Abstract
Nonhuman primates have been used for biomedical research for several decades. The high level of genetic homology to humans coupled with their outbred nature has made nonhuman primates invaluable preclinical models. In this review, we summarize recent advances in our understanding of the nonhuman primate immune system, with special emphasis on studies carried out in rhesus macaque (Macaca mulatta). We highlight the utility of nonhuman primates in the characterization of immune senescence and the evaluation of new interventions to slow down the aging of the immune system. Antioxid. Redox Signal. 14, 261–273.
Introduction
The immune system must overcome daily challenges from pathogens to protect the body from infection. The success of the immune response to infection relies on its ability to sense and evaluate microbial threats, coordinate the elimination of the threat, and limit damage to host tissues. This delicate balance is achieved through coordinated action of innate and adaptive arms of the immune system (Fig. 1). The main distinguishing characteristic of these two branches of the immune response is the way they recognize antigens. Whereas innate immunity relies on germline-encoded receptors to sense the presence of pathogens, adaptive immunity utilizes a highly diverse set of receptors generated through somatic mutation and recombination that are tailored to specific pathogens. The second major defining characteristic of the adaptive immune system is the development of immunological memory that manifests itself as increased functionality and frequency of responding cells upon re-exposure to the same antigen.
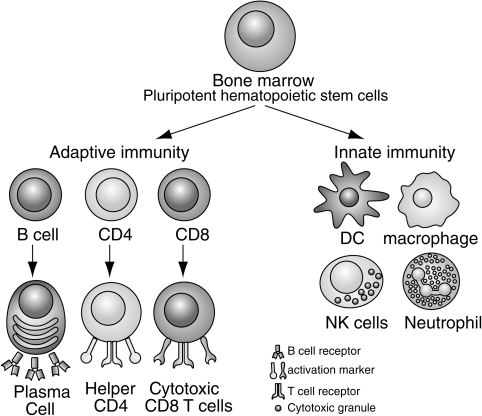
FIG. 1.
The immune system can be broadly divided into innate and adaptive branches. This figure highlights the key cellular components of each branch. Innate immunity is mostly mediated by dendritic cells (DC), natural killer (NK) cells, macrophages, and neutrophils. These cells use germline-encoded receptors to recognize pathogens. Adaptive immune response is mediated by T and B cells that express antigen receptors that recognize specific pathogens. The functions of these cells are discussed in more detail throughout the review.
Most of our understanding of how the immune system functions comes from studies utilizing specific pathogen free (SPF) laboratory rodents. SPF mice have been invaluable in the characterization of the cellular and molecular events that shape the development of the immune system and its response to challenges. Careful and methodical analysis using rodent model systems has led to key discoveries of cellular components of the immune system and have improved our understanding of how these components interact during steady state and when challenged during infection. Rodents offer several experimental advantages, including ease of genetic manipulation and a vast array of tools and resources. However, the inbred nature of laboratory rodents, their short life span, and the scarcity of murine homologues of human pathogens restrict the successful transfer of immunological discoveries made in murine models to the clinical setting (41).
Nonhuman primates (NHP), on the other hand, share significant genetic homology as well as physiological and behavioral characteristics with humans. As such, NHP models offer a unique opportunity to carry out mechanistic studies in a species that closely mimics human biology but yet can be maintained under tight laboratory conditions. NHP have been an invaluable resource in the dissection of mechanisms of pathogenesis and in testing vaccine efficacy to several pathogens. These studies have been greatly facilitated in recent years by significant advances in our understanding of the NHP immune system, especially in old world monkeys such as the rhesus macaque. In this review, we will summarize our current understanding of both the innate as well as the adaptive immune system of the rhesus macaque, while emphasizing parallels with the human immune system. Our knowledge of the innate arm of the NHP immune system is rather limited, thus, we will only present the salient features of studies pertaining to this area. On the other hand, our understanding of adaptive immunity in primates is much more thorough and will therefore be discussed in more depth. Lastly, we will review age-related changes in immune function (immune senescence) and interventions currently examined in the NHP model.
Innate Immunity
The innate immune system is the first line of defense against pathogens and it utilizes several mechanisms of pathogen recognition which can be generally grouped into three areas: a) recognition of microbial nonself; b) recognition of missing self; and c) recognition of altered self (61). Several immune cell subsets play a critical role in mediating innate immune responses. These include neutrophils, natural killer (NK) cells, dendritic cells (DC), and macrophages. Recognition of microbial entities relies on the detection of conserved molecular patterns referred to as pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) of the innate immune system as molecular signatures of infection. The two best-characterized families of PRRs are the toll-like receptors (TLR) and the retinoic acid-inducible gene (RIG-I)-like RNA helicases (RLHs). Following ligand recognition, these receptors initiate an intracellular signaling cascade leading to the production of several antimicrobial molecules that interfere with pathogen replication and spread. Furthermore, innate immune cells activate the adaptive arm of the immune system through the action of soluble mediators and antigen processing and presentation (60). In this section, we will focus our discussion on DC and NK cell function in rhesus macaques, as these subsets have been relatively well characterized.
Human dendritic cells are identified as CD3negCD20negHLA-DRposCD14neg and can be divided into two distinct populations: a) myeloid, or conventional, DCs (mDC), which are identified as CD11cposCD123dim (72); and b) plasmacytoid DCs (pDCs), which are characterized as CD11cnegCD123bright (72). Myeloid DCs mainly function to process and present antigens to naïve T cells and produce IL-12 upon activation; however, these cells generate very little of the antiviral cytokine interferon α (IFNα) in response to viral infection (89). In contrast, plasmacytoid DCs, produce vast amounts of IFNα in response to viral infection (15). These two major DC subsets have been identified in rhesus macaques using the same surface markers (22, 25, 96) (Fig. 2). More importantly, these DC subsets share functional similarities and general cytokine response upon activation and viral infection (22, 96). Since DCs represent <1% of total PBMCs in humans and NHP (22, 48), several methods have been developed to expand them both in vitro and in vivo. Human and NHP DCs can also be generated ex vivo from CD34pos progenitor cells or CD14pos monocytes cultured under specific cytokine conditions (38, 76), which facilitates functional studies. The administration of the hematopoietic growth factor Flt3 ligand (Flt3L) in vivo increases the frequency of circulating immature DCs by approximately 10–12-fold (25, 96). This has important implications for improved DC-targeting vaccine strategies to prevent viral infections (49).
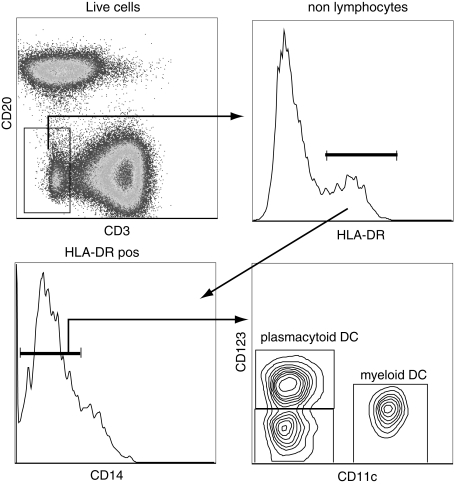
FIG. 2.
Identification of dendritic cell subsets in rhesus macaques. Dendritic cells (DCs) can be identified as nonlymphocytes (CD3negCD20neg) that express MHC-II molecules (HLA-DRpos) and lack the expression of CD14. DCs can be subdivided into myeloid DC and plasmacytoid DC based on the expression of CD11c and CD123. A minor subset does not express either of these markers.
