Summary
Contrary to the reduction of depressive-like behavior observed in several strains of cytokine receptor knockout mice, mice lacking the specific receptor for interleukin (IL)-15 showed increased immobility in tail suspension and modified forced swimming tests. There was also a reduction in social interactions. The hippocampus of the IL15Rα knockout mice had decreased mRNA for 5-HT1A, increased mRNA for 5-HT2C, and region-specific changes of serotonin reuptake transporter (SERT) immunoreactivity. Fluoxetine (the classic antidepressant Prozac, which inhibits 5-HT2C and SERT) reduced the immobility of the IL15Rα knockout mice in comparison with their pretreatment baseline. Together with the unchanged performance of the IL15Rα knockout mice on the rotarod, this response to fluoxetine indicates that the immobility reflects depression. Wildtype mice responded to IL15 treatment with improvement of immobility induced by forced swimming, whereas the knockout mice failed to respond. Thus, the cognate IL15 receptor is necessary for the antidepressive activity of IL15. In ex-vivo studies, IL15 decreased synaptosomal uptake of 5-HT, and modulated the expression of 5-HT2C and SERT in cultured neurons in a dose- and time-dependent manner. Thus, the effect of IL15 on serotonin transmission may underlie the depressive-like behavior of IL15Rα knockout mice. We speculate that IL15 is essential to maintain neurochemical homeostasis and thereby plays a role in preventing neuropsychiatric symptoms.
Keywords: IL15, IL15Rα, brain, neurotransmitter, SERT, depression, 5-HT
Introduction
This study addresses the functions of interleukin (IL)-15 in the brain by analyses of neural behavior and serotonin transmission in the hippocampus of knockout (KO) mice lacking a functional IL15Rα, the specific receptor for IL15. IL15 is a 14 kD cytokine produced by essentially all types of cells in the body. It belongs to the 4-helix-bundle family of cytokines, plays an essential role in immune cell functions, muscle and bone growth, and adiposity (Satoh et al. 1998;Fehniger and Caligiuri 2001;McInnes and Gracie 2004;Budagian et al. 2006). Few studies have addressed the role of IL15 in the central nervous system (CNS). IL15 is expressed in glial cells (Lee et al. 1996), and it enhances non-rapid eye movement sleep in rabbits after intracerebral administration (Kubota et al. 2001). IL15 or its receptors can be induced by proinflammatory and autoimmune challenges at the blood-brain barrier and different CNS regions (Pan et al. 2008b; 2009;Hsuchou et al. 2009b;Wu et al. 2010c;2010d). In patients with multiple sclerosis, IL15 concentrations are increased in their cerebrospinal fluid (Rentzos et al. 2006).
IL15 binds to its specific receptor IL15Rα as well as to its co-receptors IL2Rβ and IL2Rγ that can also be used by other cytokines in this family. Typically, IL15 binding recruits the high-affinity heterotrimeric receptor complex and results in JAK/STAT signaling in the target cells (Pereno et al. 2000;Fehniger and Caligiuri 2001)). We have shown that IL15Rα KO mice have deficits in hippocampal dependent memory and GABA transmission (He et al. 2010a). and disturbed circadian rhythms of thermoregulation, locomotor activity, and energy metabolism (He et al. 2010b). Opposite to what would be expected, the IL15Rα KO mice show astrogliosis and microgliosis in the hippocampus, and exhibit reduced normal anxiety in open field and elevated plus maze tests (Wu et al. 2010a). By contrast, IL15 ligand KO mice show more severe symptoms in experimental autoimmune encephalomyelitis (EAE), whereas IL15 treatment has protective effects after peripheral injection (Wu et al. 2010c). These results suggest that IL15 and its signaling through IL15Rα may play an essential role in normal CNS functions.
IL15Rα in the brain can be upregulated by lipopolysaccharide (Pan et al. 2008b) and EAE (Hsuchou et al. 2009b). However, IL15 appears to have opposite functions from proinflammatory cytokines. Tumor necrosis factor α (TNF), interferons, IL1β, and IL6 have all been shown to promote sickness behavior and depression (Dantzer et al. 2008). Both TNF and IL1β exacerbate hippocampal dependent memory in a dose-dependent manner (Rachal et al. 2001;Yirmiya et al. 2002;Goshen et al. 2007). IL15 and these proinflammatory cytokines can all cross the blood-brain barrier to exert CNS actions (Gutierrez et al. 1993;Banks and Kastin 1996;Pan et al. 1997a;1997b;2006;Pan and Kastin 2002). We have also observed that IL15Rα KO mice show fearlessness and improved performance in the open field and elevated plus maze tests, indicating that an intact IL15Rα may be essential for anxiety-like behavior (Wu et al. 2010a). By contrast, TNF KO mice show increased anxiety, reduced immobility in forced swimming, and greater serotonin turnover, and are thought to exhibit inappropriate coping responses when facing severe stressful situations, rather than reduced depression (Yamada et al. 2000). Both TNFR1 KO and TNFR2 KO mice show a reduction of depression with increased mobility in the forced swimming test (Simen et al. 2006). Therefore, we speculated that IL15Rα KO mice exhibit increased depression, opposite to that seen in KO mice lacking proinflammatory cytokines or their receptors. This would suggest that IL15 acts as an endogenous antidepressive cytokine. The findings from the KO mice reported here are supported by studies in normal mice and tissue preparations treated with IL15.
The cognate cytokine IL2, which shares two receptor subunits with IL15, has shown effects in anxiety and exploratory behavior after microinjection into the striatum. It dose-dependently increases rearing activity and open arm entries in the elevated plus maze test (Pawlak and Schwarting 2006). In bipolar patients during the manic phase, there is an increase of soluble IL2R concentrations in plasma (Tsai et al. 2001). Cancer patients receiving chronic subcutaneous IL2 show significantly increased scores on the clinical scale of depression and psychasthenia accompanied by an increase in conversion hysteria and psychopathic deviation, suggesting that IL2 has psychoactive properties (Pizzi et al. 2002). This further strengthens the rationale to study the neurobehavioral and biochemical changes of IL15Rα KO mice in this study.
Materials and Methods
1. Measurement of depressive-like behavior in mice
All studies were performed following a protocol approved by the Institutional Animal care and Use Committee. IL15Rα KO mice (B6;129X1-IL15ratm1Ama/J, stock number 003723 from Jackson Laboratory, Bar Harbor, ME) were studied at age 2.5 – 3.5 months along with their matched controls (B6.129SF2/J, stock number 101045, also from Jackson Laboratory) (n = 9 /group). The mice were housed in the animal care facility for at least three weeks before testing. Female and male mice were studied separately. The mice were subjected to several behavioral test regimens with one-week intervals between the tests (n = 9 /group, 9 – 12 wk old).
The water wheel test is a modified Porsolt forced swim test more suitable for mice (Nomura et al. 1982;Kastin et al. 1984) with minor modifications. The apparatus consisted of a plexiglass water tank (19×10×13.5 cm) with a water wheel in its center. The water wheel was made of a plexiglass shaft (diameter 3 cm, length 10 cm) with 8 paddles (width 0.5 cm). The tank was filled with water (25 ± 1 °C) to a height of 9 cm, with the paddle blades just resting on the surface. After the mouse was placed in the water tank, the number of rotations of the water wheel was counted for the next 5 min.
The tail suspension test was performed as described previously (Steru et al. 1985). The mouse was subjected to a short-term inescapable stress of being suspended by the tail. This resulted in an immobile posture that varies in duration depending on the state of depression. A horizontal bar was placed 60 cm above the counter. The tail of the mouse was taped to the bar. Climbing behavior and immobility were videotape monitored for 6 min. The duration of immobility (defined as the absence of all movements except for those required for respiration) in each minute was measured, and the cumulative immobility at 2, 4, and 6 min intervals is presented.
A simplified social interaction test paradigm (Signoret et al. 2000) was used. A test female mouse was introduced to a cage where another female mouse resided. The number of sniffs by the test mouse was recorded over the course of 5 min. Groups of IL15Rα KO and control mice were studied simultaneously (n = 9 /group). This test used only female mice, since hyperactivity and aggression in male mice could invalidate part of the test.
To rule out the difference in locomotor activity and coordination between the groups that might have accounted for the difference in immobility tests, KO and wildtype controls (n = 10 /group, all females) were subjected to the rotarod test (Columbus Instruments, Columbus, OH). The rotarod was accelerated from 4 to 40 rpm in 5 min, and the latency of the mouse to fall off was recorded. In the pretraining period on the accelerating rotarod, a mild electrical shock was applied when the mouse fell onto the floor. Each mouse went through three consecutive trials, and the longest time on the rotarod was used for analysis.
To determine the effect of antidepressant treatment on mouse behavior, groups of IL15Rα KO and wildtype mice (n = 9 /group) were subjected to pretesting in the tail suspension module, rested for 1 wk, and then were injected with fluoxetine (20 mg/kg) intraperitoneally. Thirty min later, the mice were retested and monitored for immobility during the 6 min duration of tail suspension. Each group contained 5 male and 4 female mice; since there was no gender difference, the data were combined. The dose and dosing regimen of fluoxetine are based on the literature that fully characterizes its antidepressive activity, serum and brain concentrations, and serum protein binding (Holladay et al. 1998).
To determine whether IL15 treatment directly affects depressive-like behavior, normal B6 mice (n = 9 /group, 3 m old) were subjected to the Nomura water wheel test and then received IL15 (100 ng/mouse/d intraperitoneally for 3 days, R & D Systems, Minneapolis, MN). They were tested again 24 h after the last dose of treatment. To compare and show the specificity of the response, IL15Rα KO mice (n = 5 / group, 2 m old) were treated in the same way.
