Abstract
In the human body, over 1000 different G protein-coupled receptors (GPCRs) mediate a broad spectrum of extracellular signals at the plasma membrane, transmitting vital physiological features such as pain, sight, smell, inflammation, heart rate and contractility of muscle cells. Signaling through these receptors is primarily controlled and regulated by a group of kinases, the GPCR kinases (GRKs), of which only seven are known and thus, interference with these common downstream GPCR regulators suggests a powerful therapeutic strategy. Molecular modulation of the kinases that are ubiquitously expressed in the heart has proven GRK2, and also GRK5, to be promising targets for prevention and reversal of one of the most severe pathologies in man, chronic heart failure (HF). In this article we will focus on the structural aspects of these GRKs important for their physiological and pathological regulation as well as well known and novel therapeutic approaches that target these GRKs in order to overcome the development of cardiac injury and progression of HF.
Introduction
GRK regulation of GPCRs is an agonist-dependent event that occurs via receptor phosphorylation and subsequent disruption of signaling. This process, termed desensitization, is initiated by interaction of GRKs with activated receptor proteins at the plasma membrane and primarily serine residues located within the C-terminal domain of the GPCR are phosphorylated [1]. Further, phosphorylation of receptors leads to lower active coupling to the heterotrimeric G protein and a loss of signaling. Moreover, GRK-mediated phosphorylation of a GPCR leads to recruitment of arrestins and subsequent endocytosis and internalization of the receptor resulting in a net down-regulation of expression [1]. One of the most studied patterns of GPCR desensitization is that which occurs for β-adrenergic receptors (β-ARs) in the failing heart as the loss of β-AR coupling and down-regulation seen in HF is a cardinal characteristic of this pathological condition and importantly, appears to correlate with GRK2 and GRK5 up-regulation [2,3]. Overall, the kinase activity and protein expression of GRKs is differentially regulated in physiological and pathological conditions and their regulation of specific GPCR signals make them an interesting target to manipulate in order to alter intracellular signaling pathways in cells to potentially elucidate novel drug targets. In this review, we will focus on features that have rendered some GRKs (primarily GRK2 and GRK5) in diseased myocardium to be considered as two promising targets in HF, and how their inhibition might be implemented.
I Structural Aspects of GRK Targeting
The GRKs form a family of seven mammalian members (GRK1-GRK7) that share a similar basic structure with an N-terminal, a central catalytic domain and a distinguishing C-terminal domain. The latter domain divides the GRKs into three sub-family types [4]. GRK1 and GRK7, also called the retinal opsin kinases make up one family and these two GRKs are expressed only in the eye. The second family is known as the βARK (for β-adrenergic receptor kinase) family and made up of GRK2 and GRK3, which were first characterized and identified in studies using β2-ARs. GRK2 and GRK3 share the characteristic that within their C-tail there is a pleckstrin homology (PH) domain that mediates phosphoinositol-4,5-bisphosphonate (PIP2) and G protein βγ-subunit (Gβγ) binding and regulation [5]. Lastly, the GRK4-like subfamily consisting of GRK4, GRK5 and GRK6, which in contrast to the other subtypes, are highly associated with the plasma membrane through N-terminal and C-terminal basic amino acid residue motifs [5]. The distribution and differential regulation of expression of GRKs probably forms the foundation of their specificity in vivo: Importance in the cardiovascular system was attributed to GRK2, GRK3 and GRK5, since they are expressed ubiquitously but most highly in the myocardium and endothelial cells. However, gene ablation of only GRK2 led to a distinct phenotype with embryonic lethality at day 15 [6], revealing a critical role for this kinase in development [7] and proving that in contrast with other GRKs it is not redundant.
