Abstract
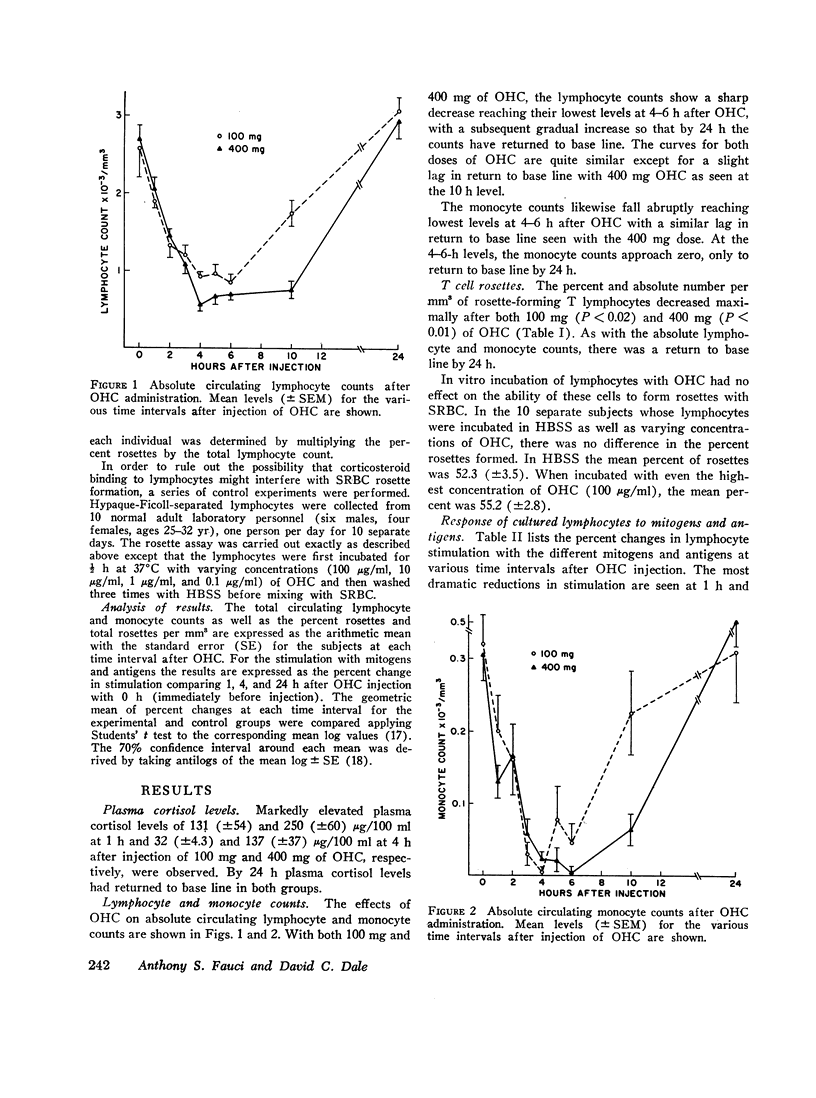
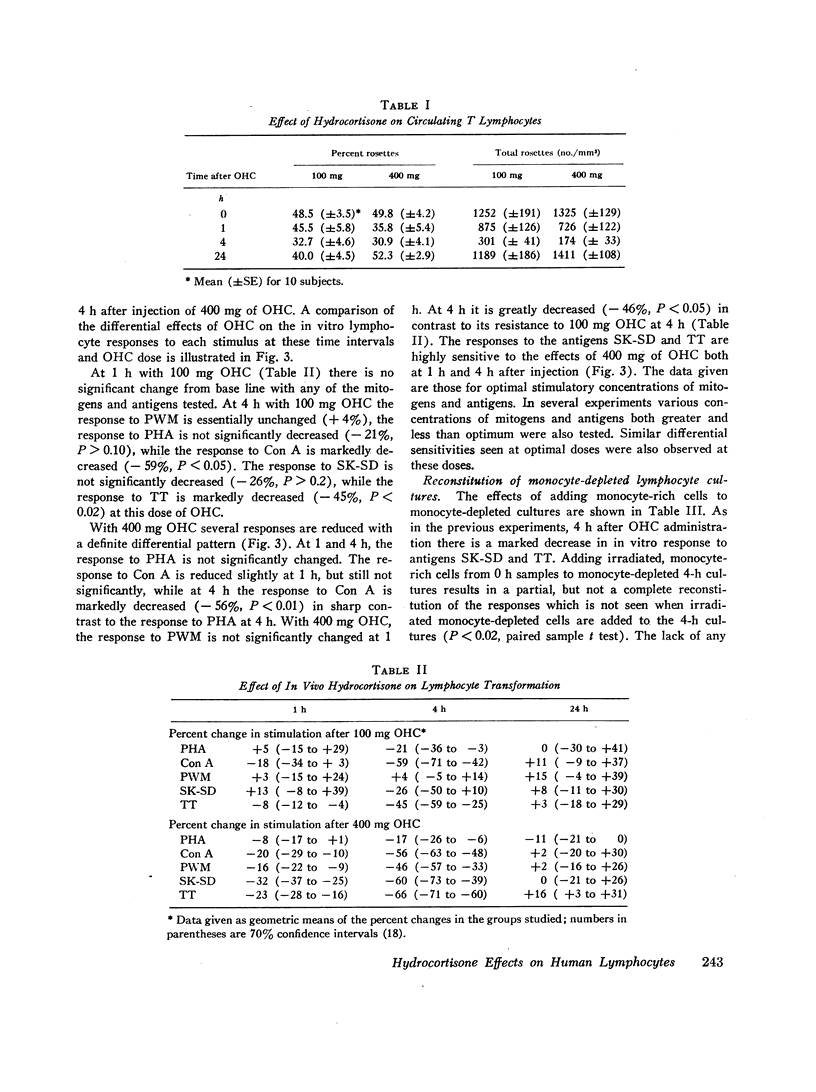
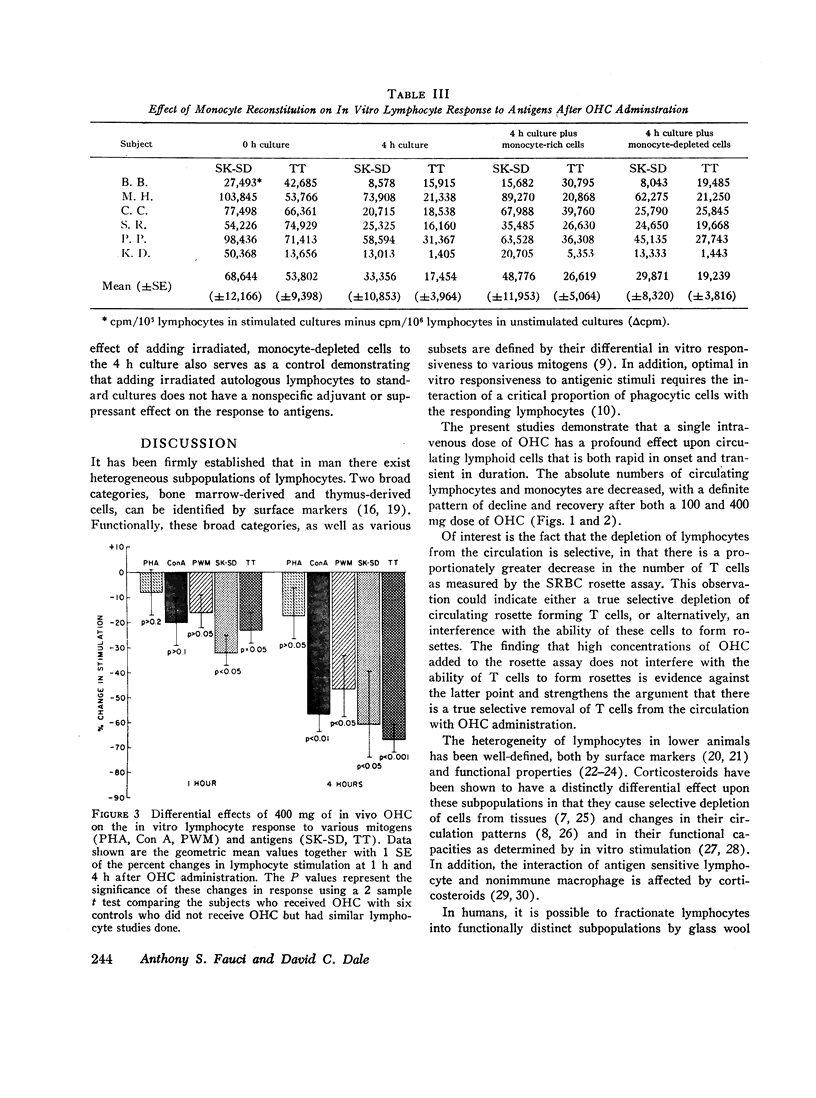
This study was designed to determine the effect of in vivo hydrocortisone on subpopulations of lymphoid cells in normal humans. Subjects received a single intravenous dose of either 100 mg or 400 mg of hydrocortisone, and blood was drawn at hourly intervals for 6 h, and then again at 10 and 24 h after injection. Profound decreases in absolute numbers of circulating lymphocytes and monocytes occurred at 4-6 h after both 100 mg and 400 mg of hydrocortisone. Counts returned to normal by 24 h. The relative proportion of circulating thymus-derived lymphocytes as measured by the sheep red blood cell rosette assay decreased maximally by 4 h and returned to base line 24 h after hydrocortisone. There was a selective depletion of functional subpopulations of lymphocytes as represented by differential effects on in vitro stimulation with various mitogens and antigens. Phytohaemagglutinin response was relatively unaffected, while responses to concanavalin A were significantly diminished. Responses to pokeweed mitogen were unaffected by 100 mg of hydrocortisone, but greatly diminished by 400 mg of hydrocortisone. In vitro responses to the antigens streptokinase-streptodornase and tetanus toxoid were markedly diminished by in vivo hydrocortisone. Reconstitution of monocyte-depleted cultures with autologous monocytes partially corrected the diminished response to antigens. This transient selective depletion of monocytes and subsets of human lymphocytes by a single dose of hydrocortisone is most compatible with a redistribution of these cells out of the circulation into other body compartments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVORNKITTI S., KANGSADAL P., SATHIRAPAT P., OONSOMBATTI P. Reversion and reconversion rate of tuberculin skin reactions in correction with the use of prednisone. Dis Chest. 