2. Liquid chromatography-tandem mass spectrometry (LC-MS) analysis
The concentrations of serotonin (5-HT) and its main metabolite 5-hydroxyindoleacetic acid (5-HIAA) were determined in snap frozen hippocampal homogenates from IL15Rα KO and wildtype mice (n = 5 /group, each sample representing one mouse). Liquid chromatography-tandem mass spectrometry (LC-MS) analysis was performed by a contractual service (Drumetix Laboratories, Greensboro, NC). The analysts were blinded to treatment groups. Tissues added with 5M ammonium acetate were homogenized on ice by sonication. Two hundred microliters of the homogenate was transferred into a new tube followed by addition of 20 µL of 20 µM 5-HT-d4 as internal standard. After centrifugation at 10,000 rpm, the supernatant of the samples was injected onto a Primesep 200 column (5 µm, 2.1×50 mm) (SIELC Technologies, Prospect Heights, IL). A gradient elution from 0.2 % formic acid 20 mM ammonium acetate in water to 0.2 % formic acid 20 mM ammonium acetate in methanol was conducted at a flow rate of 0.25 ml/min. The run time was 9.5 minutes in total. The signals were detected by multiple reaction monitoring with electrospray interface in positive ion mode. The concentration of 5-HT was quantified by use of a standard curve ranging from 0.02 to 50 µM.
3. Immunohistochemistry (IHC)
To determine the changes of SERT in the hippocampus, IL15Rα KO mice were anesthetized by intraperitoneal urethane, perfused intracardially with phosphate-buffered saline (PBS) and 3 % paraformaldehyde, and processed for IHC as described previously (Pan et al. 2008a;Hsuchou et al. 2009a). A rabbit polyclonal anti-SERT antibody (AB9726, Millipore, Temecula, CA) was used for the staining. The specificity of the signal was confirmed by the lack of fluorescence in sections incubated with secondary antibody only.
4. Quantitative PCR (qPCR)
Total RNA from the hippocampi and GT1-7 cells was extracted with the RNeasy mini kit. After digestion with DNase I to eliminate trace amounts of DNA contamination, the total RNA was purified with an RNA clean up kit (Zymo Research, Orange, CA) and quantified at 260 nm with a Bio-Rad spectrometer (Hercules, CA). Reverse transcription of the total RNA was conducted with a High Capacity cDNA Transcription Kit (Applied Biosystems, Foster City, CA). Real-time PCR amplification of 5-HT1A, 5-HT2C, 5-HT7 receptors, SERT, and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was measured with Power SYBR Green PCR Master Mix (Applied Biosystems). The level of expression of the target genes was normalized to that of GAPDH in the same sample. All primers used for amplification are listed in Table 1.
Table 1.
GenBank accession number and sequences of primers used in real-time PCR
| Gene | GenBank Accession # | Forward Primer (FP) and Reverse Primer (RP) |
|---|---|---|
| 5-HT1A | NM_008308.3 | FP: CTGTGCTGCACTTCGTCCAT |
| RP: GGTCGGTGATTGCCCAGTAC | ||
| 5-HT2C | NM_008312.3 | FP: GGATTTCACTAGATGTGCTATTTTCAA |
| RP: GCTACATACCGGTCCAGCGATA | ||
| 5-HT7 | NM_008315.2 | FP: CGCCATGGACGTCATGTG |
| RP: AGGTACCTGTCGATGCTGATCA | ||
| SERT | AF013604 | FP: GTCCGAGGTGGCCAAAGAC |
| RP: GCTATTGCCTCCGCATATGTG | ||
| GAPDH | XM_001473623.1 | FP: TGTGTCCGTCGTGGATCTGA |
| RP: CCTGCTTCACCACCTTCTTGA |
5. Effect of IL15 treatment on synaptosomal uptake of [3H]5-HT
Synaptosomes were prepared from the forebrain (including hippocampus and the entorhinal cortex where the perforant path originates) of normal mice following standard protocols (Inazu et al. 2001). In 96-well plates (n = 5 /group), synaptosomes (2 mg/ml, 100 µl/well) were treated with IL15 (10 ng/ml) or PBS vehicle for 30 min at 37 C. [3H]5-HT (Perkin Elmer, Boston, MA, 100 nM final concentration) was added and incubated for another 20 min with constant agitation. Each sample contained triplicated wells, and their values were averaged to obtain the value for the particular sample. Nonspecific binding was determined by inclusion of two additional groups in the presence of 1000-fold excess of unlabeled 5-HT. Synaptosomal uptake of [3H]5-HT was collected after rapid filtration of the mixture on glass fiber-B filters by use of a cell harvester. Radioactivity was determined after addition of Betaplate scintillation cocktail, on a 1450 MicroBeta TriLux Microplate Scintillation and Luminescent counter (Perkin Elmer).
In the study to determine the effect of IL15Rα deletion on 5-HT uptake, synaptosomes where obtained from the forebrain of wildtype or KO mice (n = 5 /group). The synaptosomes were incubated with [3H]5-HT for 30 min at 37 °C as described above, along with additional groups to determine nonspecific binding. The synaptosomal internalized and unbound portions of [3H]5-HT were separated by filtration, and the radioactivity on glass fiber-B filters (synaptosomal fraction) was determined.
6. Effect of IL15 treatment on 5-HT and SERT expression in culture GT1-7 neurons
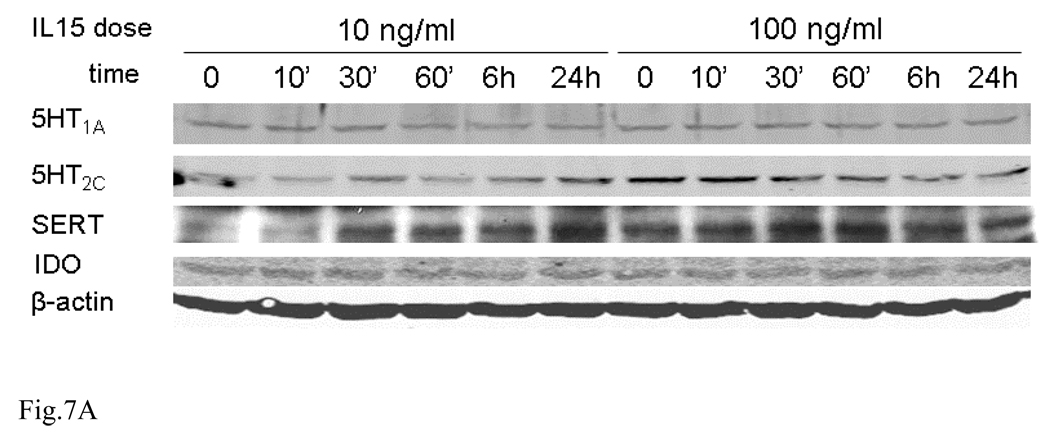
The immortalized mouse hypothalamic neuronal cell line GT1-7 was maintained in DMEM supplemented with 10 % fetal bovine serum, penicillin (100 U/ml) and streptomycin (0.1 mg/ml), and was incubated in 5 % CO2 at 37 °C. Before IL15 treatment, GT1-7 cells were serum starved for 16 h in DMEM medium without any antibiotics. Afterwards, cells were treated with IL15 at either 10 or 100 ng/ml, and harvested at 0, 10 min, 30 min, 1 h, 6 h, and 24 h with protein lysis buffer containing protease inhibitor cocktail. The 0 min was the untreated control. Protein lysates were cleared by ultracentrifugation, and quantified by bicinchoninic acid assay. Forty µg of protein was electrophoresed and transferred to a nitrocellulose membrane. The following antibodies were used for western blotting (WB): rabbit polyclonal against the 46 kD 5-HT1A (1:500, Abcam, Cambridge, MA), the 51 kD 5-HT2C (1:300, Abcam), the 65 kD SERT (1:400, Abcam), and a mouse monoclonal antibody against the 42 kD indoleamine 2,3 dioxygenase (IDO), an enzyme catalyzing the degradation of the essential amino acid L-tryptophan to N-formylkynurenine. The antibody was from Santa Cruz Biotechnology (Santa Cruz, CA) and used at 1:200 dilution. The internal control, β-actin, was also probed. Relative expression of the 5-HT related proteins was quantified by densitometric analysis by use of NIH Image J program, and expressed as the gray density of target protein /β-actin ratio.
For mRNA analysis, the cells were harvested at 0, 10 min, 30 min, 1 h and 3 h after IL15 stimulation (n = 3 /time point). The 0 time was the untreated control. The cells were incubated with RNA lysis buffer containing β-mercaptoethanol and collected for further gene expression analysis.
7. Statistics
Means are presented with their standard errors. The data were analyzed by two-way or one-way analysis of variance (ANOVA) or t-test where appropriate.