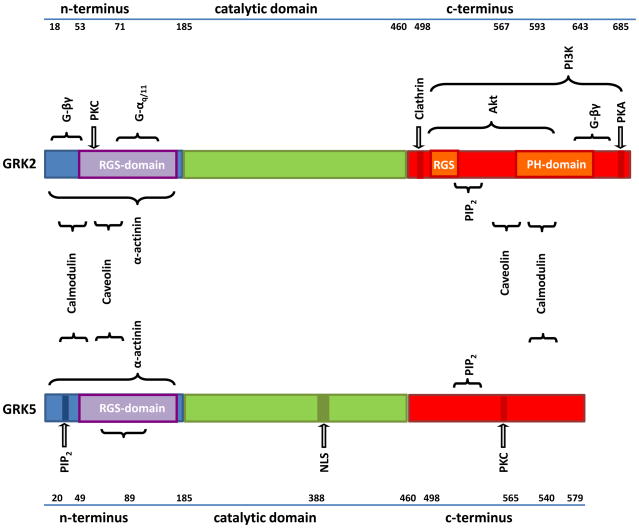
GRKs (Figure 1) have a similar in length N-terminus (183–188aa) that share considerable homology, which for at least GRK2 and GRK5 contains an RGS (Regulator of G protein Signaling) region that has been suggested to be able to regulate GPCRs by interaction with Gα proteins in a phosphorylation-independent manner [8–10], although its GTPase activity is not high as in classical RGS proteins. The N-terminus is also believed to be responsible for receptor recognition, which renders it an important structure for selective targeting of GPCRs. GRK2 for instance has an α-actinin binding domain in the N-terminus [11], which also contains a putative second Gβγ binding site at the N-terminus [12]. The basic structure of the catalytic domain for kinase activity is highly preserved and bears significant homology to other serine-threonine kinases. The C-terminal region contains phosphorylation sites and key determinants of the cellular location and interaction with lipids and/or membrane proteins, such as binding to PIP2 and Gβγ [13,14]. The distinct structural characteristics within different GRK subfamilies form the basis for potential therapeutic intervention. For example, the primary approach studied to date in disruption of GRK2 binding to Gβγ and this can prevent membrane translocation and subsequent GPCR phosphorylation. This can be accomplished most efficiently with a peptide called the βARKct, which consists of the C-terminal region of GRK2 (amino acid residues 495–689).
Figure 1. Structural aspects of GRK regulation and potential intervention.
Similarities in the tri-domain structure of GRK2 and GRK5 show possible overlapping interaction with regulators and effectors. PIP2: Phospho-inositol-2-phosphatase, PKC: Phosphokinase C, PKA: Protein-Kinase A, PI3K: Phosphatidyl-inositol-2,4,5-Kinase, NLS: Nuclear localization sequence, PH: pleckstrin homology.
II Differential Regulation of GRKs
GRKs control their target GPCRs upon agonist activation via conformational changes that elicit GRK activation. Importantly, the recruitment of any given GRK is not random as formerly assumed, but quite specified by either cytosolic or membrane-associated localization as well as the availability and expression pattern of GRKs. These characteristics of GRKs present us with multiple valuable possibilities for potential targeting. For example, the RGS domain inherent in GRK2 and GRK3 is deemed an important feature with potential for therapeutic targeting [15]. Moreover, protein-protein interactions and phosphorylation-dependent conformational changes within the RGS and PH domains form the basis for regulation of catalytic activity of GRKs. GRK2 and GRK3 activity is enhanced by facilitation of binding to Gβγ and PKA-mediated phosphorylation at Ser670 of GRK2 can also enhance kinase activity [16]. Further, modulation of GRK2 and GRK3 can also occur via mitogen-activated kinases (MAPKs) and complexes of these GRKs with Ca2+/Calmodulin can inhibit GRK activity through a presumed negative feedback loop [17]. Phospholipase C (PLC) and src activity can enhance the kinase action of GRK2 as well [18,19]. An important example of specific regulatory effects between subfamilies of GRKs resides with complex formation with Ca2+/Calmodulin, which inhibits all non-visual GRKs, but shows significantly higher affinity binding to GRK5 than for GRK2 [20]. Further, GRKs exhibit a motif that allows caveolin binding which will decrease basal GRK activity [8]. Interestingly, PI3-Kinase is recruited to the plasma membrane through interaction with GRK2 and interception of this process at the binding part (PIK domain) can prevent β-AR internalization, thus providing another potential target for intervention in HF [21].
