). In addition, almost 100% of cells were positive for CD29 (100%) and CD73 (98.3%). Notably, cells were nearly negative for the monocyte/macrophage antigen CD14 (2.0%) and only 13.5% positive for CD45. In addition, 2.2% and 1.8% were positive FITC- and PE-conjugated isotype-matched controls (Fig.4B).
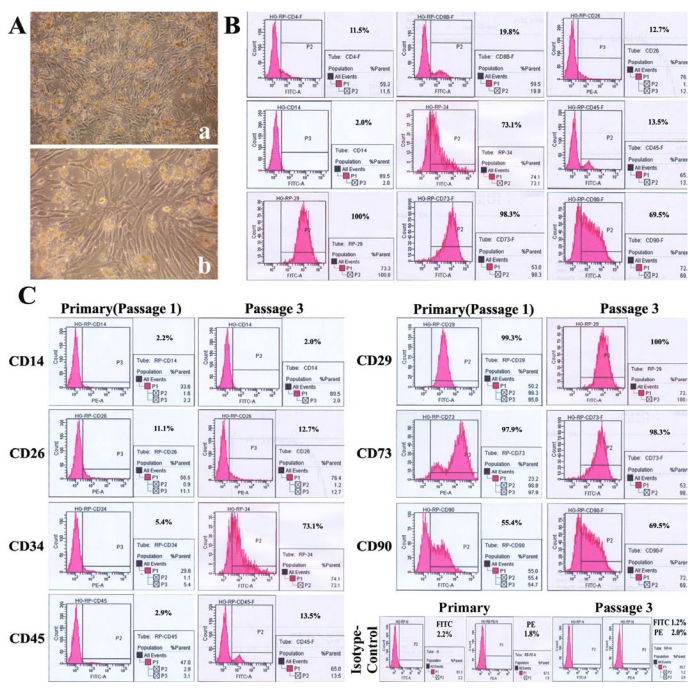
Fig 4.
PBMMP takes majority parts of mixed culture, shows an enhanced expression ofCD34 but maintained expression of mesenchymal markers during culture. (A) In the late phase of primary mixed culture, CD14- cell-derived fibroblast and stromal-like cells occupied the most areas. (B) Mixed cells were positive for T lymphocytes markers CD4 (11.5%), CD8b (19.8%), CD26 (12.7%) (also expressed in macrophage), hematopoietic stem cell markers CD34 (73.1%), CD90 (69.5%), hematopoietic marker CD45 (13.5%), msenchymal markers CD29 (100%), CD73 (98.3%), and negative for Monocyte/macrophage antigen CD14 (2.0%). FITC- and PE-conjugated isotype-matched controls were positive in 2.2% and 1.8%. (C) Significant increase of CD34 expression (from 5.4% to 73.1%), slight increase of CD45 (from2.9% to 13.5%) and CD90 (from 55.4% to 69.5%), maintained expression of CD14 (2.2% and2.0%), CD29 (99.3% and 100%) and CD73 (97.9% and 98.3%) between the primary and 3rd passage of mixed culture. FITC- and PE-conjugated isotype-matched controls in primary culture were positive in 1.2% and 2.0%. (Magnification 40×, 100×)
In comparison between the primary cells and the cells at 3rd passage in mixed culture, CD34 expression was significantly increased (from 5.4% to 73.1%), but those of CD45 (from 2.9% to 13.5%) and CD90 (from 55.4% to 69.5%) were slightly increased, and the expressions of CD14 (2.2% and 2.0%), mesenchymal markers CD29 (99.3% and 100%) and CD73 (97.9% and 98.3%) remained stable. In addition, 1.2% and 2.0% of cells were positive for FITC- and PE-conjugated isotype-matched controls in primary culture (Fig.4C). These results demonstrate PBMMPs' derivation from CD14- cells and their features of both hematopoietic and mesenchymal stem cells. The enhanced CD34 expression indicates their maturation, and the slightly increased expression of CD45 doesn't support the hematopoietic origin of the predominant population in the culture.