Results
1. IL15Rα KO mice show increased immobility in tests for depressive-like behavior
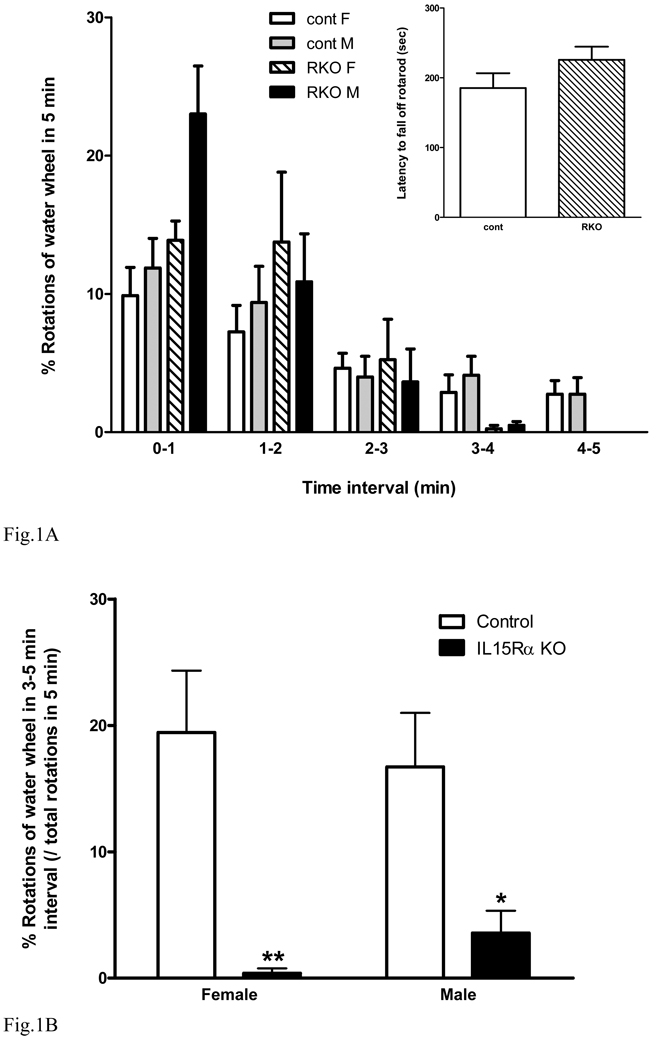
Porsolt forced swimming is a classical test to determine depressive-like behavior (Cryan et al. 2002). Since some strains of mice do not show optimal responses in this test in our past experience, we used the Nomura water wheel test as a modified forced swimming test (Nomura et al. 1982;Kastin et al. 1984). The number of full rotations of the water wheel paddled by the mice was recorded. In female and male KO and wildtype groups (n = 8 /group), all mice showed more activity at the beginning of the 5 min test but more time floating immobile at the end, indicating the lack of basal deficit in locomotion. There was no difference between female and male mice of the same strain. At 3–4 min, there was a reduction of paddling time in the KO mice. It was so dramatic that at 4–5 min both female and male KO mice showed no purposeful movement (Fig. 1A). The rotarod test, an additional measure of locomotor coordination, showed that the increased immobility of the KO mice was not caused by a reduction of muscle strength or locomotor activity since there was no significant difference [F(1,17) = 1.96, p > 0.05] between female KO and wildtype mice in the accelerating rotarod. The falling latency was greater than 3 min (inset fig. 1A), and the KO mice seem to stay on the rotating rod even longer though there was no statistically significant difference.
Fig. 1.
Performance of IL15Rα KO mice in tests to evaluate depressive-like behavior. (A) In the Nomura water wheel test, there were strain and time differences in paddling the water wheel (n = 8 /group). The IL15R KO mice paddled more in the first 2 min, but showed no activity in the last 2 min. Inset: rotarod test in female KO and wildtype mice showing no difference in latency to fall. (B) In the Nomura water wheel test, both male and female KO mice showed a reduction of paddling and a corresponding increase of immobility in the last 2 min. (C) In the tail suspension test, the KO mice had a longer duration of immobility at all time intervals tested (0–2, 0–4, and 0–6 min). (D) In the social interaction test, The KO mice had a significant reduction of social interactions with their cage mates. *: p < 0.05; **: p < 0.01; ***: p < 0.005.
Two-way analysis of variance was performed on the activity data in the last 2 min (interval of 3 – 5 min), a standard time to access immobility in the Nomura water wheel test. There was an overall effect but no interaction between strain and gender [F(1,28) = 0.77, p > 0.05]. There was a significant effect of strain [F(1,28) = 22.9, p < 0.0001], whereas gender had no effect [F(1,28) = 0, p > 0.05]. The KO-induced change was significant in both female (p < 0.01) and male (p < 0.05) mice (Fig. 1B). Thus, the KO mice showed a major increase of immobility in comparison with the control strain.
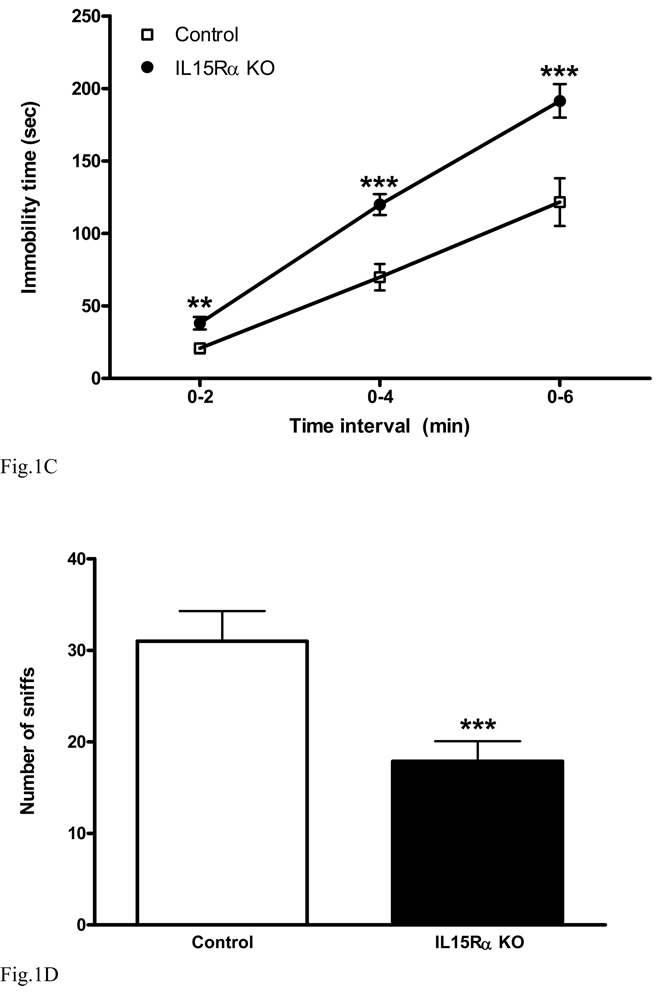
In the tail suspension test (Steru et al. 1985), a control mouse typically attempts to reverse its body position by lateral movements. Immobility is defined as an absence of initiated movements. Groups of IL15Rα KO mice and wildtype controls (n = 9 /group) were observed and scored continuously for 6 min. The cumulative immobility at three time intervals is shown in figure 1C. The KO mice showed an increase of immobility at all times, including 0–2 min [F(1,17) = 10.3, p < 0.01], 0 –4 min [F(1,17) = 18, p < 0.005], and 0–6 min [F(1,17) = 11.6, p < 0.005].
In the social interaction test (Fig. 1D), the IL15Rα KO mice had a significant reduction of sniffing interactions during the 5 min observation period [F(1,16) = 11.9, p < 0.005]. Overall, these three tests reflect different aspects of the depressive-like behavior of the IL15Rα KO mice.
2. Defective serotonin (5-HT) transmission in the hippocampus of IL15Rα KO mice
In hippocampal homogenates, the level of 5-HT was 3.56 ± 0.27 nmol/g in the wildtype mice and 3.12 ± 0.11 nmol/g in the KO mice. The decrease was not statistically significant. There was no difference in its major metabolite 5-HIAA or the 5-HT/5-HIAA ratio. A repeat study with another set of samples (n = 5 /group) also failed to show significant changes.
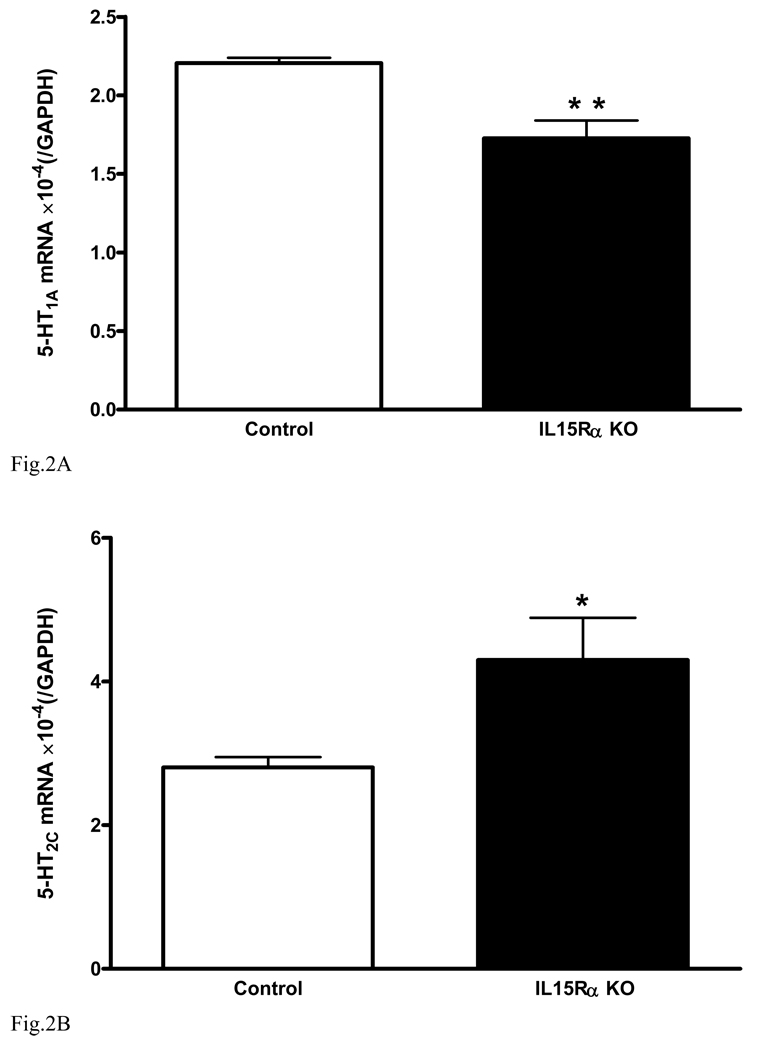
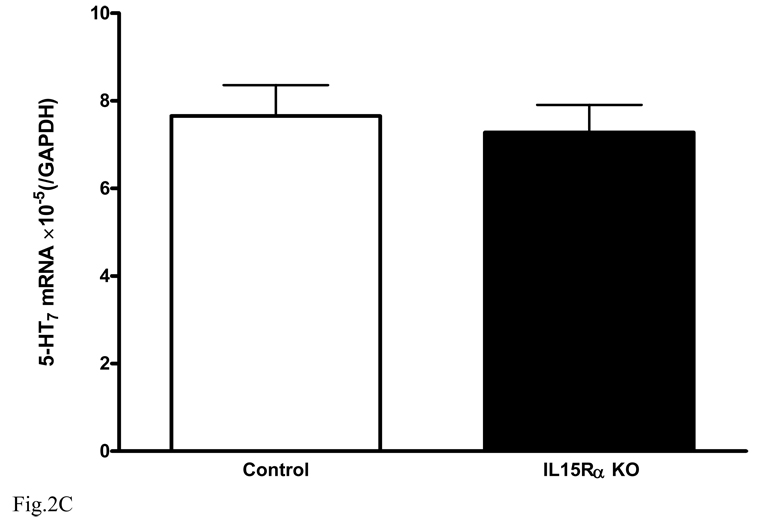
In the hippocampus of the KO mice, there was a reduction of serotonin receptors known to mediate antidepressive effects. The mRNA for 5-HT1A (Fig. 2A) was significantly lower in the hippocampus of the KO mice [F(1,7) = 12.9, p < 0.01]. By contrast, the mRNA for 5-HT2C (Fig. 2B) was increased [F(1,8) = 6.1, p < 0.05]. Activation of 5-HT1A alleviates depression whereas activation of 5-HT2C contributes to depression. These changes contrast with the lack of significant changes in 5-HT7 [F(1,8) = 0.16, p > 0.05, Fig. 2C].