Taken together, regulation of activity at the N-terminus, possibly also phosphorylation-independent mechanisms mediated by the RGS-domain, and at multiple targets contained in the C-terminal region of GRKs provide ample alternatives for therapeutic targeting. The recent findings regarding specific patterns of binding and expression of GRKs as well as improved specificity of application have led to the assumption, that interference with one GRK alone is feasible and promising. However, the complexity of differential regulation, multiple interaction and redundancy of these kinases may pose a Gordian knot.
III Therapeutic Targeting of GRKs in Cardiovascular System and in HF
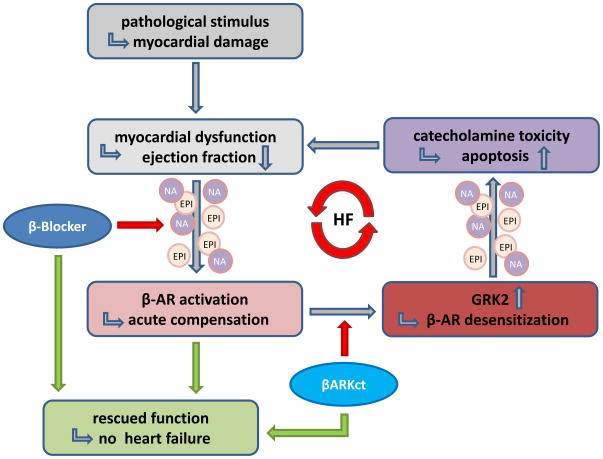
GRK2 can phosphorylate most GPCRs in vitro [1], but in vivo experiments done in transgenic mice have shown receptor specificity towards attenuation of cardiac β-ARs and Angiotensin II (AngII) receptor signaling and GRK2 does not appear to desensitize adenosine receptors or α1-ARs [22,23]. In the heart, ventricular contractile function is enhanced by sympathetic stimulation of β-AR mediated adenylyl cyclase activity and Protein kinase A (PKA) actions [24]. Sympathetic overstimulation due to enhanced tissue and circulating catecholamine neurotransmitters (epinephrine and norepinephrine) occurs as a compensatory mechanism to diminished contractile performance, subsequently leading to GRK2 upregulation and receptor desensitization. GRK2 activity and protein expression is increased 2–3-fold in the failing heart [25,26] and its contribution to the pathological mechanism of HF has been verified in numerous animal models (reviewed in [27]). It appears that GRK2 upregulation in compromised myocardium feeds into a vicious cycle of self-perpetuating dysregulation of the AR system and subsequent myocardial malfunction since desensitized receptors cause more agonist to be delivered to stimulate the failing heart (Figure 2).
Figure 2. Mechanism of catecholamines in development and progression of heart failure.
GRK inhibition by βARKct as well as interference with catecholamine induction by β-blocker therapy enable termination of the vicious cycle if adrenergic over-stimulation in HF.
Several studies have shown that inhibition or a reduction of GRK2 activity in cardiomyocytes can be beneficial for cardiac performance and geometry and actually reverses the above noted vicious cycle in HF [28]. Most recently this has been demonstrated clearly in mice and rats using two independent approaches: First, using conditional, cardiomyocyte-specific knock-out (KO) mice, a significant lowering of myocyte GRK2 expression prevented progression towards HF after a myocardial infarction (MI) and actually reversed HF and adverse remodeling when the loss of GRK2 was induced after ventricular dysfunction was evident [29]. In a second recent study, the βARKct was delivered to the hearts of rats with post-MI HF using adeno-associated virus serotype-6 (AAV6) and this gene therapy approach with the Gβγ-inhibitory peptide resulted in a chronic, clinical-like rescue of the HF phenotype [30]. The translation of GRK2 for human HF is proceeding with pre-clinical large animal AAV-βARKct gene therapy studies under way both in sheep [31] and pigs (Koch, unpublished studies).
Another promising recent result of targeting GRK2 via inhibition of Gβγ binding was carried out with developed small molecule inhibitors of this interaction [32]. Two membrane-permeable compounds, M119 and Gallein, were used and both improved cardiomyocyte contractility as well as cAMP generation and had a beneficial effect on cardiac function and remodeling in an animal model of isoproterenol-induced HF [32]. Interestingly, the administration of the non-selective β-blocker propranolol completely inhibited the M119 effect, suggesting specificity of this compound towards β-AR signaling and the potential regulation by GRK2. It will be important to determine whether or how these compounds modulate downstream signaling of Gβγ independent of GRK2 and whether they can be translated to the clinical condition since they will inhibit Gβγ-GRK2 everywhere and not just in the heart, which is a potential advantage of gene therapy using the βARKct.