Expression of TLRs within different subsets of macaque DCs exactly mimics expression in human DCs but differs significantly from murine DCs (48). Specifically, plasmacytoid pDCs isolated from rhesus macaques express TLR-7 and TLR-9 and respond to viral infection by producing large amounts of IFNα (22, 48), and by upregulating surface expression of CD86 (48). Furthermore, macaque pDCs constitutively express high levels of interferon regulatory factor-7 (IRF-7) (22), which is necessary for driving transcription of IFN-α. Similar to their human counter part, rhesus macaque myeloid DCs, express TLR-3, −4, −7, and −8, and respond to agonists for each of the 4 TLRs by upregulating surface expression of CD40 and CD86 (48). These studies provide important justification for the use of NHP to evaluate TLR ligands as adjuvants in vivo.
Human NK cells are identified as CD3negCD56pos cells and can be subdivided based on the expression of CD16 (52). The majority of blood and spleen resident NK cells are CD16pos (85%–90%); they are highly cytotoxic and secrete moderate amounts of inflammatory cytokines. The remaining NK cells are CD16neg; these cells cannot kill target cells but they secrete large amounts of inflammatory cytokines (52). Similarly, NK cells in rhesus macaques are CD3neg, and can be divided into a major cytolytic population that expresses high levels of CD16 and a minor cytokine-producing population expressing low levels of CD16 (Fig. 3). One major difference is that macaque NK cells, but not human NK cells, express high levels of CD8α (81).
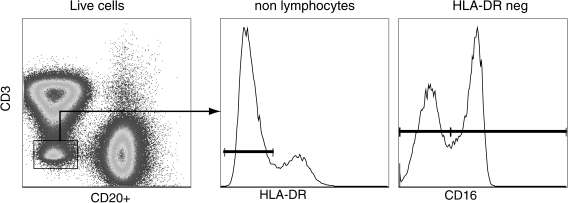
FIG. 3.
Identification of circulating natural killer cells in rhesus macaques. Peripheral blood resident NK cells can be identified as CD3negCD20negDRneg cells. The NK cells can then be subdivided into two subsets based on the expression of CD16: cytolytic NK cells (CD16pos); and inflammatory-cytokine producing NK cells (CD16neg).
NK cells mediate the recognition of missing and altered self through the expression of inhibitory and activation receptors (21). Recognition of MHC molecules is mediated by the killer inhibitory receptors (KIR), CD94/NKG2 complex (9). Homologues of the human CD94/NKG2 family members have been identified in rhesus macaques using molecular biology as well as flow cytometry tools (56). Specifically, rhesus NKp80, NKG2A, and NKG2D are expressed to similar levels as on human NK cells (59). More importantly, cytolytic assays demonstrated that NKp80 and NKG2A displayed similar cytolytic and inhibitory functions, respectively, as their human NK counterparts (59). Recent studies indicated that the gene expression of these markers could be modulated by SIV infection (65).
Some clinical studies have suggested that NK cells can impact the progression and severity of HIV disease (1). However, this hypothesis could not be directly addressed in clinical studies. The development of a humanized monoclonal antibody against CD16 had facilitated in vivo depletion studies in rhesus macaques (20). The administration of this antibody in vivo depleted over 88% of the NK cells (identified as CD3neg, CD16pos, CD159apos) from circulation. Compared to control monkeys infected with SIV, NK depletion resulted in higher peak SIV viral loads especially at times when NK depletion was most notable (20). In macaques previously infected with SIV, administering the human anti-CD16 monoclonal antibody was also successful in depleting 90% of NK cells and NK cell lytic function from PBMCs, but the depletion was shorter than in uninfected macaques, and it brought about little change with respect to viral loads (20). These data suggest that NK cells do not modulate AIDS severity in rhesus macaques.
Adaptive Immunity
The adaptive immune branch is composed of B and T lymphocytes, which unlike cells of the innate immune system, can generate a response tailored to specific pathogens. This specificity is acquired through the expression of diverse, clonally distributed antigen receptors on T and B lymphocytes. The initial diversity is produced in primary lymphoid organs (the thymus in the case of T cells and the bone marrow in the case of B cells) through a series of gene recombination events; further diversification occurs by somatic hypermutation of the B-cell receptor (BCR, antibody) and by functional diversification of effector T cells.
B cell compartment
Naïve B cells are produced in the bone marrow where B cell precursors undergo rearrangement and expression of the heavy and light chains that constitute the BCR. Upon antigen encounter, activated naïve B cells undergo vigorous proliferation, isotype class switching (from IgM to IgG, IgA, or IgE) and somatic hypermutation, thereby differentiating into antigen-experienced memory B cells producing high affinity antibodies (95). Human peripheral naïve and memory B cells can be distinguished based on the expression of CD27, a member of the tumor necrosis factor receptor family as well as the expression of IgD (95). Using these two markers, naïve B cells are identified as CD27posIgDpos, whereas memory B cells are CD27pos IgDneg. In addition, a third subset of B cells found in circulation expresses both CD27 and IgD (7). This subset has been shown to carry somatically mutated variable regions indicative of the fact that it is a transitional memory subset (106).
Similarly, B cells are identified in NHP based on the expression of CD20 and MHC class II molecules (such as HLA-DR). Rhesus macaque B cells can be subdivided into three subsets as described for humans based on the expression of CD27 and IgD (Fig 4). Furthermore, activation of rhesus macaque B cells results in the upregulation of CD80, CD86, and CD40, as described for humans (97). A high degree of conservation of BCR heavy and light chain variable regions between humans and macaques has facilitated molecular analysis of the B cell maturation in rhesus macaques (10, 36, 55). These tools facilitated studies of B cell repertoire maturation during simian immunodeficiency virus infection, and demonstrated that, as described for humans, germinal centers support antigen-driven somatic hypermutation of antibodies that follows a pattern and a rate of nucleotide substitution and selection that closely mimics that described for humans (57).
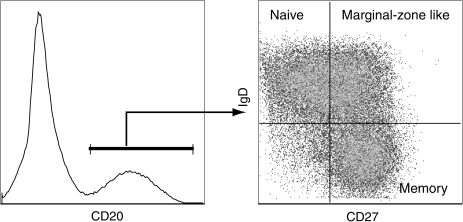
FIG. 4.
Delineation of B cell subsets in rhesus macaques. As described for humans, circulating B cells can be subdivided into three subsets based on the expression of CD27 and IgD. Naive B cells are identified as IgDposCD27neg and memory B cells are IgDnegCD27pos. In addition to these two main subsets, a third transitional subset referred as marginal zone-like B cells can be identified as IgDpos CD27pos. Both memory and marginal-zone like B cells contain somatic mutations in their B cell receptors, indicative of the fact that they have responded to antigens.
T cell repertoire
T cells identified based on the expression of the signaling complex CD3 are broadly divided into αβ CD4 and CD8 T cells (90%), as well as γδ T cells (10%). T cells recognize antigens in the form of small peptides bound to major histocompatibility (MHC) class I or class II molecules. CD8 T cells, commonly known as cytotoxic T cells, recognize foreign peptide bound to MHC-I molecules and have evolved to monitor for and eliminate tumor cells and cells harboring intracellular pathogens. CD4 T cells, also known as helper T cells, recognize foreign peptides bound to MHC-II and secrete a broad range of cytokines, which play a crucial role in the maturation of the B cell response as well as the development and establishment of the CD8 T cell response.