Fig. 2.
Level of mRNA expression of serotonin receptors in hippocampus (n = 5 /group). In comparison with the wildtype controls, the KO mice showed (A) reduced 5- HT1A, (B) increased 5-HT2C, and (C) unchanged 5-HT7. *: p < 0.05; **: p < 0.01.
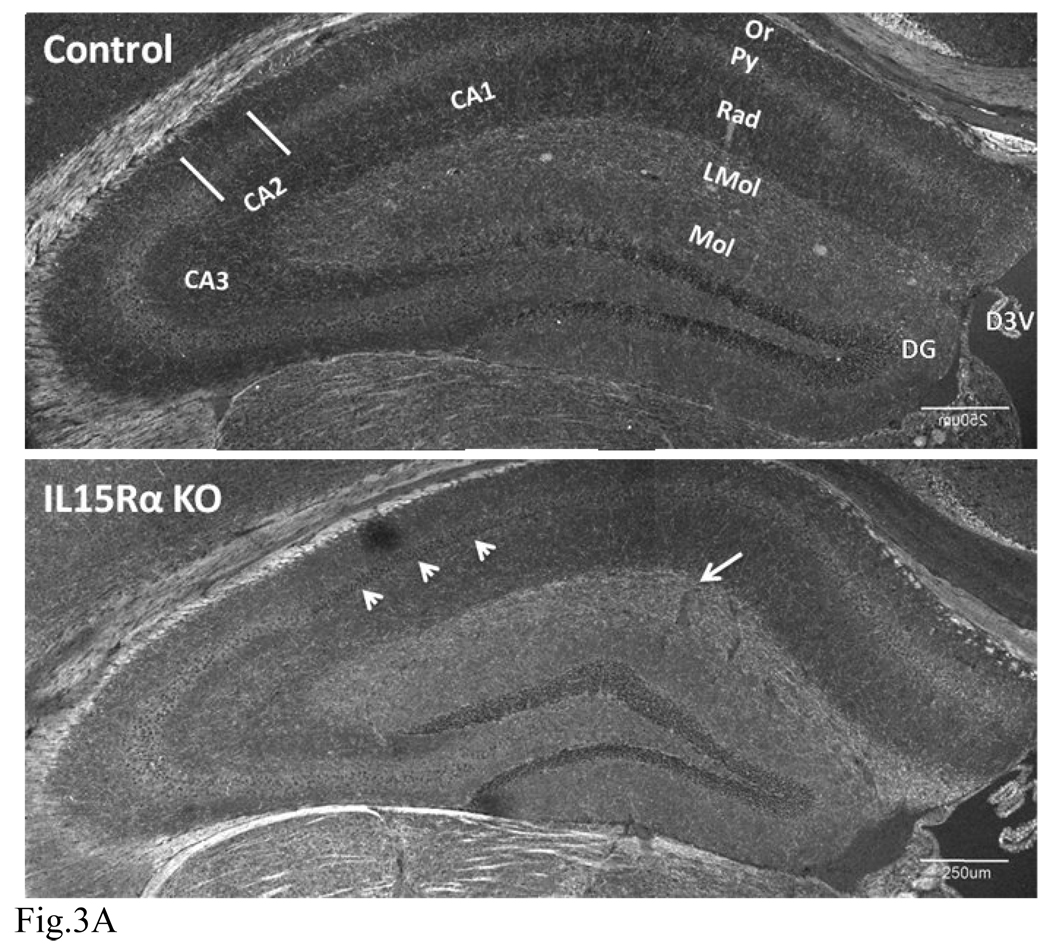
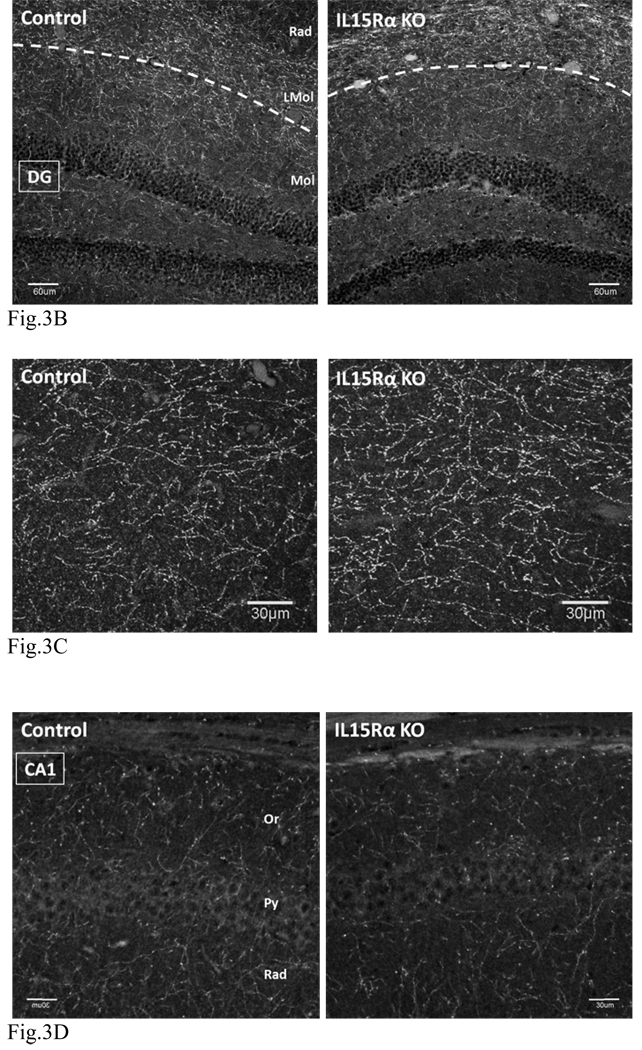
We next performed IHC of SERT in the KO and control mice (n = 3 /group). In the wildtype mice, SERT immunoreactivity was most prominent in nerve fibers in the lacunosum-molecular layer. A lower density of SERT staining was seen throughout other areas of the hippocampus, with the exception of the pyramidal layer. Besides immunopositive fibers, neuronal cell bodies in the cornus ammons (CA1, CA2 and CA3) also showed SERT immunoreactivity. In the KO mice, there was an increase of SERT immunofluoresence in the lacunosum-molecular layer (LMol) as compared with the normal mice. However, there was a decrease of neuronal soma staining in the pyramidal layer as well as fiber staining in the oriens and radiate layers of the CA1 that are adjacent to CA2. Figure 3(A–F) shows representative overviews of hippocampi in three different sets of mice. Overall, the increase of SERT in the LMol traversed by the perforant path suggests an increased input from the entorhinal cortex to the hippocampus. Axons of the perforant path synapse in the LMol, with dendrites projecting from the pyramidal layer, particularly CA1.
Fig. 3.
Distribution of SERT immunofluorescence in the hippocampus as shown by confocal microscopy. CA: cornus ammons. D3V: dorsal 3rd ventricle. DG: dentate gyrus. LMol: stratum lacunosum-moleculare. Mol: stratum moleculare of dentate gyrus. Or: stratum oriens. Py: pyramidal cell layer. Rad: stratum radiatum.
(A) In normal mice (upper panel), SERT immunoreactivity was mainly present in fibrous processes but can also be seen in neuronal soma. The highest level of expression was in the LMol. SERT (+) fibers could be seen throughout the hippocampus except in the pyramidal layer, which showed amorphous and weaker SERT immunoreactivity. In the KO mice (lower panel), there was increased SERT intensity in the LMol (arrow). Decreased neuronal soma and fibrous staining of SERT can be seen in the CA1 pyramidal layer adjacent to CA2 (arrow heads). Scale bar: 250 µm. Confocal aperture (C.A.) of scan unit for obtaining image: 400 µm.
(B) Higher resolution images showing no significant change in SERT staining in the DG (below the dash line) between KO (right panel) and control (left panel). There was increased SERT fiber density in the LMol in the KO. Scale bar: 60 µm. C.A.: 400 µm.
(C) Enlarged area of the LMol shown in (B). There was a greater increase of SERT fiber density in the KO (right panel) than the control (left panel). Scale bar: 30 µm. Z stacking image of 21 confocal slices. C.A.: automatic setting.
(D) CA1 region distal to CA2, showing a lack of significant change of SERT signal between the control (left panel) and KO (right panel). Scale bar: 30 µm. C.A.: 400 µm.