These results reveal that PBMMPs are the predominant population in the mixed culture although a small amount of T lymphocytes and monocyte-derived CD34+/CD45+ progenitors are included, and cells after more than 3 passages could basically represent the characteristics of CD14- cell-derived population. Considering the mesenchymal morphology and phenotypes, we speculate the PBMMPs' derivation from mesenchymal lineage.
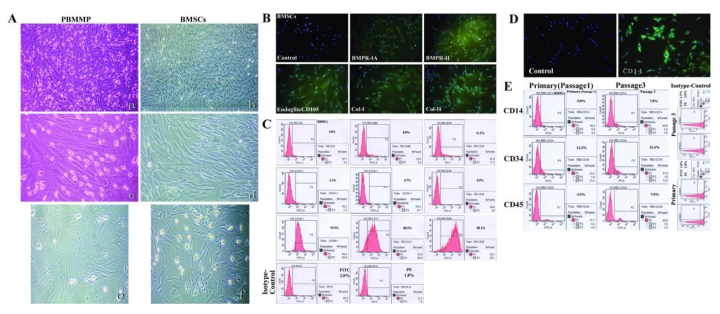
PBMMPs have Similar Morphology and Phenotypes to BMSCs
The stromal-like morphology urges us to explore the similarity between PBMMPs and BMSCs, also known as bone marrow mesenchymal stem cells (BM-MSCs). Just like the mixed culture of PBMCs, BMSCs also had cell populations derived from hematopoietic and mesenchymal lineage, and hematopoiesis originated cells decreased by passaging. We compared these two kinds of cells. The appearance was very similar at the 3rd passage in clone formation and cell morphology (Fig.5A), and BM-MSCs had same positive staining of BMPR-IA/BMPR-II/Endoglin (CD105)/Col-I/Col-II (Fig.5B).
Fig 5.
PBMMP has similar morphology and phenotypes to BMSCs. (A) The appearance of PBMMP and BMSCs was very much alike at the 3rd passage from cloneformation to cell morphology. (B) BMSCs had same positive staining of BMPR-IA/BMPR-II/Endoglin (CD105)/Col-I/Col-IIwith PBMMP. (C) BMSCs showed positive for CD4 (4.0%), CD8b (8.9%) and CD26 (11.2%), negative for hematopoietic cell markers CD14 (2.3%), CD34 (0.7%), CD45 (0.9%), and highly positive forCD29 (93.9%), CD73 (88.5%) and CD90 (98.1%). FITC- and PE-conjugated isotype-matchedcontrols were positive in 2.0% and 1.8%. (D) CD14 was stained positive in some culture of BMSCs mixed with monocytes. (E) Enhanced expression of CD45 is in relation to CD14, and CD34 expression maintained in the initial level indicated monocytes' proliferation and they are CD34- in the primitive period, the way like that in peripheral blood. (Magnification 40×, 100×)
Flow cytometry of BMSCs showed cells less positive for T lymphocytes markers CD4 (4.0%), CD8b (8.9%) and CD26 (11.2%), and almost negative for hematopoietic cell markers CD14 (2.3%), CD34 (0.7%) and CD45 (0.9%). But these cells were strong positive for CD29 (93.9%), CD73 (88.5%) and CD90 (98.1%). In addition, 2.0% and 1.8% of cells were positive for FITC- and PE-conjugated isotype-matched controls. (Fig.5C). These results indicate the different phenotypes between mixed culture (mainly PBMMPs), BMSCs have hematopoietic cell markers CD34, and both BMSCs and PBMMPs are derived from CD14- progenitors.