Since the host cannot predict the precise pathogen derived peptides that it will encounter, it relies on the generation and maintenance of a diverse T cell repertoire. During T cell development, TCR chains are generated through the stochastic recombination of noncontiguous TCR variable (V), diversity (D), joining (J), and constant (C) genes in the case of the β chain or V–J–C recombination in the case of the α chain. The human TCR β chain locus encodes 46 functional β chain variable segments, 2 diversity segments, 13 J segments and 2 constant regions. The TCR α chain locus encodes 41 functional V genes and ∼ 57 J genes. T cell repertoire diversity is further enhanced by random nucleotide deletions and additions at the junctions between these segments, as well as the pairing of different α and β chains. Theoretical estimates place the number of potential TCR combinations at 1015 (28), but additional selection mechanisms in the thymus limit this number such that the actual T cell repertoire diversity of naïve human T cells is estimated at 2.5 × 107 (4). The TCR contacts the peptide:MHC complexes using six hypervariable regions called the complementarity-determining regions (CDRs). CDR 1 and 2 contact mostly the MHC molecule, thus playing a critical role in orienting the TCR over the peptide:MHC complex. The segment of the T cell receptor chain that spans VDJ junctional region is called the CDR3. Structural studies have demonstrated that this region directly contacts the peptide bound by MHC molecules and thus plays a critical role in dictating the specificity of the TCR.
Our understanding of NHP T cell repertoire dynamics has been limited by the paucity of reagents. Only a handful of antibodies directed against human variable regions of the T cell receptor chains are available, and only 50% of these antibodies cross-react against rhesus TCR variable genes, which limits our ability to quantitatively assess changes in T cell populations. However, advances in molecular analysis technology allowed us to qualitatively assess T cell repertoire diversity in NHP. Thus far, 25 Vβ and 30 Vα genes have been sequenced and identified in rhesus macaques (17, 19, 43). Furthermore, as described for humans, two constant β and α genes were identified. All identified genes share significant homology (∼ 90% on average) with their human counterparts (54, 98)
T cell repertoire diversity in humans and rodents is qualitatively measured by spectratyping (4). This technology provides a global assessment of T cell repertoire diversity by displaying the distribution of CDR3 lengths of either the TCR α or β chain. In healthy young individuals, almost all TCR Vβ or Vα families display a Gaussian distribution of CDR3 lengths (74). However, antigenic exposure and disease result in the selective expansion of specific T cell populations, which in turn skews the Gaussian distribution of CDR3 lengths (11, 100). In some extreme cases, the CDR3 profile is dominated by a single sequence, a phenomenon referred as oligoclonal expansion. Similarly, healthy NHPs have a diverse T cell repertoire characterized by a Gaussian distribution of βCDR3 lengths (26). This pattern is disturbed during chronic viral infections such as SIV (18), where T cell repertoire analysis clearly revealed dominance of T cells expressing specific Vβ segments. In some instances, specific CDR3 sequences dominated the SIV-specific T cell repertoire and these monoclonal populations were stable for several months (108). More recently, a more powerful technique for assessing T cell repertoire has emerged wherein antigen-specific T cells are isolated and all potential CDR3 sequences determined in an unbiased manner (30). This approach was recently applied to analyze the CD4 T cell repertoire in response to rhesus cytomegalovirus (RhCMV) infection (79). The authors found that during acute infection, the RhCMV-specific CD4 T cell response was highly polyclonal. Over time, and with repeated exposure to RhCMV, which establishes a chronic infection, RhCMV-specific CD4+ T cells exhibited skewed hierarchies dominated by two or three clonotypes that were stable for several months. Recently, studies have shown that T cell repertoire also undergoes a significant shrinkage in aged rhesus macaques punctuated by the appearance of T cell clonal expansions (63) as described for elderly humans (78, 86).
The efficacy of the T cell response also relies on the presence of multiple MHC alleles that can present a large pool of peptides for potential T cell recognition. MHC molecules also play a crucial role in the selection of the T cell repertoire. Humans have three MHC class I loci: HLA-A, B, and C; and three MHC class II loci: HLA-DP, DQ, and DR. Each locus can encode multiple alleles. This polymorphism is especially concentrated in the peptide-binding portion of the molecule (42). Diversity within the human MHC loci is based on amino acid differences as well as the inheritance of specific set of HLA molecules from each parent with little crossover. Rhesus macaques have two MHC class I loci: A and B; and three MHC class II loci: DP, DQ, and DR (32, 73). Unlike humans, rhesus can have several MHC class I alleles on each chromosome and significant crossover can occur resulting in increased haplotype diversity.
There have also been significant advances in our ability to characterize functional diversity of the T cell response in rhesus macaques. Specifically, our ability to measure T cell production of several cytokines simultaneously has significantly improved over the last few years in rhesus macaques. One of the most commonly used methods is intracellular cytokine staining (ICS) where cells are stimulated with either intact virus, viral lysate, or peptide libraries in the presence of a chemical that blocks cytokine secretion (103). This approach enumerates cells that have produced a specific cytokine in response to stimulation. More importantly, due to improvements in flow cytometry that facilitate the analysis of several parameters simultaneously, this method allows the detection of T cells that can simultaneously produce multiple cytokines, which has been shown to be a critical determinant for disease outcome during chronic viral infections in human and nonhuman primates (47, 66). Significant advances in identifying T cells that can specifically recognize SIV-derived peptides bound to specific MHC class I molecule have also been made (35, 85). The ability to analyze the phenotype, structural and functional diversity of a specific T cell population has significantly improved our understanding of the T cell response to viral infection in rhesus macaques.
T cell homeostasis
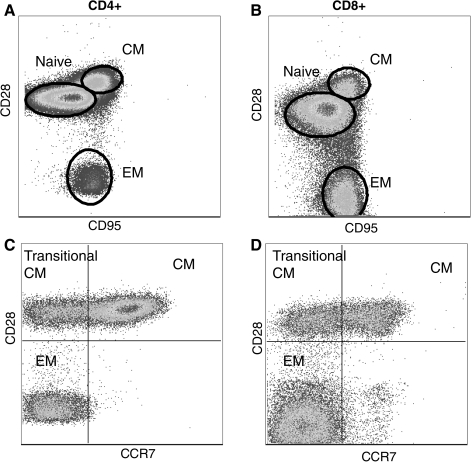
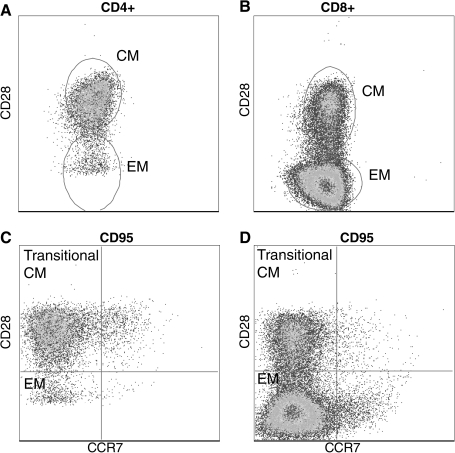
The maintenance of a structurally and functionally diverse T cell repertoire is a dynamic process governed by thymic output and the exposure to antigen and cytokines, which modulate T cell survival, proliferation and death. A rigorous analysis developed over several years has demonstrated that human CD4 and CD8 T cells can be subdivided into naïve, central memory (CM) and effector memory (EM). A similar effort has led to the delineation of naïve, CM and EM CD4 and CD8 T cells in rhesus macaques using CD28 and CD95 as the primary cell surface markers (77) (Fig. 5).Using these markers, naïve cells were defined as CD95negCD28pos, CM cells were defined as CD95posCD28pos, and EM cells were defined as CD95posCD28neg. Since the original description, this differentiation has been refined based on the expression of the chemokine receptor CCR7 as described for humans with CD95posCD28negCCR7neg being terminally differentiated EM T cells and CD95posCD28posCCR7pos representing CM T cells. This leaves CD95posCD28posCCR7neg and CD95posCD28negCCR7pos as transitional populations (75) (Fig 5). In accordance with this definition, CCR7pos CM T cells circulate in blood and lymphoid organs but are excluded from nonlymphoid tissues such as the lung where only CCR7negCD28posCD95pos cells are detected (75) (Fig 6).