(E) CA1 region adjacent to CA2, showing an apparent reduction of SERT in the pyramidal layer, stratum oriens, and stratum radiatum, when the KO (right panel) is compared with the control (left panel). The change is indicated by arrowheads. Scale bar: 30 µm. C.A.: 400 µm.
(F) CA3 region, showing no significant change between the control (left panel) and KO (right panel). Scale bar: 30 µm. C.A.: 400 µm.
The mRNA for SERT in hippocampal homogenates did not differ between the KO (2.88 ± 0.62) and control (3.58 ± 0.30) groups (n = 5 /group). This may be a result of region-specific changes (increase in LMol but decrease in DG and Mol). It may also suggest that the changes of SERT mainly occur at the post-transcriptional level during receptor trafficking.
3. Response of IL15Rα KO mice to fluoxetine (Prozac), a classical SSRI antidepressant
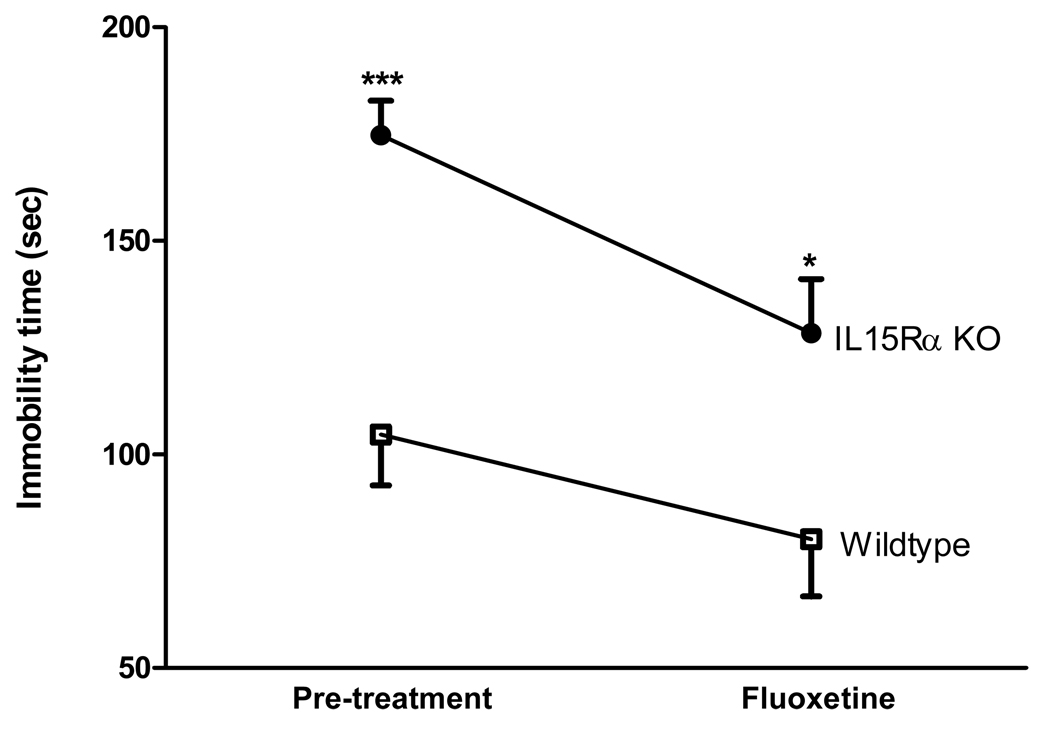
Altered SERT turnover has been shown with citalopram, a selective serotonin reuptake inhibitor (SSRI) (Lau et al. 2008). To test whether SSRI modulates the depressive-like behavior in the IL15Rα KO mice, two groups of mice were studied (n = 9 /group). Wildtype and KO mice were subjected to the tail suspension test both before and after fluoxetine treatment. In the wildtype mice, fluoxetine did not induce a significant change in the time of immobility [F(1,16) = 1.9, p > 0.05]. In the KO mice, there was a significant treatment effect with reduction of immobility [F(1,15) = 10.1, p < 0.01]. IL15Rα KO mice showed a significantly longer duration of immobility than the wildtype mice in both pre- (p < 0.005) and post-tests (p < 0.05). Thus, fluoxetine treatment resulted in a significant improvement in the IL15Rα KO mice during the test period of 6 min (Fig. 4).
Fig. 4.
Effects of the serotonin reuptake transport inhibitor fluoxetine on time of immobility in the tail suspension test (n = 9 /group). In the KO mice, fluoxetine decreased the time of immobility when the mice were retested 30 min later (p < 0.05). In the wildtype mice, fluoxetine did not induce significant changes. *: p < 0.05; ***: p < 0.005 when the KO mice were compared with the respective control group.
4. IL15 treatment improves immobility
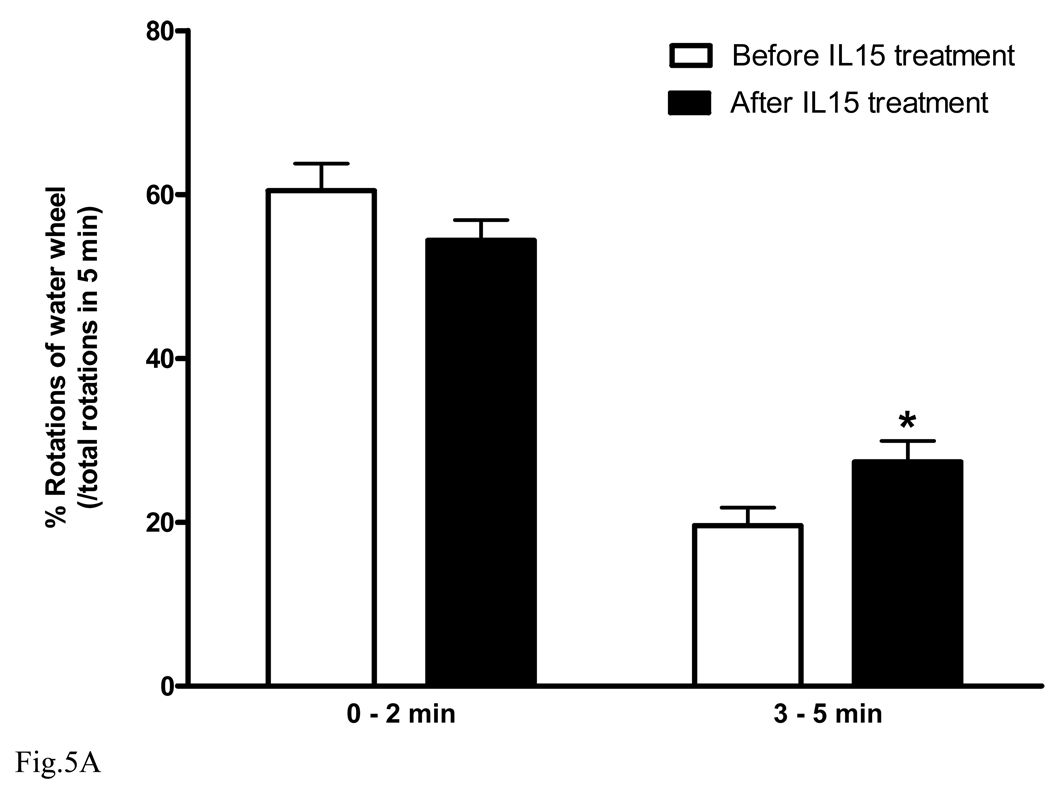
In mice, depressive-like behavior can be induced by forced swimming (Lucki 1997;Cryan et al. 2002;Gourley et al. 2008;Pouladi et al. 2009). We used the Nomura water wheel test to induce depressive-like behavior in normal adult C57 mice. Mice were subjected to the Nomura water wheel pre-test. Afterwards, mice were treated with IL15 (100 ng/mouse ip daily) for 3 consecutive days (n = 9) and subjected to the test again. Results showed that IL15 treatment significantly increased the number of wheel rotations in comparison with the pretreatment baseline (Fig. 5A, p < 0.05).
Fig. 5.
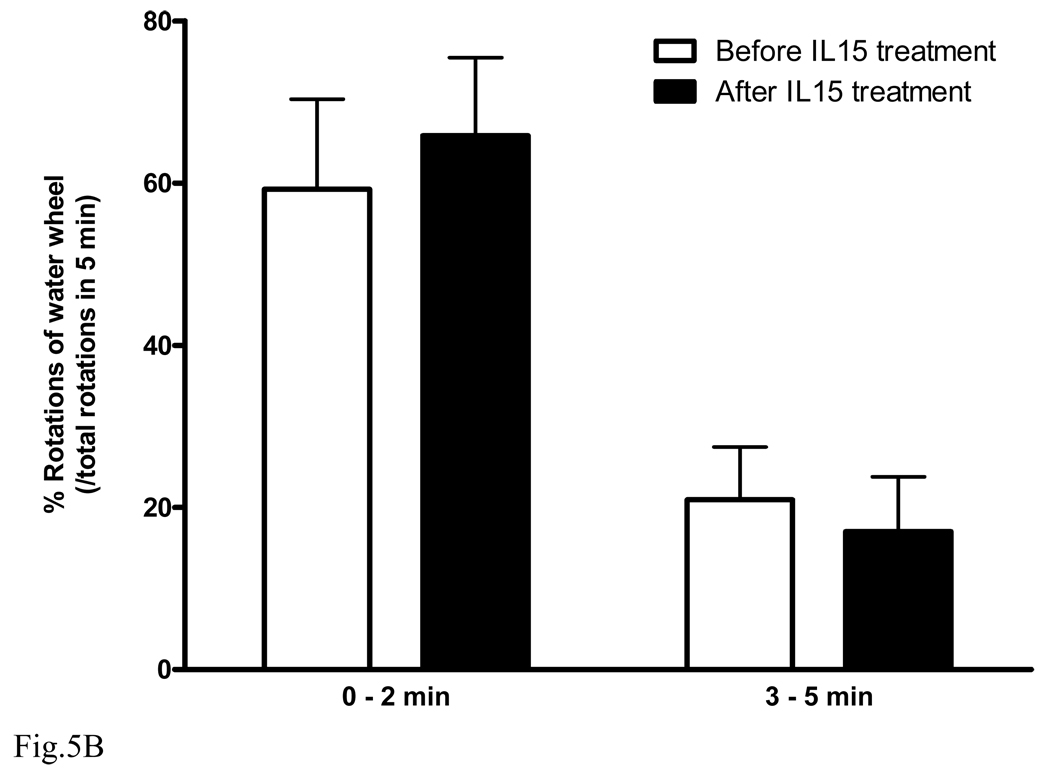
Effect of IL15 treatment on wildtype and IL15Rα KO mice. After IL15 treatment for 3 days, B6 mice showed significantly improved performance in the Nomura water wheel test for depression (A, n = 9 /group). By contrast, IL15Rα KO mice did not show any improvement (B). *: p < 0.05.