Finally, concerning GRK2 in HF, it was found that GRK2 expression is up-regulated the adrenal gland when the heart is failing, where it regulates sympathetic nervous system activity via catecholamine production and secretion in the adrenal medulla which is under a negative-feedback loop via α2-ARs [33]. It appears that enhanced GRK2 activity desensitizes α2-ARs in the adrenal gland and promotes enhanced catecholamine release through this loss of negative feedback and interestingly, adenoviral-mediated βARKct gene delivery to the adrenal gland resulted in up-regulation of α2-ARs and lowered circulating catecholamine levels in a HF model and this moderate decrease in sympathetic activity provided significant improvement of cardiac function [34].
GRK5 is a member of the GRK4-like subfamily of GRKs and, classically, has been defined by its properties of more avid membrane association compared to GRK2 [23]. GRK5 in vivo can desensitize β-ARs and adenosine receptors but does not appear to desensitize AngII receptors [22,23]. Moreover, in contrast to GRK2, GRK5 contains a nuclear localization sequence (NLS), which imparts translocation to the nucleus [35]. Of importance to the failing heart, it was recently found that in response to hypertrophic stimuli, GRK5 nuclear accumulation in myocytes is enhanced and nuclear GRK5 has specific activity of histone deactylases (HDACs) that lift functional repression of genes associated with cardiac hypertrophy [36]. This provides the first direct evidence that in vivo, a GRK can have non-GPCR functions that are actually relevant to pathophysiology and this provides a novel function of GRK5 to target. As an example, when a mutant GRK5 was overexpressed that could not enter the nucleus, pressure overload hypertrophy was not maladaptive [36], indicating that limiting the nuclear activity of GRK5 is indeed a potential target for potential HF therapy.
Another interesting difference of GRK5 compared to GRK2 is that in humans, there are 4 polymorphisms for GRK5, one of which substitutes amino acid leucine for glutamine at position 41 (Leu41) in close proximity to a regulatory domain at the N-terminus (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=17098707), and leads to enhanced desensitization of β2-AR upon agonist treatment [37]. In vitro data support the hypothesis that this variant leads to accelerated desensitization of β1-adrenergic signaling and was protective in a transgenic mouse subjected to chronic isoproterenol infusion-induced HF [38]. The afforded protection from ventricular remodeling caused by catecholamine overstimulation was similar to that of a β-blocker. In a clinical setting, patients who suffer from HF that are carrying this allele, which occurs 10-fold higher in the African American population, were observed in a longitudinal study and indeed, the Leu41 polymorphism had a protective effect comparable to that of β-blocker therapy, measured as transplant-free survival [38]. Since 40% of African Americans carry the allele coding for Leu41, this might explain the generally disappointing response of this population to treatment with β-blockers. These intriguing findings suggest a larger trial and the need for elucidating the regulatory mechanisms of GRKs to improve and individualize patient care. There does not appear to be any relevant mutations for GRK2 in humans, which when coupled with the embryonic lethality in the GRK2 KO mice, may underlie its overall importance in physiology.
GRK3 is also worth mentioning since it is widely distributed including in the heart. GRK3 was identified early and has been studied extensively, and its highest expression levels are in olfactory epithelium and leukocytes, but also present in the lung, heart and adipose tissue [39]. The in vivo activity of GRK3 represents a concrete example of the specificity of GRKs towards GPCRs, which is questioned often. A study in transgenic mice with cardiac-specific GRK3 overexpression exhibited normal β-AR and AngII receptor signaling, which is in contrast to GRK2 although the two GRKs are highly homologous and both regulated via Gβγ binding [22]. GRK3 activity was found to desensitize thrombin and α1B-AR signaling in the heart [22], rendering this GRK a potential target in vascular pathology and hypertension.