1960 Jul;38:51–55. doi: 10.1378/chest.38.1.51. [DOI] [PubMed] [Google Scholar]
- Balow J. E., Rosenthal A. S. Glucocorticoid suppression of macrophage migration inhibitory factor. J Exp Med. 1973 Apr 1;137(4):1031–1041. doi: 10.1084/jem.137.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Nussenzweig V. Theta-bearing and complement-receptor lymphocytes are distinct populations of cells. Science. 1971 Jul 9;173(3992):154–156. doi: 10.1126/science.173.3992.154. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. In vitro approaches to the mechanism of cell-mediated immune reactions. Adv Immunol. 1971;13:101–208. doi: 10.1016/s0065-2776(08)60184-4. [DOI] [PubMed] [Google Scholar]
- Boldt S., Skinner A. M., Kornfeld S. Studies of two subpopulations of human lymphocytes differing in responsiveness to concanavalin A. J Clin Invest. 1972 Dec;51(12):3225–3234. doi: 10.1172/JCI107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. 3. Evidence for interaction between two types of thymus-derived cells. J Exp Med. 1972 Apr 1;135(4):764–779. doi: 10.1084/jem.135.4.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Moorhead J. W., Benner W. H. Corticosteroids and lymphoid cells in vitro. I. Hydrocortisone lysis of human, guinea pig, and mouse thymus cells. J Lab Clin Med. 1971 Oct;78(4):499–507. [PubMed] [Google Scholar]