FIG. 5.
Delineation of rhesus macaque T cell subsets. T cells can be broadly subdivided into three subsets based on the expression of CD28 and CD95. Naive T cells are identified as CD28posCD95neg, central memory T cells are CD28posCD95neg, and effector memory T cell are CD28negCD95neg (A and B). The definition of memory T cells can be further refined based on the expression of CCR7 as described for human T cells. (C) and (D) show CD95pos CD4 and CD8 memory T cells. The expression of CCR7 together with CD28 allows the identification of central memory, effector memory, and transitional central memory T cells that are differentiating to effector memory.
FIG. 6.
Nonlymphoid T cells do not express CCR7. T cells isolated from bronchial alveolar lavage are all memory phenotype based on CD95 expression. Furthermore, BAL-resident T cells lack the expression of CCR7.
Studies in rodents have identified IL-7 and IL-15 as key players in T cell homeostasis (93). IL-7 and MHC contact are critical for the survival of naïve T cells. These signals do not induce differentiation but rather a low level of homeostatic proliferation and promote the survival of these cells. Memory T cells on the other hand, do not require MHC contact for survival (12), but do require both IL-7 and IL-15 (80). T cell homeostasis in rhesus macaques is governed by similar requirements as those described for rodents. The genes encoding rhesus IL-7 and IL-15 sequences have been identified and cloned. The gene shares significant genetic (96%), amino acid and significant functional homology with the human/rodent homologues (3). Administration of IL-15 in rhesus macaques increased the generation of antiviral memory T cells and their IFNγ production (101). The administration of recombinant rhesus IL-7 induces proliferation of naïve and central memory T cells (75), whereas the administration of IL-15 enhanced proliferation of effector memory T cells (75). These studies have provided crucial pre-clinical data for trials to improve anti-viral and anti-tumor immunity.
Immune Senescence in Rhesus
Aging of the immune system results in a diminished immune response to infection and vaccination. Consequently, aged individuals suffer greater morbidity and mortality from infectious diseases than adult individuals. This state of diminished immune function is commonly referred to as immune senescence. Clinical studies have defined several hallmarks of immune senescence that have been collectively referred as the “immune risk phenotype” (IRP), which has been found to correlated with longevity (53). Although immune senescence impacts both innate and adaptive branches of the immune system, most studies to date strongly suggest that changes in adaptive immunity and more specifically the T cell compartment are largely responsible for this diminished immunity in the elderly (71).
The most striking age-related difference is the loss of naïve T cells (37) largely due to diminished thymic output, which significantly decreases after the sixth decade of life (70). This is further compounded by a life-long exposure to pathogens that results in significant conversion of naïve T cells to memory T cells (27). The loss of naïve T cells is accompanied by the accumulation of terminally differentiated memory T cells, especially CD8 T cells that have lost CD28 expression (99). This shift towards memory T cells results in a reduced diversity of the T cell repertoire, which is further compounded by the appearance of T cell clonal expansions (TCE) (78, 86). Another hallmark of immune senescence is the loss of T cell proliferative capacity (67). Aging of the immune system is accompanied by an increase in the production of inflammatory cytokines especially IL-6 and TNF-α (29). This phenomenon, often referred as “inflammaging”, is believed to contribute to the development of several chronic diseases such as Alzheimer's and osteoporosis (29).
The vast majority of our understanding of mechanisms underlying immune senescence comes from studies utilizing laboratory strains of SPF rodents. However, there are fundamental differences between the rodent and human immune system that complicate the transfer of findings between the two species. For instance, loss of CD28 expression on T cells, especially CD8 T cells, is one of the hallmarks of immune senescence in humans. Murine T cells, however, do not lose CD28 expression with age (24), thus rendering the identification of comparable T cell subsets difficult. The NHP model offers the highly desirable combination of increased life span and higher genetic homology to humans. Aging studies using NHP have been traditionally limited to the investigation of age-related changes in behavior, cognitive function, and reproduction. Although this model was severely underutilized for immune senescence studies, this picture is rapidly changing with the development of new immunological tools and protocols for longitudinal systemic analysis of immunity in this animal model. Furthermore, this model is amenable to longitudinal extensive experimentation and simultaneous sampling of several organs.
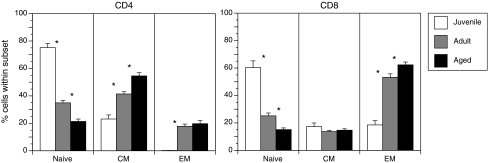
Recent studies have shown that rhesus macaques undergo immune senescence similar to what has been described for humans (44). There is a progressive loss of naïve T cells and a concomitant accumulation of memory phenotype T cells with increasing age (Fig. 7), especially CD28negCD8pos EM T cells (44, 63). The accumulation of terminally differentiated T cells results in narrowing of the T cell repertoire, which is compounded by the appearance of T cell clonal expansions identified based on the TCR β chain CDR3 polymorphism (63). In fact, the frequency of naïve T cells was inversely correlated to the frequency of TCE in aged monkeys (23). As described for humans, several factors contribute to the loss of naïve T cells in the aged rhesus macaque. In addition to thymic involution and decreased lymphopoeisis in the bone marrow, recent studies suggest that increased T cell turnover in the aged host significantly contributes to the depletion of naïve T cell reserves by increasing their conversion to memory cells (23). The study compared homeostatic proliferation rate within the three major subsets of T cells of adult and aged rhesus macaques using in vivo BrdU pulse/chase studies. BrdU incorporation as well as the expression of the cell cycle marker Ki-67 were elevated in peripheral naïve T cells, especially within the CD8 subset indicative on increased homeostatic proliferation. These data are consistent with clinical studies that reported an increase in CD4 T cell proliferation with age that dramatically amplified after 70 years of age (70). Furthermore, as described for humans, we detected an increase in frequency of T cells that secrete the inflammatory cytokines IFNγ and TNFα following polyclonal stimulation in rhesus macaques.
FIG. 7.
Advanced age is accompanied by severe loss of naive T cells in rhesus macaques. Frequency of naive, central memory (CM), and effector memory (EM) T cells was determined based on the expression of CD28 and CD95 in juvenile (∼1 year of age, n = 12), adult (5–10 years of age, n = 35) and aged (>17 years of age, n = 25). Cross sectional analysis indicates that, as described for humans, increasing age is associated with the loss of circulating naive T cells. Thus, rhesus macaques are a robust model to study mechanisms underlying T cell senescence.
Rejuvenation of the Aged Immune System in NHP
Recent estimates predict that by 2020, 30% of the American population will be at least 55 years of age. Thus, greater research efforts are needed to gain a deeper understanding of immune senescence and to design interventions to reduce its impact and improve quality of life. Our increased understanding of the aging rhesus macaque immune system has led to the acquisition of a new animal model where interventions to delay or reverse the aging of the immune system can be investigated in a highly relevant preclinical setting. In this section, we review several interventions.
Caloric restriction
One such intervention that showed promise in revitalizing the immune system, and as such has been the focus of recent efforts, is caloric restriction (CR) (58). CR delays the aging process in several short-lived species such as rodents, worms, and yeast. Furthermore, CR delayed the onset/reduced the severity of immune senescence in rodents. Specifically, CR preserved frequency of naïve T cells (16), maintained T cell proliferative capacity (39) most likely by enhancing apoptosis of senescent cells (91), and improved the T cell response to influenza (34). CR reduced the levels of circulating IL-6 and TNFα (33, 90), which in turn decreased the incidence of autoimmune diseases (45, 46) and cancer (102, 105). Several epidemiological studies strongly suggested that CR would have similar benefits in humans (83, 107). To rigorously address this question, studies in nonhuman primates were initiated at the National institute on Aging (50) as well as the Wisconsin National Primate Research Center (82). Reports from both studies showed that CR exerted many of the beneficial physiological effects observed in rodents such as improved cardiovascular and glucoregulatory function (14, 51).