Conclusions
Here we have integrated well-established concepts and recent research developments that render GRK2 and GRK5 promising targets for novel HF therapy. The emerging knowledge of intrinsic interactions between GRKs with certain GPCRs as well as particular patterns of GRK regulation by other kinases, protein binding and autophosphorylation delivers a picture of both challenging complexity and exceeding potential. Translational development of GRK2-Gβγ signaling as a potent target in HF has reached the pre-clinical stage in the case of viral-mediated βARKct gene therapy and novel discovery of small peptide inhibitors of this mechanism mark a further step towards future clinical application. Moreover, the role of GRK5 has been proven remarkable by its specific characteristic nuclear translocation affecting pathological hypertrophy and its importance in the clinical setting will further expand when increasing knowledge of its singular gene-drug interactions will prove useful in day-to-day practice.
Table 1.
Therapeutic targets in cardiovascular disease states/heart failure
| Target | Strategic approach | Hypothesis/Rationale | Group | References |
|---|---|---|---|---|
| GRK2 | Inducible cardio-specific ablation of GRK2 | Prevention of heart failure progression | Koch | [29] |
| GRK2 | Gene therapy with the inhibitory peptide bARKct | Inotropic effect to improve contractility | Koch | [30] |
| GRK2 | Selective inhibition of Gβγ subunits via a small peptide | Disruption of GRK2 recruitment and activity | Blaxall | [32] |
| GRK2 | βARKct expression in adrenal medulla | Inhibition of SNS activity and catecholaminergic toxicity | Koch | [34, 40] |
| GRK2 | Inhibition of GRK2-mediated PI3Kγ membrane association | Reduced β-AR internalization | Rockman | [41, 42] |
| GRK5 | GRK5 polymorphism at Leu41 leads to protection from catecholamine induced | Leu41 expression protects from progression of HF | Dorn | [38] |
| GRK5 | GRK5 nuclear localization provides HDAC kinase effect to induce MEF2 | Inhibtion of GRK5 leads to reduced MEF2 activated hypertrophy | Koch | [36] |
Acknowledgments
WJ Koch is supported by NIH grants R37 HL61690, R01 HL56205, R01 HL088503, P01 HL091799, and P01 HL075443 (Project 2). H Brinks was supported by a developing scientist grant of the Swiss National Science Foundation (SNF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pitcher JA, et al. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 2.Iaccarino G, et al. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 3.Ping P, et al. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am J Physiol. 1997;273 (2 Pt 2):H707–717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- 4.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 5.Penela P, et al. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69 (1):46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Jaber M, et al. Essential role of β-adrenergic receptor kinase-1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matkovich SJ, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99 (9):996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 8.Carman CV, et al. Selective regulation of Galpha(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem. 1999;274 (48):34483–34492. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]
- 9.Sallese M, et al. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: direct interaction of kinase N terminus with activated galphaq. Mol Pharmacol. 2000;57 (4):826–831. [PubMed] [Google Scholar]
- 10.Dhami GK, et al. Phosphorylation-independent regulation of metabotropic glutamate receptor signaling by G protein-coupled receptor kinase 2. J Biol Chem. 2002;277 (28):25266–25272. doi: 10.1074/jbc.M203593200. [DOI] [PubMed] [Google Scholar]
- 11.Freeman JL, et al. alpha-Actinin is a potent regulator of G protein-coupled receptor kinase activity and substrate specificity in vitro. FEBS Lett. 2000;473 (3):280–284. doi: 10.1016/s0014-5793(00)01543-x. [DOI] [PubMed] [Google Scholar]
- 12.Eichmann T, et al. The amino-terminal domain of G-protein-coupled receptor kinase 2 is a regulatory Gbeta gamma binding site. J Biol Chem. 2003;278 (10):8052–8057. doi: 10.1074/jbc.M204795200. [DOI] [PubMed] [Google Scholar]
- 13.Pitcher JA, et al. G protein-coupled receptor kinases. Annual Reviews in Biochemistry. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 14.DebBurman SK, et al. G protein-coupled receptor kinase GRK2 is a phospholipid-dependent enzyme that can be conditionally activated by G protein betagamma subunits. J Biol Chem. 1996;271 (37):22552–22562. doi: 10.1074/jbc.271.37.22552. [DOI] [PubMed] [Google Scholar]
- 15.Willets JM, et al. Specificity of g protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell m3 muscarinic acetylcholine receptor signaling. Mol Pharmacol. 2003;64 (5):1059–1068. doi: 10.1124/mol.64.5.1059. [DOI] [PubMed] [Google Scholar]
