Abstract
The functional and morphologic pattern of superficial nephron development was studied in guinea pigs ranging in age between 2 h and 38 days. Concomitent measurements of total kidney function and glomerular counts were also performed.
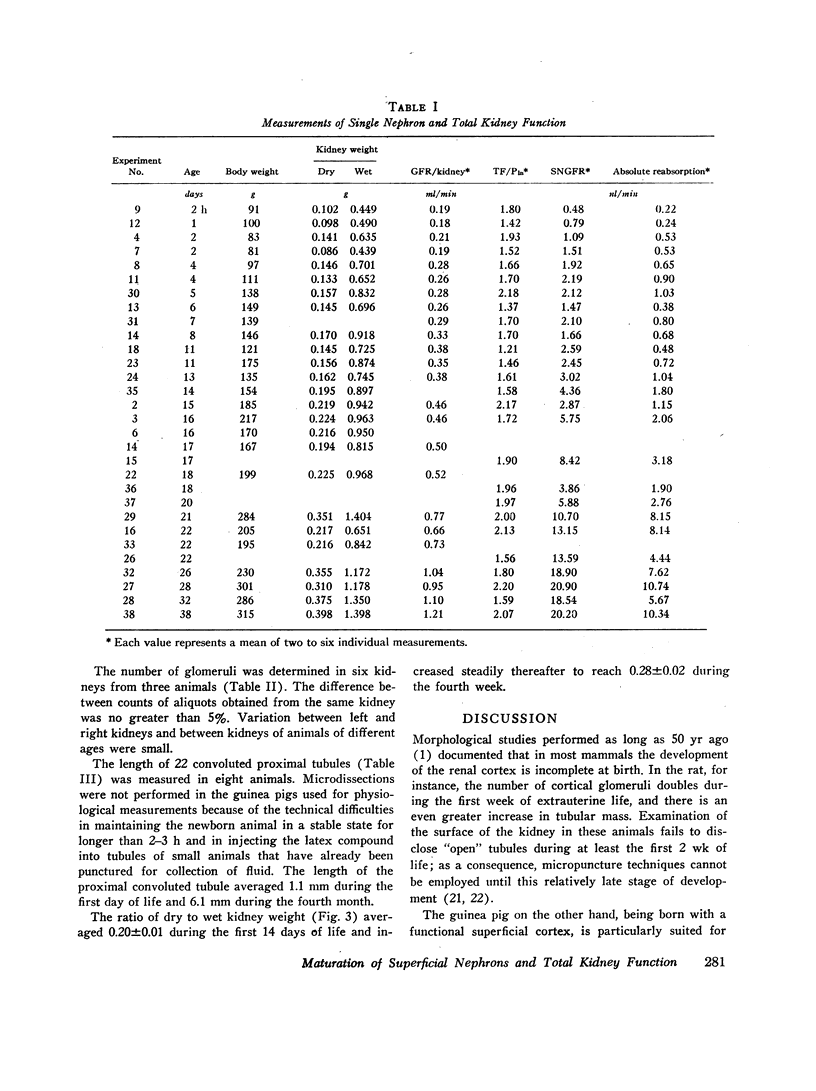
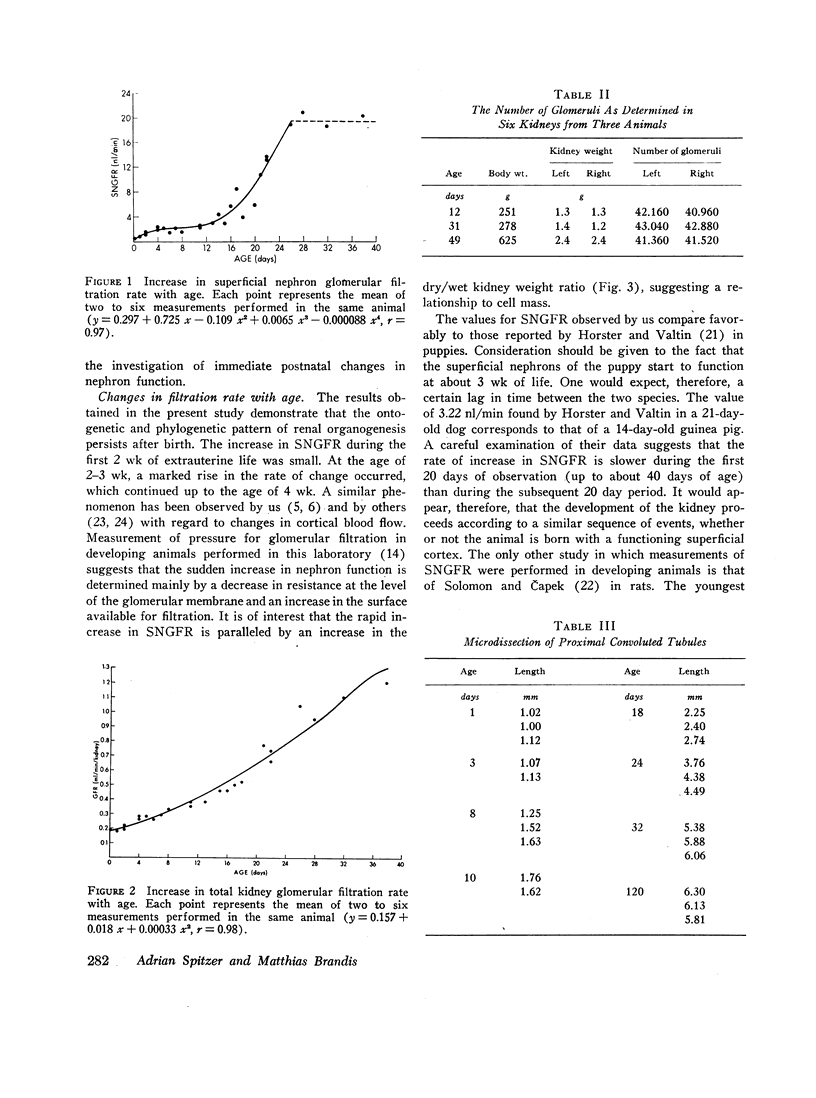
Superficial nephron glomerular filtration rate was found to increase from 0.92 to 19.32 nl/min. The filtration rate of the entire kidney rose from 0.19 to 1.31 ml/min. During the first 15 days of life the average rate of increase in glomerular filtration rate per nephron (0.48 nl/min·day), obtained by dividing the increase in total kidney glomerular filtration rate by glomerular number, was more than twice the observed rate of increase in the superficial nephrons (0.21 nl/min·day). During the remainder of the first month, the increase in superficial nephron glomerular filtration rate (0.97 nl/min·day) was greater than the average increase for all nephrons (0.71 nl/min·day). Thus, the initial increase in total kidney glomerular filtration rate was primarily a consequence of the activity of the deep nephrons, whereas during the ensuing period the superficial nephrons appeared to be the sole contributors to the change in total kidney glomerular filtration rate.
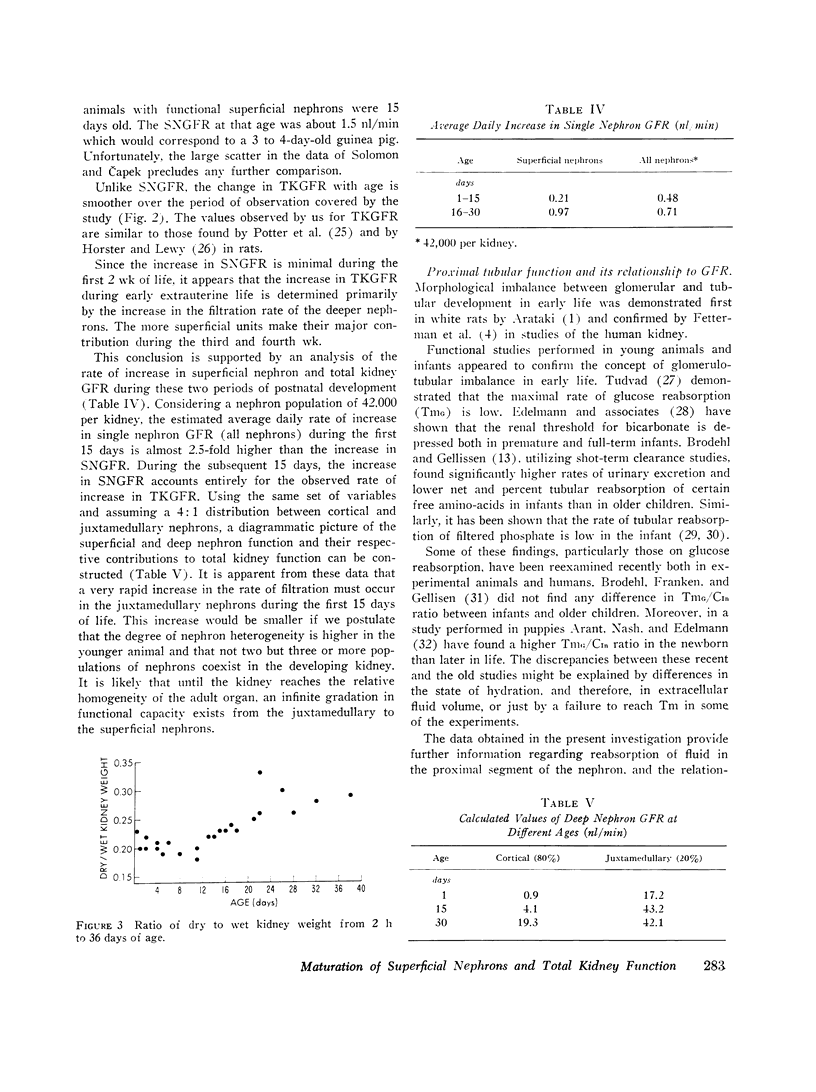
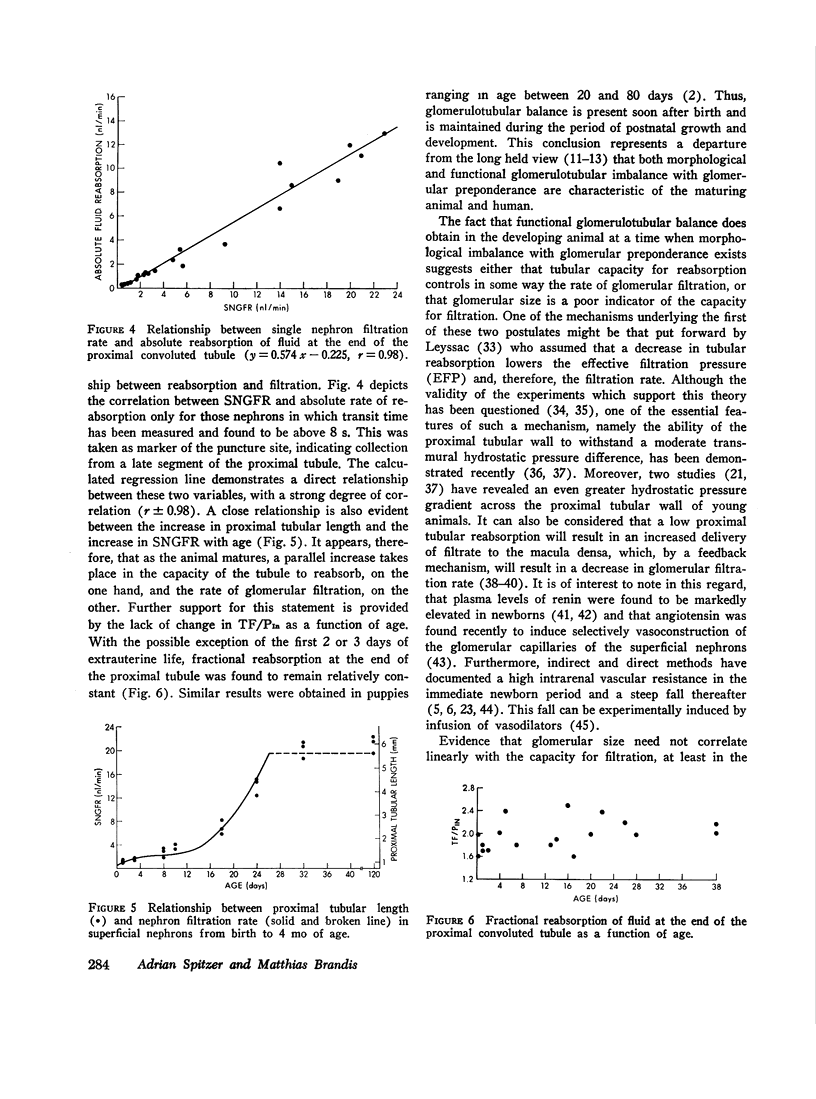