We have recently investigated the impact of caloric restriction on T cell senescence in the rhesus macaque cohort established at the National Institute on Aging (62, 64). Our studies examined the severity of T cell senescence in male and female rhesus macaques that have been calorically restricted since early age (1–2 years), early adulthood (5–7 years), or advanced age (>17 years) (62, 63). We showed that adult-onset CR preserved naïve T cells, a diverse T cell repertoire, T cell proliferative capacity, and reduced the frequency of memory T cells that secreted pro-inflammatory factors IFNγ and TNFα in response to CD3 stimulation (63). These findings strongly suggest that immune response to infection/vaccination would be more robust in CR animals. However, these findings differed from earlier reports that suggested a decrease in immune function in CR rhesus macaques (40, 84, 104). The duration of CR as well as the age at onset differed significantly between the earlier studies and our reported data. We examined animals that have started caloric restriction during early adulthood and were maintained continuously on this diet for 14 years, whereas earlier studies examined animals after a short period of CR (∼2 years) and whose age at onset was somewhere between 8–14 years.
To determine whether age at CR onset modulates its impact on immune senescence, we measured several immune senescence parameters in animals who were calorically restricted either early (1–2 years) or late (>17 years) in life (62). Our analysis revealed that juvenile-onset CR (JO-CR) in male rhesus macaques resulted in a significant increase in the frequency of terminally differentiated effector memory CD4 and CD8 T cells and the reduction of T cell repertoire diversity. Furthermore JO-CR increased the frequency of CD4 and CD8 T cells that secreted IFNγ and TNFα in response to CD3 stimulation, and reduced T cell proliferative capacity. Old onset CR resulted in reduced T cell proliferative response to stimulation in the absence of any detectable changes in T cell subset distribution. Taken together, these data strongly suggest that there is an optimal window for the initiation of CR. The delay of immune senescence by adult onset CR would theoretically lead to improvement, whereas juvenile onset CR would be expected to result in a diminished immune response to infection or vaccination. Studies are currently underway to address this critical question.
IL-7 therapy
The loss of naïve T cells is probably the most dramatic change that occurs with increasing age. Since naïve T cells are the host's reserve to respond to new pathogens, there is increased focus on thymic regeneration in the elderly (5). One of the leading candidates considered is IL-7 therapy. As discussed earlier, IL-7 promotes the survival of thymocytes, naïve and central memory T cells. Studies in rodents showed that IL-7 administration enhanced reconstitution of peripheral T cell repertoire (13, 94), and improved thymic output in aged mice (2). More recent studies tested whether IL-7 treatment can overcome CD4 depletion in SIV-infected NHP (8). These findings prompted studies in aged rhesus macaques to determine whether IL-7 treatment could improve thymic output and the immune response to vaccination (6).
Data from these studies indicated the administration of recombinant IL-7 to aged animals results in a transient increase in the number of circulating CD4 and CD8 T cells that returned to baseline 10 weeks after the last treatment dose. IL-7 administration also increased thymic output as measured by the frequency of T cell receptor excision circles (TRECs). These episomal circular DNA fragments are the result of gene recombination events that take place at the TCR locus. Since TRECs are not amplified during cell division, they are often used as an indirect measure of thymic output (31). Interestingly, despite a transient increase in TREC numbers, there was no change in the frequency of naïve T cells following IL-7 treatment (6). This phenomenon can be explained by rapid conversion of recent thymic emigrants to central memory T cells (69). Alternatively, the administration of IL-7 could promote the trafficking of recent thymic emigrants and naïve T cells from lymphoid tissue into the blood. IL-7-treated animals also generated a more robust antibody response following vaccination with influenza (6). However, the antibody titer eventually decreased to the same set point as that observed in control animals. These preliminary studies suggest a promising role for IL-7 treatment, but the optimal schedule of administration and dosage remain to be determined. A recent clinical study showed that IL-7 administration to refractory cancer patients resulted in the same outcomes reported for the aged primates including increased number of circulating CD4 and CD8 T cells, higher TREC numbers, and improved T cell repertoire diversity (92).
Keratinocyte growth factor:
Another intervention aimed at rejuvenating the thymus is the administration of human keratinocyte growth factor (KGF). Initial studies in rodents showed that the KGF protein has considerable potential to increase thymopoeisis and improve T-cell-dependent antibody responses in aged mice (68). This outcome prompted recent studies in primates to evaluate the ability of KGF to promote thymic regeneration following irradiation and stem cell transplantation. The results from the study show that KGF-treated animals showed a well-preserved thymic structure and output which translated into a broader T cell repertoire and improved antibody responses compared to control animals (87, 88)
Conclusion
The evaluation of medical therapies requires animal models that faithfully recapitulate the physiological changes that occur during the initiation, progression, and resolution of disease in humans. NHPs are invaluable models for the purposes of understanding pathogenesis, immunity and vaccine development due to their: a) high degree of genetic similarity to humans; b) natural susceptibility to a wide range of pathogens that are closely related to those that infect humans; and c) their susceptibility to human pathogens. The advances in our understanding of the NHP immune system will certainly accelerate our ability to define immune correlates of protection against infectious diseases and develop novel vaccine strategies that are better suited for vulnerable populations such as the elderly. Unfortunately, we do not yet have a simple test that signals the onset of immune senescence other than age. Therefore, the identification and validation of biomarkers in an outbred model will be essential. Advances to our understanding of the rhesus immune system coupled with improved methodologies to manipulate different cellular components of this model will certainly accelerate our ability to uncover mechanisms of immune dysregulation and determine effective interventions.
Abbreviations Used
- BCR
B-cell receptor
- CM
central memory
- CR
caloric restriction
- DC
dendritic cells
- EM
effector memory
- IRF-7
interferon regulatory factor-7
- KGF
keratinocyte growth factor
- NHP
nonhuman primates
- NK
natural killer
- PAMPs
pathogen-associated molecular patterns
- PBMCs
peripheral blood mononuclear cells
- PRRs
pattern recognition receptors
- RhCMV
rhesus cytomegalovirus
- RLHs
retinoic acid-inducible gene (RIG-I)-like RNA helicases
- SPF
specific pathogen free
- TCR
T cell receptor
- TLR
toll-like receptors
- TRECs
T cell receptor excision circles
Acknowledgments
This work was supported by NIH grants 5P51 RR00163-47, 5P51 RR00163-48 and American Federation for Aging Research award# A07136 to I. Messaoudi, and CA132638-01 to S. Wong
Bridget Robinson is supported by NIH training grant T32 AI007472-12
We would like to thank Dr. Delphine Malherbe for critical perusal of this manuscript and Andrew Townsend for assistance with figure preparations.