Enhanced Expression of CD45 is in relation to CD14 in BMSCs
In culture of BMSCs mixed with hematopoietic stem cells, CD14 staining showed positive (Fig.5D). We compared hematopoietic markers CD14, CD34 and CD45 in the primary cells and cells at the 3rd passage. Results showed the enhanced expression of CD45 was in relation to CD14, and CD34 expression remains at the initial level. Positive staining for FITC- and PE-conjugated isotype-matched controls were found in 1.8% and 2.8% of primary cells, 1.5% and 2.0% of cells at the 3rd passage (Fig.5E). These findings suggest enhanced expression of CD45 represents the proliferation of monocytes, and they are CD34- in the primitive period, which was similar to that in peripheral blood.
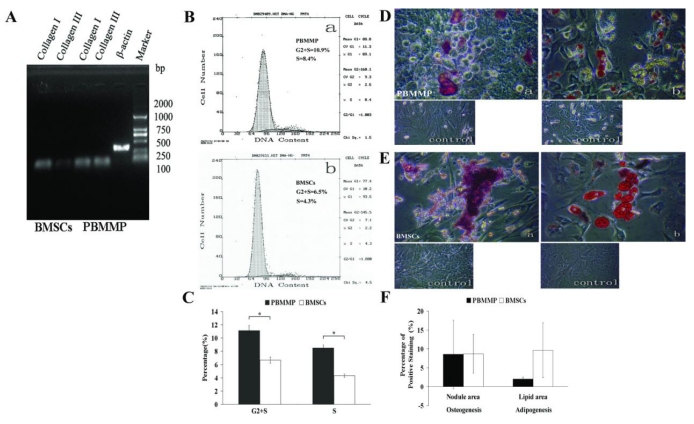
PBMMP has Increased Proliferation, Collagen Secretion and Similar Multi-differentiation Potential compared with BMSCs
Expressions of Col-I and Col-III were detected in both kinds of cells, and results showed a seemly active ability to secrete ECM of PBMMPs (Fig.6A). Cell cycle analysis showed the percent of PBMMP and BMSCs in G2+S phase were 10.9 and 6.5, respectively, especially the proportion of PBMMPs in S phase was 8.4, which was nearly double to that of BMSCs in S phase (4.3) (Fig.6B). Analysis showed statistically significant difference (P<0.05), which confirmed a significantly increased proliferation of PBMMP compared with BMSCs (Fig.6C).
Fig 6.
PBMMP has more active proliferation, collagen secretion and similar multi-differentiation Potential compared with BMSCs. (A) Gene expression of Col-I and Col-III were detected in both kinds of cells, but it seemed like the production of Col-III in PBMMP was more than that of BMSCs, which indicates an active ability to secrete ECM of PBMMP. (B) Representative cell cycle analysis showed the percent of G2+S phase in PBMMP and BMSCs were 10.9 and 6.5 seperately, especially the percent of S phase in PBMMP was 8.4, nearly double to the percent of S phase in BMSCs (4.3). (C) The results of three tests were analyzed and showed a difference of statistical significance (P<0.05), which proved a more active proliferation capability of PBMMP than BMSCs. (D) When cultured under specific induction media, PBMMP possessed osteogenic potential and stained positive by Alizarin Red S. (E) PBMMP also possessed adipogenic potential and stained positive by Oil red-O after induction. (F) Randomly selection of five fields to count the percentage of positive staining area by the software ImageJ and analyzed by spss13.0. Although the induction of BMSCs showed larger calcium nodules and more lipid droplets formation, there were no differences of statistical significance (P>0.05), which indicated similar differentiation potential of PBMMPs and BMSCs. (Magnification 200×)
When cultured under specific induction media, PBMMPs possessed osteogenic and adipogenic potentials and were positive in Alizarin Red S and Oil red-O staining (Fig.6D, E). In order to compare the multi-differentiation potential between these two groups, we randomly selected five fields to calculate the percentage of positive area by ImageJ software. Although the induction of BMSCs showed larger calcium nodules and more lipid droplets, there were no statistically significant differences (P>0.05), which indicated a similar differentiation potential between PBMMPs and BMSCs (Fig.6F).