- Coburg A. J., Gray S. H., Katz F. H., Penn I., Halgrimson C., Starzl T. E. Disappearance rates and immunosuppression of intermittent intravenously administered prednisolone in rabbits and human beings. Surg Gynecol Obstet. 1970 Nov;131(5):933–942. [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol. 1972 Mar;108(3):841–844. [PubMed] [Google Scholar]
- Craddock C. G., Longmire R., McMillan R. Lymphocytes and the immune response. 2. N Engl J Med. 1971 Aug 12;285(7):378–384. doi: 10.1056/NEJM197108122850705. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Kamin R. M., Fudenberg H. H. Human lymphocyte response to phytomitogens in vitro: normal, agammaglobulinemic and paraproteinemic individuals. J Immunol. 1969 Dec;103(6):1185–1195. [PubMed] [Google Scholar]
- Esteban J. N. The differential effect of hydrocortisone on the short-lived small lymphocyte. Anat Rec. 1968 Nov;162(3):349–356. doi: 10.1002/ar.1091620309. [DOI] [PubMed] [Google Scholar]
- Farid N. R., Munro R. E., Row V. V., Volpé R. Peripheral thymus-dependent (T) lymphocytes in Graves's disease and Hashimoto's thyroiditis. N Engl J Med. 1973 Jun 21;288(25):1313–1317. doi: 10.1056/NEJM197306212882502. [DOI] [PubMed] [Google Scholar]
- Folb P. I., Trounce J. R. Selective pharmacological inhibition of lymphocyte stimulation. Lancet. 1971 Jul 24;2(7717):221–222. doi: 10.1016/s0140-6736(71)90941-x. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. A., Claman H. N. Bone marrow and spleen: dissociation of immunologic properties by cortisone. Science. 1970 Mar 13;167(3924):1515–1517. doi: 10.1126/science.167.3924.1515. [DOI] [PubMed] [Google Scholar]
- Loriaux D. L., Guy R., Lipsett M. B. A simple, quick, solid-phase method for radioimmunoassay of plasma estradiol and of plasma cortisol of late pregnancy. J Clin Endocrinol Metab. 1973 Apr;36(4):788–790. doi: 10.1210/jcem-36-4-788. [DOI] [PubMed] [Google Scholar]