References
- 1.Alter G. Altfeld M. NK cells in HIV-1 infection: Evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265:29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew D. Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 3.Ansari AA. Mayne AE. Onlamoon N. Pattanapanyasat K. Mori K. Villinger F. Use of recombinant cytokines for optimized induction of antiviral immunity against SIV in the nonhuman primate model of human AIDS. Immunol Res. 2004;29:1–18. doi: 10.1385/IR:29:1-3:001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arstila TP. Casrouge A. Baron V. Even J. Kanellopoulos J. Kourilsky P. Diversity of human alpha beta T cell receptors. Science. 2000;288:1135. doi: 10.1126/science.288.5469.1135a. [DOI] [PubMed] [Google Scholar]
- 5.Aspinall R. Mitchell W. Reversal of age-associated thymic atrophy: Treatments, delivery, and side effects. Exp Gerontol. 2008;43:700–705. doi: 10.1016/j.exger.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Aspinall R. Pido–Lopez J. Imami N. Henson SM. Ngom PT. Morre M. Niphuis H. Remarque E. Rosenwirth B. Heeney JL. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10:5–17. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- 7.Bar–Or A. Oliveira EM. Anderson DE. Krieger JI. Duddy M. O'Connor KC. Hafler DA. Immunological memory: Contribution of memory B cells expressing costimulatory molecules in the resting state. J Immunol. 2001;167:5669–5677. doi: 10.4049/jimmunol.167.10.5669. [DOI] [PubMed] [Google Scholar]
- 8.Beq S. Nugeyre MT. Ho Tsong Fang R. Gautier D. Legrand R. Schmitt N. Estaquier J. Barre-Sinoussi F. Hurtrel B. Cheynier R. Israel N. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol. 2006;176:914–922. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- 9.Biassoni R. Natural killer cell receptors. Adv Exp Med Biol. 2008;640:35–52. doi: 10.1007/978-0-387-09789-3_4. [DOI] [PubMed] [Google Scholar]
- 10.Bible JM. Howard W. Robbins H. Dunn–Walters DK. IGHV1, IGHV5 and IGHV7 subgroup genes in the rhesus macaque. Immunogenetics. 2003;54:867–873. doi: 10.1007/s00251-003-0536-2. [DOI] [PubMed] [Google Scholar]
- 11.Bour H. Puisieux I. Even J. Kourilsky P. Favrot M. Musette P. Nicolas JF. T-cell repertoire analysis in chronic plaque psoriasis suggests an antigen-specific immune response. Hum Immunol. 1999;60:665–676. doi: 10.1016/s0198-8859(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 12.Boyman O. Purton JF. Surh CD. Sprent J. Cytokines and T-cell homeostasis. Curr Opinion Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Bradley LM. Haynes L. Swain SL. IL-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–176. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Cefalu WT. Wagner JD. Bell-Farrow AD. Edwards IJ. Terry JG. Weindruch R. Kemnitz JW. Influence of caloric restriction on the development of atherosclerosis in nonhuman primates: Progress to date. Toxicol Sci. 1999;52:49–55. doi: 10.1093/toxsci/52.2.49. [DOI] [PubMed] [Google Scholar]
- 15.Cella M. Jarrossay D. Facchetti F. Alebardi O. Nakajima H. Lanzavecchia A. Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon [see comments] Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 16.Chen J. Astle CM. Harrison DE. Delayed immune aging in diet-restricted B6CBAT6 F1 mice is associated with preservation of naive T cells. J Gerontol A Biol Sci Med Sci. 1998;53:B330–337. doi: 10.1093/gerona/53a.5.b330. discussion B338–339. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZW. Kou ZC. Shen L. Reimann KA. Letvin NL. Conserved T-cell receptor repertoire in simian immunodeficiency virus-infected rhesus monkeys. J Immunol. 1993;151:2177–2187. [PubMed] [Google Scholar]
- 18.Chen ZW. Li Y. Zeng X. Kuroda MJ. Schmitz JE. Shen Y. Lai X. Shen L. Letvin NL. The TCR repertoire of an immunodominant CD8+ T lymphocyte population. J Immunol. 2001;166:4525–4533. doi: 10.4049/jimmunol.166.7.4525. [DOI] [PubMed] [Google Scholar]
- 19.Cheynier R. Henrichwark S. Wain–Hobson S. Sequence of the rhesus monkey T-cell receptor beta chain diversity and joining loci. Immunogenetics. 1996;43:83–87. doi: 10.1007/BF00186610. [DOI] [PubMed] [Google Scholar]
- 20.Choi EI. Reimann KA. Letvin NL. In vivo natural killer cell depletion during primary simian immunodeficiency virus infection in rhesus monkeys. J Virol. 2008;82:6758–6761. doi: 10.1128/JVI.02277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christopher E. Andoniou JDC. Mariapia A. Degli–Esposti M. Killers and beyond: NK-cell-mediated control of immune responses. Eur J Immunol. 2008;38:2938–2942. doi: 10.1002/eji.200838882. [DOI] [PubMed] [Google Scholar]
- 22.Chung E. Amrute SB. Abel K. Gupta G. Wang Y. Miller CJ. Fitzgerald–Bocarsly P. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin Diagn Lab Immunol. 2005;12:426–435. doi: 10.1128/CDLI.12.3.426-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicin–Sain L. Messaoudi I. Park B. Currier N. Planer S. Fischer M. Tackitt S. Nikolich–Zugich D. Legasse A. Axthelm MK. Picker LJ. Mori M. Nikolich–Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci USA. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clambey ET. van Dyk LF. Kappler JW. Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunol Rev. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 25.Coates PT. Barratt–Boyes SM. Zhang L. Donnenberg VS. O'Connell PJ. Logar AJ. Duncan FJ. Murphey–Corb M. Donnenberg AD. Morelli AE. Maliszewski CR. Thomson AW. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 26.Currier JR. Stevenson KS. Kehn PJ. Zheng K. Hirsch VM. Robinson MA. Contributions of CD4+, CD8+, and CD4+CD8+ T cells to skewing within the peripheral T cell receptor beta chain repertoire of healthy macaques. Hum Immunol. 1999;60:209–222. doi: 10.1016/s0198-8859(98)00109-8. [DOI] [PubMed] [Google Scholar]
- 27.Czesnikiewicz–Guzik M. Lee WW. Cui D. Hiruma Y. Lamar DL. Yang ZZ. Ouslander JG. Weyand CM. Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis MM. Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 29.De Martinis M. Franceschi C. Monti D. Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 30.Douek DC. Betts MR. Brenchley JM. Hill BJ. Ambrozak DR. Ngai KL. Karandikar NJ. Casazza JP. Koup RA. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J Immunol. 2002;168:3099–3104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 31.Douek DC. Koup RA. Evidence for thymic function in the elderly. Vaccine. 2000;18:1638–1641. doi: 10.1016/s0264-410x(99)00499-5. [DOI] [PubMed] [Google Scholar]
- 32.Doxiadis GG. Heijmans CM. Otting N. Bontrop RE. MIC gene polymorphism and haplotype diversity in rhesus macaques. Tissue Antigens. 2007;69:212–219. doi: 10.1111/j.1399-0039.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 33.Effros RB. Svoboda K. Walford RL. Influence of age and caloric restriction on macrophage IL-6 and TNF production. Lymphokine Cytokine Res. 1991;10:347–351. [PubMed] [Google Scholar]
- 34.Effros RB. Walford RL. Weindruch R. Mitcheltree C. Influences of dietary restriction on immunity to influenza in aged mice. J Gerontol. 1991;46:B142–147. doi: 10.1093/geronj/46.4.b142. [DOI] [PubMed] [Google Scholar]