The increase in superficial nephron glomerular filtration rate was found to correlate closely with the increase in proximal tubular length. Functional glomerulotubular balance was maintained throughout the entire period of renal maturation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. E., Lipham E. M., Gottschalk C. W. Hydrostatic pressure in the rat kidney. Am J Physiol. 1972 Oct;223(4):975–983. doi: 10.1152/ajplegacy.1972.223.4.975. [DOI] [PubMed] [Google Scholar]
- Arturson G., Groth T., Grotte G. Human glomerular membrane porosity and filtration pressure: dextran clearance data analysed by theoretical models. Clin Sci. 1971 Feb;40(2):137–158. doi: 10.1042/cs0400137. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. Pressures in cortical structures of the rat kidney. Am J Physiol. 1972 Feb;222(2):246–251. doi: 10.1152/ajplegacy.1972.222.2.246. [DOI] [PubMed] [Google Scholar]
- Brodehl J., Gellissen K. Endogenous renal transport of free amino acids in infancy and childhood. Pediatrics. 1968 Sep;42(3):395–404. [PubMed] [Google Scholar]
- CALCAGNO P. L., RUBIN M. I. RENAL EXTRACTION OF PARA-AMINOHIPPURATE IN INFANTS AND CHILDREN. J Clin Invest. 1963 Oct;42:1632–1639. doi: 10.1172/JCI104848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMADIAN R. V., SHWAYRI E., BRICKER N. S. ON THE EXISTENCE OF NON-URINE FORMING NEPHRONS IN THE DISEASED KIDNEY OF THE DOG. J Lab Clin Med. 1965 Jan;65:26–39. [PubMed] [Google Scholar]
- Dean R. F., McCance R. A. Phosphate clearances in infants and adults. J Physiol. 1948 Mar 15;107(2):182–186. doi: 10.1113/jphysiol.1948.sp004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann C. M., Soriano J. R., Boichis H., Gruskin A. B., Acosta M. I. Renal bicarbonate reabsorption and hydrogen ion excretion in normal infants. J Clin Invest. 