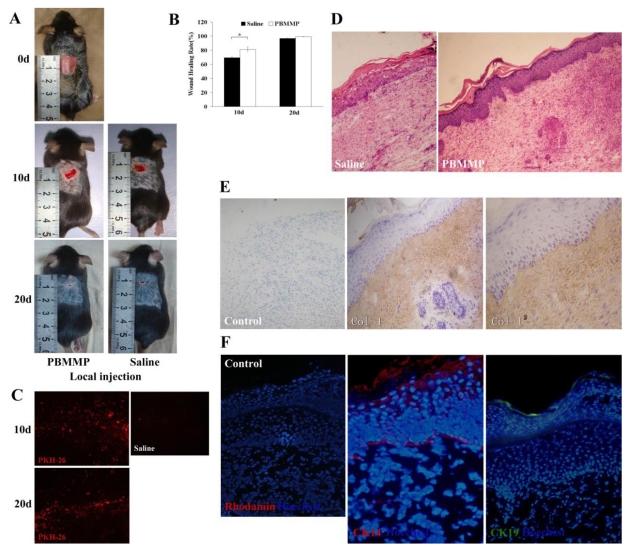
PBMMPs Accelerate Wound Repair through Collagen Deposition and Epithelialization
On the 10th and 20th day after transplantation, there was less unclosed wound in PBMMPs group compared with control group (Fig.7A). We randomly selected five mice from each group, calculate the percentage of wound area by ImageJ software. Results showed a statistically significant difference in healing rate on the 10th day, but not on the 20th day. Red fluorescence of PKH-26 in the healing tissue suggested the survival of transplanted cells (Fig.7C).
Fig 7.
PBMMP accelerates skin repair through collagen deposition and epithelialization. (A) On the 10th and 20th day after transplantation, there was less unclosed wound in PBMMP group compared with the control group. (B) There are statistical differences of healing rate between PBMMP group and control group on the 10th day (P<0.05), but without statistical differences on the 20th day (P>0.05) (n=5). (C) Red fluorescence of PKH-26 detected in the healing tissue displayed the survival of the transplanted cells. (D) In PBMMP group, regenerated epidermal cells distributed uniformly in different layers and the ridge became distinct in epidermis. In control group, a few epidermal cells distributed discontinuously in the basal layer, scattered cells observed in the different layer and no ridges formed. Also a larger amount of ECM deposition was showed in PBMMP group. (E) Strong staining of Col-I in the dermis, which indicates PBMMP's differentiating into fibroblasts and secreting a huge amount of ECM to fill the wound. (F) Positive staining of CK14 and CK19 in the basal membrane compartment, epidermallayer and the surface of stratified epithelium. (Magnification 100×, 200×)
HE staining of the healing samples showed the number of regenerated epidermal cells in PBMMPs group was larger than that in control group. These cells distributed uniformly in different layers, and the ridge became distinct in the epidermis. In the control group, however, a few epidermal cells were found and distributed discontinuously in the basal layer, and sparsely distributed cells were observed in the different layer without any ridges (Fig.7D). Strong staining of Col-I in the dermis indicates PBMMPs' differentiation into fibroblasts and secretion of a huge amount of ECM to fill the wound, and further confirms their mesenchymal characteristics (Fig.7E). After immunofluorescence staining, we found positive staining of CK14 and CK19 in the basal membrane compartment, the epidermal layer and the surface of stratified epithelium (Fig.7F). These PBMMPs could differentiate into fibroblasts secreting ECM to heal the wound and into epithelial cells to participate in the re-epithelialization. On the contrary, there were not enough functional cells to repair the wound in control group, and thus intensified wound contraction, weak epithelialization and scar formation were found.
Discussion
One of the most interesting findings in this study is that CD14- cell-derived PBMMPs appear mesenchymal in morphology, phenotypes and gene expression, and have high expression of hematopoietic stem cell marker CD34. This is the first study showing mesenchymal progenitors with hematopoietic features, which extends the traditional understanding about the biology of MSC system.