- 35.Egan MA. Kuroda MJ. Voss G. Schmitz JE. Charini WA. Lord CI. Forman MA. Letvin NL. Use of major histocompatibility complex class I/peptide/beta2M tetramers to quantitate CD8(+) cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol. 1999;73:5466–5472. doi: 10.1128/jvi.73.7.5466-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ermert K. Mitlohner H. Schempp W. Zachau HG. The immunoglobulin kappa locus of primates. Genomics. 1995;25:623–629. doi: 10.1016/0888-7543(95)80003-5. [DOI] [PubMed] [Google Scholar]
- 37.Fagnoni FF. Vescovini R. Passeri G. Bologna G. Pedrazzoni M. Lavagetto G. Casti A. Franceschi C. Passeri M. Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 38.Garderet L. Cao H. Salamero J. Verge V. Tisserand E. Scholl S. Gorin NC. Lopez M. In vitro production of dendritic cells from human blood monocytes for therapeutic use. J Hematother Stem Cell Res. 2001;10:553–567. doi: 10.1089/15258160152509163. [DOI] [PubMed] [Google Scholar]
- 39.Grossmann A. Maggio–Price L. Jinneman JC. Wolf NS. Rabinovitch PS. The effect of long-term caloric restriction on function of T-cell subsets in old mice. Cell Immunol. 1990;131:191–204. doi: 10.1016/0008-8749(90)90246-n. [DOI] [PubMed] [Google Scholar]
- 40.Grossmann A. Rabinovitch PS. Lane MA. Jinneman JC. Ingram DK. Wolf NS. Cutler RG. Roth GS. Influence of age, sex, and dietary restriction on intracellular free calcium responses of CD4+ lymphocytes in rhesus monkeys (Macaca mulatta) J Cell Physiol. 1995;162:298–303. doi: 10.1002/jcp.1041620216. [DOI] [PubMed] [Google Scholar]
- 41.Hein WR. Griebel PJ. A road less travelled: Large animal models in immunological research. Nat Rev Immunol. 2003;3:79–84. doi: 10.1038/nri977. [DOI] [PubMed] [Google Scholar]
- 42.Hughes AL. Yeager M. Natural selection at major histocompatibility complex loci of vertebrates. Annu Rev Genet. 1998;32:415–435. doi: 10.1146/annurev.genet.32.1.415. [DOI] [PubMed] [Google Scholar]
- 43.Jaeger EE. Bontrop RE. Lanchbury JS. Structure, diversity, and evolution of the T-cell receptor VB gene repertoire in primates. Immunogenetics. 1994;40:184–191. doi: 10.1007/BF00167078. [DOI] [PubMed] [Google Scholar]
- 44.Jankovic V. Messaoudi I. Nikolich–Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): Differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 45.Jolly CA. Dietary Restriction and immune function. J Nutr. 2004;134:1853–1856. doi: 10.1093/jn/134.8.1853. [DOI] [PubMed] [Google Scholar]
- 46.Jolly CA. Fernandes G. Diet modulates Th-1 and Th-2 cytokine production in the peripheral blood of lupus-prone mice. J Clin Immunol. 1999;19:172–178. doi: 10.1023/a:1020503727157. [DOI] [PubMed] [Google Scholar]
- 47.Kannanganat S. Ibegbu C. Chennareddi L. Robinson HL. Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ketloy C. Engering A. Srichairatanakul U. Limsalakpetch A. Yongvanitchit K. Pichyangkul S. Ruxrungtham K. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008;125:18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Kwissa M. Amara RR. Robinson HL. Moss B. Alkan S. Jabbar A. Villinger F. Pulendran B. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J Exp Med. 2007;204:2733–2746. doi: 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lane MA. Ingram DK. Cutler RG. Knapka JJ. Barnard DE. Roth GS. Dietary restriction in nonhuman primates: Progress report on the NIA study. Ann NY Acad Sci. 1992;673:36–45. doi: 10.1111/j.1749-6632.1992.tb27434.x. [DOI] [PubMed] [Google Scholar]
- 51.Lane MA. Ingram DK. Roth GS. Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicol Sci. 1999;52:41–48. doi: 10.1093/toxsci/52.2.41. [DOI] [PubMed] [Google Scholar]
- 52.Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larbi A. Franceschi C. Mazzatti D. Solana R. Wikby A. Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 54.Levinson G. Hughes AL. Letvin NL. Sequence and diversity of rhesus monkey T-cell receptor beta chain genes. Immunogenetics. 1992;35:75–88. doi: 10.1007/BF00189516. [DOI] [PubMed] [Google Scholar]
- 55.Link JM. Hellinger MA. Schroeder HW., Jr. The Rhesus monkey immunoglobulin IGHD and IGHJ germline repertoire. Immunogenetics. 2002;54:240–250. doi: 10.1007/s00251-002-0468-2. [DOI] [PubMed] [Google Scholar]
- 56.Labonte ML. Letvin NL. Variable NKG2 expression in the peripheral blood lymphocytes of rhesus monkeys. Clin Exp Immunol. 2004;138:205–212. doi: 10.1111/j.1365-2249.2004.02625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margolin DH. Saunders EH. Bronfin B. de Rosa N. Axthelm MK. Goloubeva OG. Eapen S. Gelman RS. Letvin NL. Germinal center function in the spleen during simian HIV infection in rhesus monkeys. J Immunol. 2006;177:1108–1119. doi: 10.4049/jimmunol.177.2.1108. [DOI] [PubMed] [Google Scholar]
- 58.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Mavilio D. Benjamin J. Kim D. Lombardo G. Daucher M. Kinter A. Nies–Kraske E. Marcenaro E. Moretta A. Fauci AS. Identification of NKG2A and NKp80 as specific natural killer cell markers in rhesus and pigtailed monkeys. Blood. 2005;106:1718–1725. doi: 10.1182/blood-2004-12-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 61.Medzhitov R. Janeway CA., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 62.Messaoudi I. Fischer M. Warner J. Park B. Mattison J. Ingram DK. Totonchy T. Mori M. Nikolich–Zugich J. Optimal window of caloric restriction onset limits its beneficial impact on T-cell senescence in primates. Aging Cell. 2008;7:908–919. doi: 10.1111/j.1474-9726.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Messaoudi I. Warner J. Fischer M. Park B. Hill B. Mattison J. Lane MA. Roth GS. Ingram DK. Picker LJ. Douek DC. Mori M. Nikolich–Zugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci USA. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Messaoudi I. Warner J. Nikolich–Zugich D. Fischer M. Nikolich–Zugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 65.Michelle† L. LaBonte PFM. Norman† L. Letvin Evidence of NK cell dysfunction in SIV-infected rhesus monkeys: Impairment of cytokine secretion and NKG2C/C2 expression. Eur J Immunol. 2006;36:2424–2433. doi: 10.1002/eji.200635901. [DOI] [PubMed] [Google Scholar]
- 66.Miller JD. Masopust D. Wherry EJ. Kaech S. Silvestri G. Ahmed R. Differentiation of CD8 T cells in response to acute and chronic viral infections: Implications for HIV vaccine development. Curr Drug Targets Infect Disord. 2005;5:121–129. doi: 10.2174/1568005054201544. [DOI] [PubMed] [Google Scholar]
- 67.Miller RA. The aging immune system: Primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 68.Min D. Panoskaltsis–Mortari A. Kuro OM. Hollander GA. Blazar BR. Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moniuszko M. Fry T. Tsai W–P. Morre M. Assouline B. Cortez P. Lewis MG. Cairns S. Mackall C. Franchini G. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naylor K. Li G. Vallejo AN. Lee WW. Koetz K. Bryl E. Witkowski J. Fulbright J. Weyand CM. Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 71.Nikolich–Zugich J. T cell aging: Naive but not young. J Exp Med. 2005;201:837–840. doi: 10.1084/jem.20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olweus J. BitMansour A. Warnke R. Thompson PA. Carballido J. Picker LJ. Lund–Johansen F. Dendritic cell ontogeny: A human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci USA. 1997;94:12551–12556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Otting N. de Vos–Rouweler AJ. Heijmans CM. de Groot NG. Doxiadis GG. Bontrop RE. MHC class I A region diversity and polymorphism in macaque species. Immunogenetics. 2007;59:367–375. doi: 10.1007/s00251-007-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pannetier C. Cochet M. Darche S. Casrouge A. Zoller M. Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Picker LJ. Reed–Inderbitzin EF. Hagen SI. Edgar JB. Hansen SG. Legasse A. Planer S. Piatak M., Jr. Lifson JD. Maino VC. Axthelm MK. Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinchuk LM. Grouard–Vogel G. Magaletti DM. Doty RT. Andrews RG. Clark EA. Isolation and characterization of macaque dendritic cells from CD34(+) bone marrow progenitors. Cell Immunol. 1999;196:34–40. doi: 10.1006/cimm.1999.1538. [DOI] [PubMed] [Google Scholar]