1967 Aug;46(8):1309–1317. doi: 10.1172/JCI105623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FETTERMAN G. H., SHUPLOCK N. A., PHILIPP F. J., GREGG H. S. THE GROWTH AND MATURATION OF HUMAN GLOMERULI AND PROXIMAL CONVOLUTIONS FROM TERM TO ADULTHOOD: STUDIES BY MICRODISSECTION. Pediatrics. 1965 Apr;35:601–619. [PubMed] [Google Scholar]
- Granger P., Rojo-Ortega J. M., Casado Pérez S., Boucher R., Genest J. The renin-angiotensin system in newborn dogs. Can J Physiol Pharmacol. 1971 Feb;49(2):134–138. doi: 10.1139/y71-019. [DOI] [PubMed] [Google Scholar]
- Gruskin A. B., Edelmann C. M., Jr, Yuan S. Maturational changes in renal blood flow in piglets. Pediatr Res. 1970 Jan;4(1):7–13. doi: 10.1203/00006450-197001000-00001. [DOI] [PubMed] [Google Scholar]
- Heller J. The influence of lissamine green on tubular reabsorption of electrolytes and water in rats. Pflugers Arch. 1971;323(1):27–33. doi: 10.1007/BF00586563. [DOI] [PubMed] [Google Scholar]
- Hornych H., Beaufils M., Richet G. The effect of exogenous angiotensin on superficial and deep glomeruli in the rat kidney. Kidney Int. 1972 Dec;2(6):336–343. doi: 10.1038/ki.1972.117. [DOI] [PubMed] [Google Scholar]
- Horster M., Lewy J. E. Filtration fraction and extraction of PAH during neonatal period in the rat. Am J Physiol. 1970 Oct;219(4):1061–1065. doi: 10.1152/ajplegacy.1970.219.4.1061. [DOI] [PubMed] [Google Scholar]
- Horster M., Thurau K. Micropuncture studies on the filtration rate of single superficial and juxtamedullary glomeruli in the rat kidney. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):162–181. doi: 10.1007/BF00362733. [DOI] [PubMed] [Google Scholar]