- 16.Penela P, et al. Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15 (11):973–981. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 17.Pitcher JA, et al. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem. 1999;274 (49):34531–34534. doi: 10.1074/jbc.274.49.34531. [DOI] [PubMed] [Google Scholar]
- 18.Winstel R, et al. Protein kinase cross-talk: membrane targeting of the beta-adrenergic receptor kinase by protein kinase C. Proc Natl Acad Sci U S A. 1996;93 (5):2105–2109. doi: 10.1073/pnas.93.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan G, et al. c-Src tyrosine kinase binds the beta 2-adrenergic receptor via phospho-Tyr-350, phosphorylates G-protein-linked receptor kinase 2, and mediates agonist-induced receptor desensitization. J Biol Chem. 2001;276 (16):13240–13247. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- 20.Pronin AN, et al. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem. 1997;272 (29):18273–18280. doi: 10.1074/jbc.272.29.18273. [DOI] [PubMed] [Google Scholar]
- 21.Perrino C, et al. Restoration of β-adrenergic receptor signaling and contractile function in heart failure by disruption of the βARK1/phosphoinositide 3-kinase complex. Circulation. 2005;111:2579–2587. doi: 10.1161/CIRCULATIONAHA.104.508796. [DOI] [PubMed] [Google Scholar]
- 22.Eckhart AD, et al. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ Res. 2000;86 (1):43–50. doi: 10.1161/01.res.86.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Rockman HA, et al. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proceedings of the National Academy of Sciences of the. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch WJ, et al. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 25.Bristow MR, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307 (4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 26.Ungerer M, et al. Expression of beta-arrestins and beta-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74 (2):206–213. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- 27.Brinks H, Koch WJ. betaARKct: A Therapeutic Approach for Improved Adrenergic Signaling and Function in Heart Disease. J Cardiovasc Transl Res. doi: 10.1007/s12265-010-9206-6. [DOI] [PubMed] [Google Scholar]
- 28.Rockman HA, et al. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 29.Raake PW, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103 (4):413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rengo G, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119 (1):89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swain J. Improved Myocardial Mechanics After GRK2 Inhibition Molecular Cardiac Surgery With Recirculating Delivery (MCARD™) to deliver AAV6-βARKct in Sheep. Circ Suppl 2009 [Google Scholar]
- 32.Casey LM, et al. Small Molecule Disruption of G{beta}{gamma} Signaling Inhibits the Progression of Heart Failure. Circ Res. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Small KM, et al. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347 (15):1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 34.Lymperopoulos A, et al. Adrenal GRK2 up-regulation mediates sympathetic overdrive in chronic heart failure. Nature Medicine. 2007 doi: 10.1038/nm1553. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Johnson LR, et al. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol Cell Biol. 2004;24 (23):10169–10179. doi: 10.1128/MCB.24.23.10169-10179.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martini JS, et al. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105 (34):12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang WC, et al. A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors. Pharmacogenet Genomics. 2008;18 (8):729–732. doi: 10.1097/FPC.0b013e32830967e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liggett SB, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14 (5):510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Premont RT, et al. Protein kinases that phosphorylate activated G protein-coupled receptors. Faseb J. 1995;9 (2):175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 40.Lymperopoulos A, et al. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285 (21):16378–16386. doi: 10.1074/jbc.M109.077859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naga Prasad SV, et al. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by β-adrenergic receptor kinase 1. Journal of Biological Chemistry. 2001;276:18953–18959. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- 42.Perrino C, et al. Targeted inhibition of β-adrenergic receptor kinase-1-associated phosphoinositide-3 kinase activity preserves receptor signaling and prolongs survival in heart failure induced by calsequestrin overexpression. Journal of the American College of Cardiology. 2005;45:1862–1870. doi: 10.1016/j.jacc.2005.02.062. [DOI] [PubMed] [Google Scholar]