- 77.Pitcher CJ. Hagen SI. Walker JM. Lum R. Mitchell BL. Maino VC. Axthelm MK. Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 78.Posnett DN. Sinha R. Kabak S. Russo C. Clonal populations of T cells in normal elderly humans: The T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Price DA. Bitmansour AD. Edgar JB. Walker JM. Axthelm MK. Douek DC. Picker LJ. Induction and evolution of cytomegalovirus-specific CD4+ T cell clonotypes in rhesus macaques. J Immunol. 2008;180:269–280. doi: 10.4049/jimmunol.180.1.269. [DOI] [PubMed] [Google Scholar]
- 80.Purton JF. Tan JT. Rubinstein MP. Kim DM. Sprent J. Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramothea L. Webster RPJ. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–214. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramsey JJ. Colman RJ. Binkley NC. Christensen JD. Gresl TA. Kemnitz JW. Weindruch R. Dietary restriction and aging in rhesus monkeys: The University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 83.Roberts SB. Schoeller DA. Human caloric restriction for retardation of aging: Current approaches and preliminary data. J Nutr. 2007;137:1076–1077. doi: 10.1093/jn/137.4.1076. [DOI] [PubMed] [Google Scholar]
- 84.Roecker EB. Kemnitz JW. Ershler WB. Weindruch R. Reduced immune responses in rhesus monkeys subjected to dietary restriction. J Gerontol A Biol Sci Med Sci. 1996;51:B276–279. doi: 10.1093/gerona/51a.4.b276. [DOI] [PubMed] [Google Scholar]
- 85.Sauermann U. Making the animal model for AIDS research more precise: The impact of major histocompatibility complex (MHC) genes on pathogenesis and disease progression in SIV-infected monkeys. Curr Mol Med. 2001;1:515–522. doi: 10.2174/1566524013363555. [DOI] [PubMed] [Google Scholar]
- 86.Schwab R. Szabo P. Manavalan JS. Weksler ME. Posnett DN. Pannetier C. Kourilsky P. Even J. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol. 1997;158:4493–4499. [PubMed] [Google Scholar]
- 87.Seggewiss R. Einsele H. Hematopoietic growth factors including keratinocyte growth factor in allogeneic and autologous stem cell transplantation. Semin Hematol. 2007;44:203–211. doi: 10.1053/j.seminhematol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Seggewiss R. Lore K. Guenaga FJ. Pittaluga S. Mattapallil J. Chow CK. Koup RA. Camphausen K. Nason MC. Meier–Schellersheim M. Donahue RE. Blazar BR. Dunbar CE. Douek DC. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood. 2007;110:441–449. doi: 10.1182/blood-2006-12-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siegal FP. Kadowaki N. Shodell M. Fitzgerald–Bocarsly PA. Shah K. Ho S. Antonenko S. Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 90.Spaulding CC. Walford RL. Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 91.Spaulding CC. Walford RL. Effros RB. The accumulation of non-replicative, non-functional, senescent T cells with age is avoided in calorically restricted mice by an enhancement of T cell apoptosis. Mech Ageing Dev. 1997;93:25–33. doi: 10.1016/s0047-6374(96)01808-8. [DOI] [PubMed] [Google Scholar]
- 92.Sportes C. Hakim FT. Memon SA. Zhang H. Chua KS. Brown MR. Fleisher TA. Krumlauf MC. Babb RR. Chow CK. Fry TJ. Engels J. Buffet R. Morre M. Amato RJ. Venzon DJ. Korngold R. Pecora A. Gress RE. Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Surh CD. Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Tan JT. Dudl E. LeRoy E. Murray R. Sprent J. Weinberg KI. Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tarlinton D. B-cell memory: Are subsets necessary? Nat Rev Immunol. 2006;6:785–790. doi: 10.1038/nri1938. [DOI] [PubMed] [Google Scholar]
- 96.Teleshova N. Jones J. Kenney J. Purcell J. Bohm R. Gettie A. Pope M. Short-term Flt3L treatment effectively mobilizes functional macaque dendritic cells. J Leukoc Biol. 2004;75:1102–1110. doi: 10.1189/jlb.1103588. [DOI] [PubMed] [Google Scholar]
- 97.Teleshova N. Kenney J. Williams V. Van Nest G. Marshall J. Lifson JD. Sivin I. Dufour J. Bohm R. Gettie A. Pope M. CpG-C ISS-ODN activation of blood-derived B cells from healthy and chronic immunodeficiency virus-infected macaques. J Leukoc Biol. 2006;79:257–267. doi: 10.1189/jlb.0205084. [DOI] [PubMed] [Google Scholar]
- 98.Thiel C. Bontrop RE. Lanchbury JS. Structure and diversity of the T-cell receptor alpha chain in rhesus macaque and chimpanzee. Hum Immunol. 1995;43:85–94. doi: 10.1016/0198-8859(94)00149-k. [DOI] [PubMed] [Google Scholar]
- 99.Vallejo AN. CD28 extinction in human T cells: Altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 100.Velotti F. Chopin D. Gil–Diez S. Maille P. Abbou CC. Kourilsky P. Even J. Clonality of tumor-infiltrating lymphocytes in human urinary bladder carcinoma. J Immunother. 1997;20:470–478. doi: 10.1097/00002371-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 101.Villinger F. Miller R. Mori K. Mayne AE. Bostik P. Sundstrom JB. Sugimoto C. Ansari AA. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 102.Volk MJ. Pugh TD. Kim M. Frith CH. Daynes RA. Ershler WB. Weindruch R. Dietary restriction from middle age attenuates age-associated lymphoma development and interleukin 6 dysregulation in C57BL/6 mice. Cancer Res. 1994;54:3054–3061. [PubMed] [Google Scholar]
- 103.Walker JM. Maecker HT. Maino VC. Picker LJ. Multicolor flow cytometric analysis in SIV-infected rhesus macaque. Methods Cell Biol. 2004;75:535–557. doi: 10.1016/s0091-679x(04)75022-0. [DOI] [PubMed] [Google Scholar]
- 104.Weindruch R. Lane MA. Ingram DK. Ershler WB. Roth GS. Dietary restriction in rhesus monkeys: Lymphopenia and reduced mitogen-induced proliferation in peripheral blood mononuclear cells. Aging (Milano) 1997;9:304–308. doi: 10.1007/BF03341833. [DOI] [PubMed] [Google Scholar]
- 105.Weindruch R. Walford RL. Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 106.Weller S. Braun MC. Tan BK. Rosenwald A. Cordier C. Conley ME. Plebani A. Kumararatne DS. Bonnet D. Tournilhac O. Tchernia G. Steiniger B. Staudt LM. Casanova JL. Reynaud CA. Weill JC. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Willcox BJ. Willcox DC. Todoriki H. Fujiyoshi A. Yano K. He Q. Curb JD. Suzuki M. Caloric restriction, the traditional Okinawan diet, and healthy aging: The diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann NY Acad Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 108.Wilson JD. Cranage M. Cook N. Leech S. McMichael AJ. Callan MF. Evidence for the persistence of monoclonal expansions of CD8+ T cells following primary simian immunodeficiency virus infection. Eur J Immunol. 1998;28:1172–1180. doi: 10.1002/(SICI)1521-4141(199804)28:04<1172::AID-IMMU1172>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]