- Horster M., Valtin H. ostnatal development of renal function: micropuncture and clearance studies in the dog. J Clin Invest. 1971 Apr;50(4):779–795. doi: 10.1172/JCI106549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R. L. Intrarenal heterogeneity. The case for two functionally dissimilar populations of nephrons in the mammalian kidney. Am J Med. 1973 Mar;54(3):281–289. doi: 10.1016/0002-9343(73)90022-3. [DOI] [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of superficial and juxtamedullary nephrons in the rat. Am J Physiol. 1970 Jan;218(1):46–55. doi: 10.1152/ajplegacy.1970.218.1.46. [DOI] [PubMed] [Google Scholar]
- Jose P. A., Logan A. G., Slotkoff L. M., Lilienfield L. S., Calcagno P. L., Eisner G. M. Intrarenal blood flow distribution in canine puppies. Pediatr Res. 1971 Aug;5(8):335–344. doi: 10.1203/00006450-197108000-00001. [DOI] [PubMed] [Google Scholar]
- Kotchen T. A., Strickland A. L., Rice T. W., Walters D. R. A study of the renin-angiotensin system in newborn infants. J Pediatr. 1972 Jun;80(6):938–946. doi: 10.1016/s0022-3476(72)80005-2. [DOI] [PubMed] [Google Scholar]
- LEYSSAC P. P. Dependence of glomerular filtration rate on proximal tubular reabsorption of salt. Acta Physiol Scand. 1963 Jun-Jul;58:236–242. doi: 10.1111/j.1748-1716.1963.tb02644.x. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Schneider E. G., Strandhoy J. W., Willis L. R., Knox F. G. Effect of lissamine green dye on renal sodium reabsorption in the dog. J Appl Physiol. 1973 Jul;35(1):169–171. doi: 10.1152/jappl.1973.35.1.169. [DOI] [PubMed] [Google Scholar]