To show that the effect of IL15 was specific and mediated by IL15Rα, the IL15Rα KO mice were studied in parallel (Fig. 5B, n = 5). IL15 treatment did not improve the water wheel rotations of IL15Rα KO mice. This provides a negative control, further indicating the specificity of the findings on normal C57 mice.
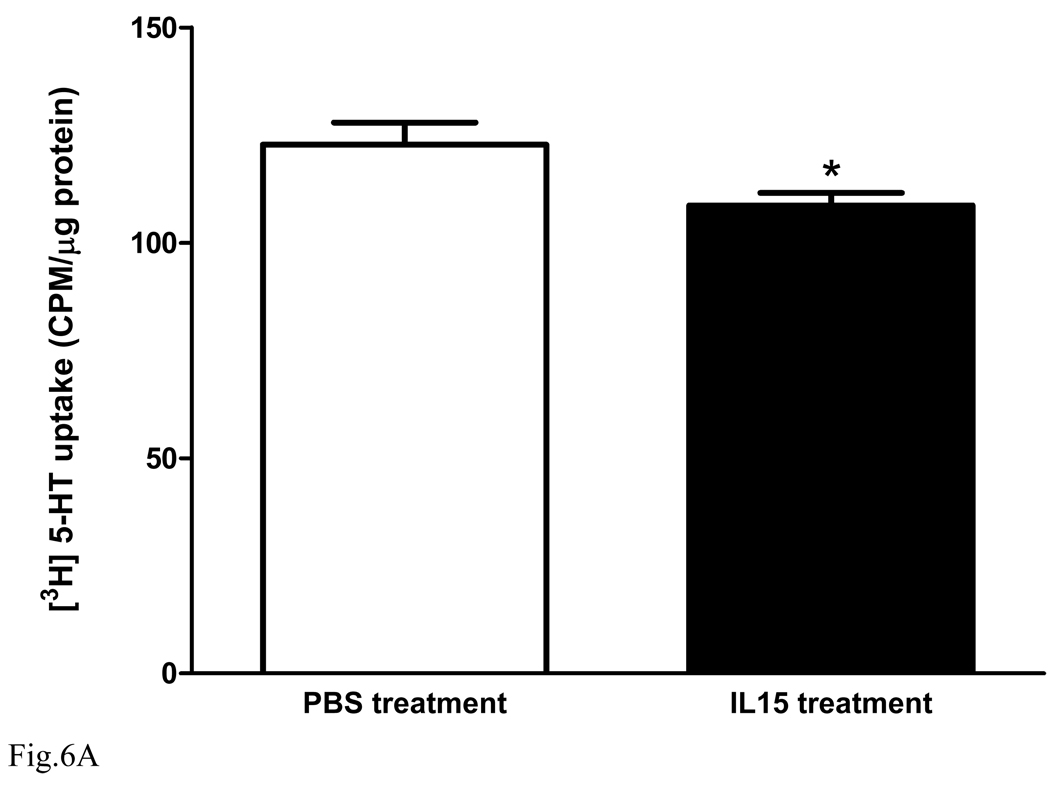
5. IL15 treatment directly reduces the synaptosomal uptake of 5-HT
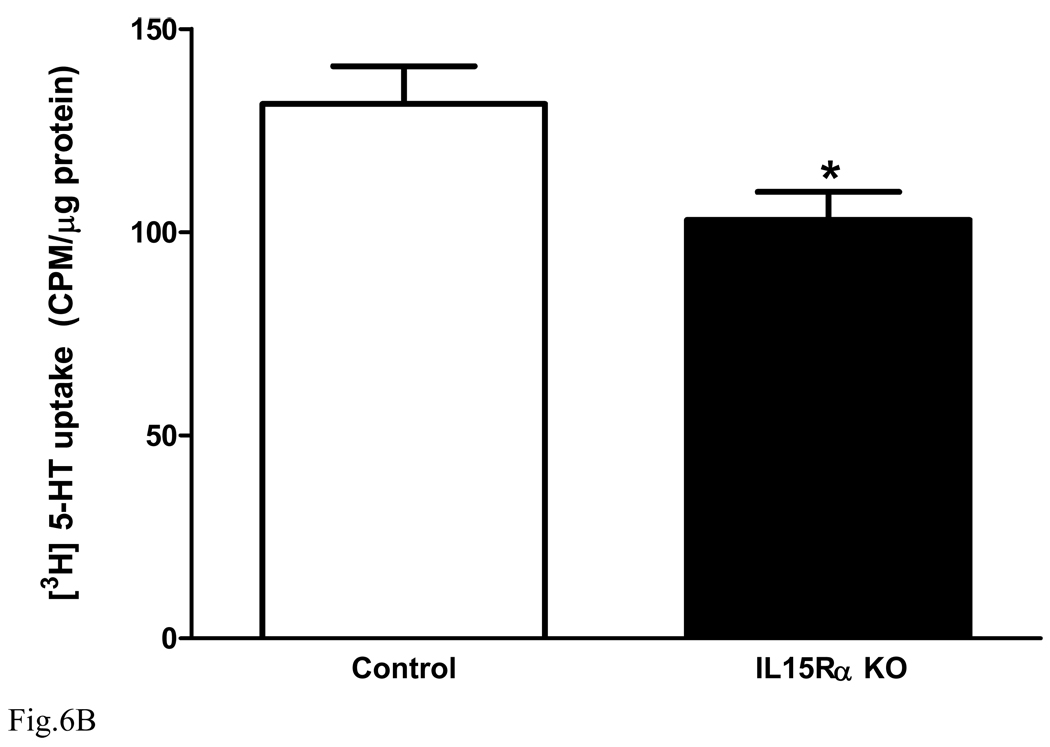
Synaptosomes from normal mouse forebrain were prepared and divided into two groups for either IL15 (10 ng/ml) or PBS treatment (n = 5 /group). After 30 min of IL15 treatment, [3H]5-HT uptake was determined in the presence of IL15 or PBS co-treatment for 20 min. Figure 6A shows that IL15 treatment induced a significant reduction of 5-HT uptake [F(1,8) = 5.7, p < 0.05]. The results suggest that IL15 increases 5-HT concentrations in the synaptic cleft and prolongs the duration of antidepressive actions. However, in the forebrain synaptosomes prepared from the KO mice (Fig. 6B), there was also a decrease of 5-HT uptake [F(1,8) = 6.1, p < 0.05]. This indicates that the KO mice showed compensatory changes to prolong 5-HT concentrations in the synaptic cleft, and that the acute treatment effects of IL15 in synaptosomes may not be mediated by IL15Rα.
Fig. 6.
Effects of IL15 treatment or IL15Rα deletion on synaptosomal [3H]5-HT uptake. (A) in synaptosomes prepared from B6 mice, IL15 (10 ng/ml) pretreatment for 30 min and co-treatment for 20 min resulted in a significant decrease of 5-HT uptake in comparison with PBS vehicle treatment (n = 5 /group). (B) [3H]5-HT uptake by synaptosomes from the KO mice was also significantly lower than that from the wildtype controls (n = 5 /group). *: p < 0.05.