- Moorhead J. W., Claman H. N. Thymus-derived lymphocytes and hydrocortisone: identification of subsets of theta-bearing cells and redistribution to bone marrow. Cell Immunol. 1972 Sep;5(1):74–86. doi: 10.1016/0008-8749(72)90085-8. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- QUITTNER H., WALD N., SUSSMAN L. N., ANTOPOL W. The effect of massive doses of cortisone on the peripheral blood and bone marrow of the mouse. Blood. 1951 Jun;6(6):513–521. [PubMed] [Google Scholar]
- Schechter G. P. McFarland W,+MACFARLAND W: Interaction of lymphocytes and a radioresistant cell in PPD-stimulated human leukocyte cultures. J Immunol. 1970 Sep;105(3):661–669. [PubMed] [Google Scholar]
- Shortman K., Byrd W. J., Cerottini J. C., Brunner K. T. Characterisation and separation of mouse lymphocyte subpopulations responding to phytohemagglutinin and pokeweed mitogens. Cell Immunol. 1973 Jan;6(1):25–40. doi: 10.1016/0008-8749(73)90003-8. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Thompson J., van Furth R. The effect of glucocorticosteroids on the proliferation and kinetics of promonocytes and monocytes of the bone marrow. J Exp Med. 1973 Jan 1;137(1):10–21. doi: 10.1084/jem.137.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L. Effect of hydrocortisone on the reactivity of thymus and spleen cells of mice to in vitro stimulation. Immunology. 1972 Nov;23(5):777–784. [PMC free article] [PubMed] [Google Scholar]
- Weston W. L., Claman H. N., Krueger G. G. Site of action of cortisol in cellular immunity. J Immunol. 1973 Mar;110(3):880–883. [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]