- McCRORY W. W., FORMAN C. W., McNAMARA H., BARNETT H. L. Renal excretion of inorganic phosphate in newborn infants. J Clin Invest. 1952 Apr;31(4):357–366. doi: 10.1172/JCI102616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbing H., Blaufox M. D., Aschinberg L. C., Silkalns G. I., Bernstein J., Spitzer A., Edelmann C. M., Jr Postnatal changes in renal glomerular blood flow distribution in puppies. J Clin Invest. 1973 Nov;52(11):2885–2895. doi: 10.1172/JCI107485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter D., Jarrah A., Sakai T., Harrah J., Holliday M. A. Character of function and size in kidney during normal growth of rats. Pediatr Res. 1969 Jan;3(1):51–59. doi: 10.1203/00006450-196901000-00007. [DOI] [PubMed] [Google Scholar]
- Spitzer A., Edelmann C. M., Jr Maturational changes in pressure gradients for glomerular filtration. Am J Physiol. 1971 Nov;221(5):1431–1435. doi: 10.1152/ajplegacy.1971.221.5.1431. [DOI] [PubMed] [Google Scholar]
- THURAU K. RENAL HEMODYNAMICS. Am J Med. 1964 May;36:698–719. doi: 10.1016/0002-9343(64)90181-0. [DOI] [PubMed] [Google Scholar]
- THURAU K., SCHNERMANN J. DIE NATRIUMKONZENTRATION AN DEN MACULA DENSA-ZELLEN ALS REGULIERENDER FAKTOR FUER DAS GLOMERULUMFILTRAT (MIKROPUNKTIONSVERSUCHE) Klin Wochenschr. 1965 Apr 15;43:410–413. doi: 10.1007/BF01483845. [DOI] [PubMed] [Google Scholar]
- Wahl M., Schnermann J. Microdissection study of the length of different tubular segments of rat superficial nephrons. Z Anat Entwicklungsgesch. 1969;129(2):128–134. doi: 10.1007/BF00522242. [DOI] [PubMed] [Google Scholar]