PBMMPs need T lymphocytes to support their growth and proliferation, which is similar to CD14+ cell-derived progenitor-like CFs. Abe et al confirm CFs' outgrowth requires cellular interaction between CD14+ cells and other peripheral blood cells, and T lymphocytes is necessary for the culture 5. However, our results showed the outgrowth of PBMMPs was partly inhibited by CD14+ cells. Only when CD14- cell fraction increased more than 4 times of that in normal condition was the clone formation obviously observed in the mixed culture. Although the cell density for seeding probably influences the clone formation, sparsely distributed cells were not observed after 7 days of culture. These results indicate the direct cell-cell interaction plays a more important role than the paracrine effect in inhibiting the differentiation of CD14- cell-derived population. We speculate this mechanism may remain a homeostasis in peripheral blood that a little mesenchymal population circulates under normal condition, and only when monocytes are consumed in the regeneration and repair can MSCs proliferate. On the other hand, large amounts of lymphocytes migrate to the wound stimulating the mobilization and proliferation of CD14- cell-derived population, which may cause disinhibition from relatively low number of monocytes and MSCs' proliferation to participate in the healing process.
CD14+ cell-derived population also shows the similar characters to CFs, which has been identified by Bucala et al in 1994 4. They share the same features of proliferating slowly, passaging difficultly in the absence of growth factor support, differentiating into macrophage-like cells in late phase, decreased expression of CD14, enhanced expression of CD34 and relatively unchanged expression of CD45 during culture, as well as the capabilities of osteogenesis and adipogenesis (unpublished data). This is the first evidence on monocyte-derived progenitors existing in rat peripheral blood. Studies have shown that monocyte-derived multipotential cells (MOMC) can used as a source of mesenchymal and endothelial progenitors 17,18 and pluripotent stem cells can be generated from a subset of peripheral blood monocytes after treatment with a high dose of M-CSF 19, which confirm that monocytes are not only phagocyte precursors but multipotent precursors for several lineages, including non-hematopoietic cells. We also found the differences between CD14+ and CD14- cell-derived populations herein and the differences in the expressions of hematopoietic markers CD14 and CD45 reveal the origins from different bone marrow compartments. Also, the apparently different morphology implies their origin. The former are cells of fusiform with a slightly plump body and two elongated thin ends, whereas the latter are more blanket-like, that is, stretched out in shape, pale in color, and without two sharp ends.
Although the mixed culture was a hybrid system including PBMMPs, lymphocytes and CD14+ monocyte-derived population, the characteristics of passaging difficultly and proliferating slowly in the latter population determine their minor role in culture system, and on the contrary, PBMMPs are the predominant population in the mixed culture. Except for the CD45 expression, which has a similar importance to CD4, CD8b and CD26, we speculate the hematopoietic characters of cultured PBMMPs are CD14-/CD34+/CD45-/CD90+. Together with the stromal morphology, similar phenotypes to BM-MSCs, PBMMPs are indicated as a novel CD14- mesenchymal population with hematopoietic features in the peripheral blood and derived from bone marrow mesenchymal cells.
Actually, BMSCs consist of heterogeneous populations of stem cells. Results showed some purified BMSCs, also known as BM-MSCs, did not express CD14, CD34 and CD45 and but highly expressed CD29, CD73 and CD90. In addition, some BMSCs that mixed with hematopoietic cells had low expressions of CD14, CD34 and CD45. The change in CD45 expression was in consistent with CD14, and CD34 remained at the initial level. It is unlikely that these CD14+ progenitors in the marrow stroma generate PBMMPs and the low CD45 expression also does not support the PBMMP's origin from hematopoietic cells including monocytes.
Although CD34 was still considered to be a marker of primitive hematopoietic stem cells, its expression doesn't remain stable. Similar to this, CD14 expression decreased during their differentiation from monocytes to other progenitors. However, the CD45 expression was stable. Our results confirmed the alteration of CD34 expression indicating the maturation of PBMMPs after leaving the blood and implanting in the local environment in the way similar to that of CD14+ monocytes-derived progenitor such as CFs, and low expression of CD45 suggested PBMMPs of mesenchymal lineage and high expression of CD45 confirmed CFs of hematopoietic lineage. Considering the high CD34 expression in mesencymal progenitors, we investigated the present of common hematopoietic-mesenchymal progenitors. A progenitor origin from hematopoietic or mesenchymal lineage can not be confirmed only by detection of CD34 expression, and then CD45 was used as a more appropriate indicator to define the hematopoietic lineage.