6. IL15 induced dose- and time-dependent changes in proteins involved in 5-HT transmission in GT1-7 cells
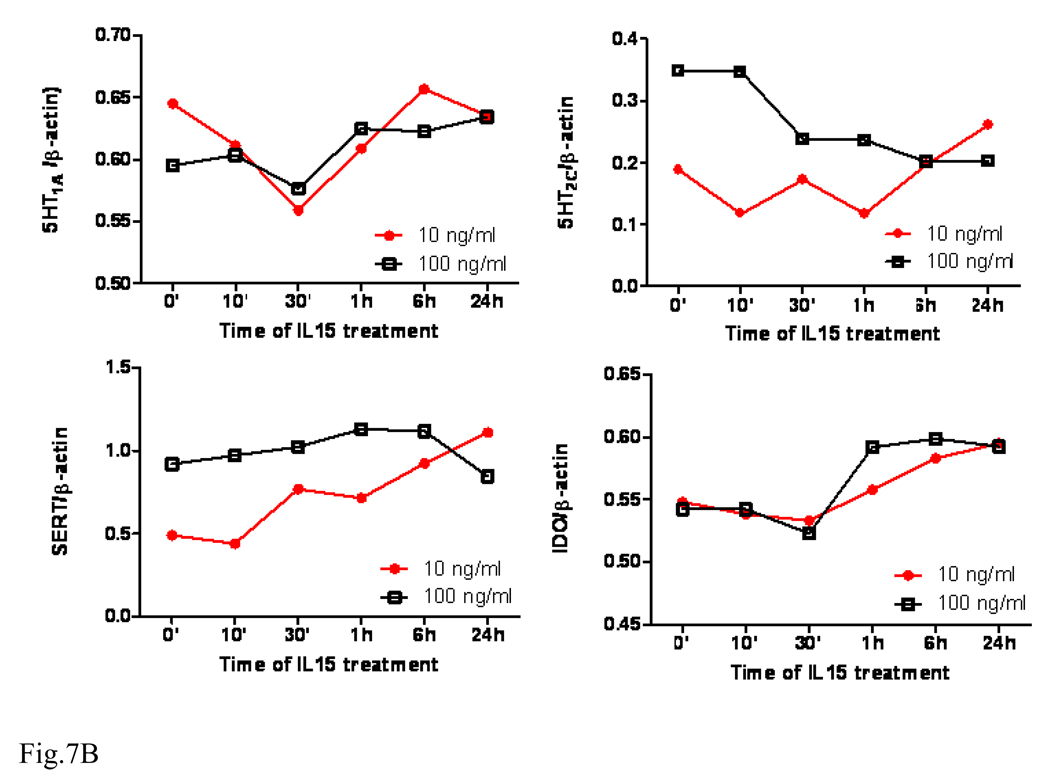
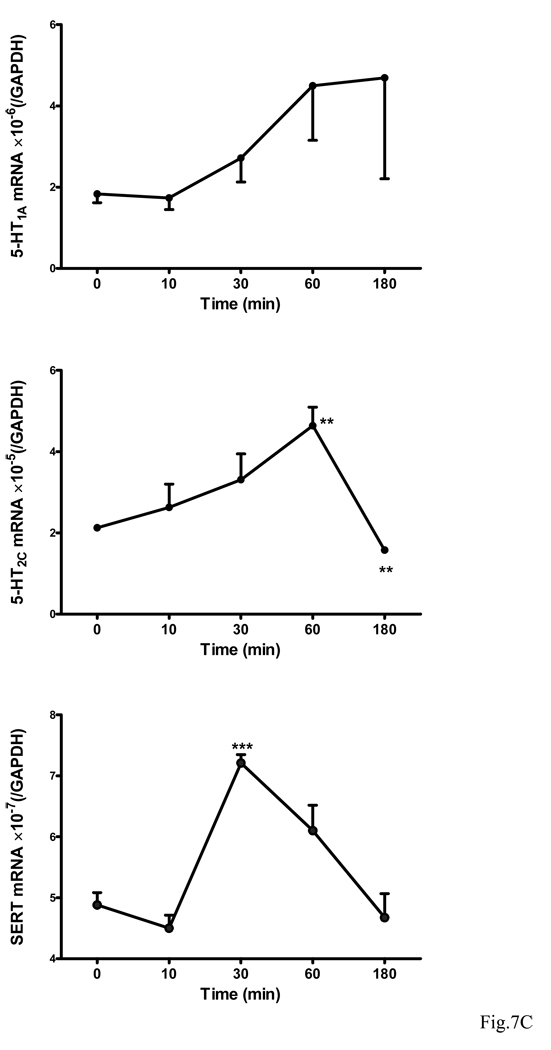
IL15 treatment appears to induce dose- and time-dependent changes of all target proteins. The level of 5-HT1A showed a transient decrease at 30 min by both doses. 5-HT1C was increased by the low dose but decreased by the high dose. SERT was increased by the low dose but unchanged by the high dose. By contrast, IDO showed an overall increase at later time points by both doses (Fig. 7A–B). Since the cell culture results showed a different trend from those from mice or synaptosomes, transcriptional regulation of these proteins was further determined by qPCR analysis. The mRNA for 5-HT1A showed a tendency to increase over time (10 min – 3 h), though it was not statistically significant. By contrast, 5-HT2C mRNA was significantly elevated during the first hour after IL15 treatment (p < 0.01). However, this increase was reversed at 3 h (p < 0.01). SERT mRNA showed a different pattern, with a significant increase at 30 min after IL15 treatment (p < 0.005) and return to baseline by 3 h (Fig. 7C).
Fig. 7.
Time course and dose-dependence of 5-HT1A, 5-HT2C, SERT, and IDO expression in GT1-7 cells upon IL15 stimulation. (A) WB shows that these four proteins have different patterns of regulation after either 10 or 100 ng/ml of IL15 from 10 min to 24 h. (B) Densitometric analysis shows the value of each protein in comparison with the internal control β-actin. (C) IL15 induced changes in the mRNA expression of 5-HT1A, 5-HT2C and SERT over time (10 min – 3 h, n = 3 /time point). **: p < 0.01; ***: p< 0.005.
Discussion
Animal models of depression approximate aspects of the depressed mood in humans (Porsolt et al. 1977;2001;Rotzinger et al. 2010). The modified Nomura water wheel test is suitable to determine behavioral changes in response to escapable stress (Nomura et al. 1982;Kastin et al. 1984) and the acute, inescapable stress of tail suspension provides a reliable test of a different aspect of immobility (Steru et al. 1985;El Yacoubi. et al. 2003;Andreasen and Redrobe 2009). In both of these tests we found that IL15Rα KO mice had a significant increase of immobility time, suggestive of a depressive phenotype. The social interaction test, reflecting social anxiety as well as other aspects of social interactions (Kennett et al. 1997), showed that these KO mice also had reduced interest in interacting with strangers.
The depressive-like behavior of IL15Rα KO mice was opposite to the results with other proinflammatory cytokines. Both TNFR1 KO and TNFR2 KO mice have reduced depression shown by increased mobility in the forced swimming test (Simen et al. 2006). IL1R1 KO mice have decreased anxiety behavior and impaired fear memory, although there are no changes in depressive-like behavior (Koo and Duman 2009). IL15/IL2-Rβ KO mice show deficits in prepulse inhibition in the acoustic startle reflex, and exhibit less anxiety in the elevated plus maze test (Petitto et al. 2002). In addition, KO mice lacking the shared gamma common chain (IL2Rγc) for IL15, IL2, and other cytokines in this family also display depressive-like behavior in forced swimming tests (Wu et al. 2010b). By comparison, the IL15Rα KO mouse shows a more profound depressive-like behavior. This represents an important “antidepressant” role for a specific cytokine receptor shown for the first time by the depressive-like behavior resulting from deletion of the receptor. Depressive-like changes in otherwise normal appearing mice suggest a prominent role of IL15Rα in maintaining normal affective behavior in the non-stressed situation.
Serotonin is the best studied neurotransmitter in depression, and SSRIs are effective in the mouse forced swim test (Redrobe et al. 2005). We chose to study three serotonin receptor subtypes representative of different mechanisms of action and different roles in depression. We focused on the hippocampus, an area known to be involved in depression (Clark et al. 2009). The results show that 5-HT1A mRNA was decreased in the hippocampus of the IL15Rα KO mice. This is consistent with reports that the absence of 5-HT1A leads to depression (Heisler et al. 1998;Zhuang et al. 1999), and that 5-HT1A binding is decreased in major depressive disorders (Savitz et al. 2009). Elevated 5-HT2C, as seen in this study, is also associated with increased depression and anxiety, whereas antagonists to 5-HT2C show anxiolytic effects in the social interaction test (Kennett et al. 1997).
The action of serotonin is terminated by reuptake, and SERT (SLC6A4) plays a major role (Chen et al. 2004). IHC of IL15Rα KO mice showed that the increased expression of SERT was region-specific and mainly present in the stratum lacunosum-moleculare where the perforant path synapses. The increased SERT (+) input to the hippocampus (mainly from the entorhinal cortex) is not inconsistent with the reduction of SERT staining in CA1 adjacent to CA2. Activation of CA1 neurons by the perforant path is limited and dependent on the generation of dendritic spikes (Jarsky et al. 2005). Fluoxetine blocks 5-HT2C action, shown by inhibition of 3H-5-HT binding to 5-HT2C in Hela cells and rat cortical membranes, and reduction of 5-HT-induced electrophysiological response of 5-HT2C in Xenopus oocytes (Ni and Miledi 1997).
SERT trafficking may not be relevant to this, and there might be multiple compensatory changes of the serotonin system in mice with embryonic IL15Rα deletion. Regardless, fluoxetine (Prozac), a classic SSRI effective in patients with depression, significantly reduced the depressive-like behavior of the IL15RKO mice. Unlike IL15, fluoxetine is obviously not dependent on the cognate receptor for IL15 (IL15Rα) for its effects on serotonin and depression. It is not clear why fluoxetine did not affect the behavior of wildtype mice even though the same treatment regimen was effective in other studies (Holladay et al. 1998;Liu et al. 2010).
The effects of IL15 on the serotonergic system may lie in the modulation of serotonin synthesis, receptors, or turnover by SERT-mediated reuptake. In the synaptosomal studies, IL15 acutely decreased 5-HT uptake. This indicates that IL15 might have decreased the intracellular trafficking of 5-HT receptors or decreased the functions of SERT. IL15 treatment studies in cultured GT1-7 neurons that are known to express serotonin receptors, however, showed that the changes were dependent on the duration of treatment, and perhaps concentrations of IL15 as well. Although cultured neurons of hypothalamic origin cannot completely duplicate in-vivo conditions, it is apparent that IL15 can directly affect the neuronal serotonin system. Interestingly, the decrease in 5-HT uptake in synaptosomes from the IL15Rα KO mice was greater than that from the wildtype controls. Since we studied pooled synaptosomes from the entire forebrain rather than those from hippocampus because of limitations in sample preparation, the results reflect the overall changes inclusive of all forebrain regions. In addition, the decrease of 5-HT uptake in the KO mice may suggest compensatory changes as a consequence of embryonic IL15Rα deletion.
It is possible that other neurotransmitter systems are also implicated in the depressive-like behavior of the KO mice. In support of this possibility, the IL15Rα KO mice also show reduction of GABA and altered GAD-67 expression in the hippocampus (He et al. 2010a), and have changes in thermoregulation and metabolic activities (He et al. 2010b). IL15 probably modulates the actions of multiple neurotransmitters by its unique effects on the solute carrier SLC6A family.