Another interesting finding is that CD34+ progenitors participate in the regeneration of epithelial cells in the skin epidermis. Wu et al have reported that implanted CD34+ bone marrow cells did not express cytokeratin and not incorporate into the epidermis and appendages in the wound 20. They indicated that BM-MSCs but not CD34+ cells could generate the cutaneous structures. Similarly, a previous study by Spees et al proved that BM-MSCs could repair the epithelial cells in vitro through differentiation and fusion 21. However, the homeostasis in normal skin is identical to that in peripheral blood and CD34 expression was also recognized in the epidermal stem cells and hair follicle stem cells, which suggests that progenitors with hematopoieitc features can regenerate the epidermal cells during the wound healing. Our results provide evidence that CD34+ mesenchymal progenitors, but probably not hematopoietic originated CD34+ progenitors, play roles in the epidermal regeneration. We have confirmed the epithelial differentiation of these cells into cells in epidermis, sebaceous gland and hair follicle (unpublished data).
This study emphasizes the difference between PBMMPs and monocyte-derived progenitors, the similarity between PBMMPs and BMSCs, suggesting PBMMPs as a kind of common hematopoietic-mesenchymal progenitors. But the physiological and pathological functions should be further studied in further experiments. Although osteogenesis and adipogenesis indicate their mesenchymal differentiation potential, the characteristic of CD34+ facilitates us to study their potential to differentiate into neural cells, cardiac muscle cells, endothelial cells, etc, and even to reconstitute lymphohematopoietic system.
Acknowledgments
The study was supported by the National High Technology Research and Development Program of China (863 Project) (Grant No: 2006AA02A119) and Fundamental Research Funds for the Central Universities.
References
- 1.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 2.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Nardi NB, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–282. [PubMed] [Google Scholar]
- 4.Bucala R, Spiegel LA, Chesney J. et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Abe R, Donnelly SC, Peng T. et al. Peripheral blood fibrocytes:differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7762. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 6.Mori L, Bellini A, Stacey MA. et al. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Wan C, He Q, Li G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J Orthop Res. 2006;24:610–618. doi: 10.1002/jor.20119. [DOI] [PubMed] [Google Scholar]
- 8.Ukai R, Honmou O, Harada K. et al. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J neurotrauma. 2007;24:508–520. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 11.Campagnoli C, Roberts IAG, Kumar S. et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 12.Zvaifler NJ, Marinova-Mutafchieva L, Adams G. et al. Mesenchumal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández M, Simon V, Herrera G. et al. Detection of stromal cells in peripheral blood progenitor cell collections from breast cancer patients. Bone Marrow Transplant. 1997;20:265–271. doi: 10.1038/sj.bmt.1700890. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus HM, Haynesworth SE, Gerson SL. et al. Human bone marrow-derived mesenchymal (stromal) progenitor cells (MPCs) cannot be recovered from peripheral blood progenitor cell collections. J Hematother. 1997;6:447–455. doi: 10.1089/scd.1.1997.6.447. [DOI] [PubMed] [Google Scholar]
- 15.Wexler SA, Donaldson C, Denning-Kendall P. et al. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsov SA, Mankani MH, Gronthos S. et al. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwana M, Okazaki Y, Kodama H. et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–845. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- 18.Kuwana M, Okazaki Y, Kodama H. et al. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells. 2006;24:2733–2743. doi: 10.1634/stemcells.2006-0026. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci USA. 2003;100:2426–2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Chen L, Scott PG. et al. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 21.Spees JL, Olson SD, Ylostalo J. et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]