In summary, we provide the first evidence that IL15Rα knockout induces a paradoxical depressive phenotype, with the mice showing increased immobility and decreased social interactions. The worsening of behavioral performance was partially reversed by fluoxetine. Concurrently, there was reduced 5-HT1A receptor expression, increased 5-HT2C, and altered distribution of SERT in the hippocampus. Synaptosomal 5-HT uptake was decreased in the KO mice and in the normal mice after IL15 treatment, suggesting that an acute effect of IL15 on SERT does not require IL15Rα. Neuronal serotonin receptors and SERT responded to IL15 with time-dependent changes. Overall, IL15 interacts with the 5-HT system to maintain mood stability. This opens a new direction for combating depression with novel strategies to boost the brain’s intrinsic antidepressive system, including cytokines like IL15.
Acknowledgements
Grant support was provided by NIH (NS62291 and NS45751 to WP, and DK54880 to AJK). We thank Dr. Zengbiao Bill Li and Drumetix Laboratories (Greensboro, NC) for contractual work on neurotransmitter measurement by mass spectrometry, Dr. Jiming Feng at LSU Veterinary School for kind support in synaptosomal uptake assays, Dr. Paul J. Pistell at the PBRC Behavioral Core Facility for access to the rotarod, and the PBRC Genomic Core Facility for the ABI 7900 equipment to perform real-time PCR studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav. Pharmacol. 2009;20:286–295. doi: 10.1097/FBP.0b013e32832c713e. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Passage of peptides across the blood-brain barrier: pathophysiological perspectives. Life Sci. 1996;59:1923–1943. doi: 10.1016/s0024-3205(96)00380-3. [DOI] [PubMed] [Google Scholar]
- Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium-and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu. Rev. Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol. Psychiatry. 2008;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- He Y, Hsuchou H, Wu X, Kastin AJ, Khan RS, Pistell PJ, Wang W-H, Feng J, Li Z, Guo X, Pan W. Interleukin-15 receptor is essential to facilitate GABA transmission and hippocampal dependent memory. J. Neurosci. 2010a;30:4725–4734. doi: 10.1523/JNEUROSCI.6160-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wu X, Khan RS, Kastin AJ, Cornélissen GG, Hsuchou H, Robert B, Halberg F, Pan W. IL15 receptor deletion results in circadian changes of locomotor and metabolic activity. J. Mol. Neurosci. 2010b;41:315–321. doi: 10.1007/s12031-009-9319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay JW, Dewey MJ, Yoo SD. Pharmacokinetics and antidepressant activity of fluoxetine in transgenic mice with elevated serum alpha-1-acid glycoprotein levels. Drug Metab Dispos. 1998;26:20–24. [PubMed] [Google Scholar]
- Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009a;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Wu X, Kastin AJ. Cessation of blood-to-brain influx of interleukin-15 during development of EAE. J. Cereb. Blood Flow Metab. 2009b;29:1568–1578. doi: 10.1038/jcbfm.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inazu M, Takeda H, Ikoshi H, Sugisawa M, Uchida Y, Matsumiya T. Pharmacological characterization and visualization of the glial serotonin transporter. Neurochem. Int. 2001;39:39–49. doi: 10.1016/s0197-0186(01)00010-9. [DOI] [PubMed] [Google Scholar]
- Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2005;8:1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Abel DA, Ehrensing RH, Coy DH, Graf MV. Tyr-MIF-1 and MIF-1 are active in the water wheel test for antidepressant drugs. Pharmacol. Biochem. Behav. 1984;21:767–771. doi: 10.1016/s0091-3057(84)80017-9. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci. Lett. 2009;456:39–43. doi: 10.1016/j.neulet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Brown RA, Fang J, Krueger JM. Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1004–R1012. doi: 10.1152/ajpregu.2001.281.3.R1004. [DOI] [PubMed] [Google Scholar]
- Lau T, Horschitz S, Berger S, Bartsch D, Schloss P. Antidepressant-induced internalization of the serotonin transporter in serotonergic neurons. FASEB J. 2008;22:1702–1714. doi: 10.1096/fj.07-095471. [DOI] [PubMed] [Google Scholar]
- Lee YB, Satoh J, Walker DG, Kim SU. Interleukin-15 gene expression in human astrocytes and microglia in culture. NeuroReport. 1996;7:1062–1066. doi: 10.1097/00001756-199604100-00022. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Gracie JA. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:392–397. doi: 10.1016/j.coph.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ni YG, Miledi R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac) Proc. Natl. Acad. Sci. U. S. A. 1997;94:2036–2040. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Shimizu J, Kinjo M, Kametani H, Nakazawa T. A new behavioral test for antidepressant drugs. Eur. J. Pharmacol. 1982;83:171–175. doi: 10.1016/0014-2999(82)90248-5. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J. Neuroimmunol. 1997a;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte Leptin Receptor (ObR) and Leptin Transport in Adult-Onset Obese Mice. Endocrinology. 2008a;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Yu C, Kastin AJ. Permeation of blood-borne IL15 across the blood-brain barrier and the effect of LPS. J. Neurochem. 2008b;106:313–319. doi: 10.1111/j.1471-4159.2008.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. TNFα transport across the blood-brain barrier is abolished in receptor knockout mice. Exp. Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- Pan W, Xiang S, Tu H, Kastin AJ. Cytokines interact with the blood-brain barrier. In: Dermietzel R, Spray DC, Nedergaard M, editors. Blood-Brain Barrier Interfaces: From Ontogeny to Artificial Barriers. Weinheim, Germany: Wiley-VCH; 2006. pp. 247–264. [Google Scholar]
- Pan W, Yu C, Hsuhou H, Khan RS, Kastin AJ. Cerebral microvascular IL15 is a novel mediator of TNF action. J. Neurochem. 2009;111:819–827. doi: 10.1111/j.1471-4159.2009.06371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Zadina JE, Harlan RE, Weber JT, Banks WA, Kastin AJ. Tumor necrosis factor α: a neuromodulator in the CNS. Neurosci. Biobehav. Rev. 1997b;21:603–613. doi: 10.1016/s0149-7634(96)00047-4. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Schwarting RK. Striatal microinjections of interleukin-2 and rat behaviour in the elevated plus-maze. Behav. Brain Res. 2006;168:339–344. doi: 10.1016/j.bbr.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Pereno R, Giron-Michel J, Gaggero A, Cazes E, Meazza R, Monetti M, Monaco E, Mishal Z, Jasmin C, Indiveri F, Ferrini S, Azzarone B. IL-15/IL-15Ralpha intracellular trafficking in human melanoma cells and signal transduction through the IL-15Ralpha. Oncogene. 2000;19:5153–5162. doi: 10.1038/sj.onc.1203873. [DOI] [PubMed] [Google Scholar]
- Petitto JM, Huang Z, Hartemink DA, Beck R., Jr IL-2/15 receptor-beta gene deletion alters neurobehavioral performance. Brain Res. 2002;929:218–225. doi: 10.1016/s0006-8993(01)03393-5. [DOI] [PubMed] [Google Scholar]
- Pizzi C, Caraglia M, Cianciulli M, Fabbrocini A, Libroia A, Matano E, Contegiacomo A, Del PS, Abbruzzese A, Martignetti A, Tagliaferri P, Bianco AR. Low-dose recombinant IL-2 induces psychological changes: monitoring by Minnesota Multiphasic Personality Inventory (MMPI) Anticancer Res. 2002;22:727–732. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2001;Chapter 8(Unit) doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- Pouladi MA, Graham RK, Karasinska JM, Xie Y, Santos RD, Petersen A, Hayden MR. Prevention of depressive behaviour in the YAC128 mouse model of Huntington disease by mutation at residue 586 of huntingtin. Brain. 2009;132:919–932. doi: 10.1093/brain/awp006. [DOI] [PubMed] [Google Scholar]
- Rachal PC, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci. Biobehav. Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Fournier A, Baker GB, Quirion R. Role of serotonin (5-HT) in the antidepressant-like properties of neuropeptide Y (NPY) in the mouse forced swim test. Peptides. 2005;26:1394–1400. doi: 10.1016/j.peptides.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Rentzos M, Cambouri C, Rombos A, Nikolaou C, Anagnostouli M, Tsoutsou A, Dimitrakopoulos A, Triantafyllou N, Vassilopoulos D. IL-15 is elevated in serum and cerebrospinal fluid of patients with multiple sclerosis. J Neurol. Sci. 2006;241:25–29. doi: 10.1016/j.jns.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31:736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Satoh J, Kurohara K, Yukitake M, Kuroda Y. Interleukin-15, a T-cell growth factor, is expressed in human neural cell lines and tissues. J. Neurol. Sci. 1998;155:170–177. doi: 10.1016/s0022-510x(97)00310-9. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol. Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry b, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Yang YY, Kuo CJ, Chen CC, Leu SJ. Effects of symptomatic severity on elevation of plasma soluble interleukin-2 receptor in bipolar mania. J. Affect. Disord. 2001;64:185–193. doi: 10.1016/s0165-0327(00)00252-4. [DOI] [PubMed] [Google Scholar]
- Wu X, Kastin AJ, He Y, Hsuchou H, Rood JC, Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav. Immun. 2010a doi: 10.1016/j.bbi.2010.06.012. PMID 20600810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kastin AJ, Hsuchou H, Pan W. The effects of IL2Rgamma knockout on depression and contextual memory. Behav. Brain Res. 2010b;213:319–322. doi: 10.1016/j.bbr.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pan W, He Y, Hsuchou H, Kastin AJ. Cerebral interleukin-15 shows upregulation and beneficial effects in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2010c;223:65–72. doi: 10.1016/j.jneuroim.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pan W, Stone KP, Zhang Y, Hsuchou H, Kastin AJ. Expression and signaling of novel IL15Rαsplicing variants in cerebral endothelial cells of the blood-brain barrier. J. Neurochem. 2010d;114:122–129. doi: 10.1111/j.1471-4159.2010.06729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, Nabeshima T. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. J. Neuroimmunol. 2000;111:131–138. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol. Learn. Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21:52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]