Abstract
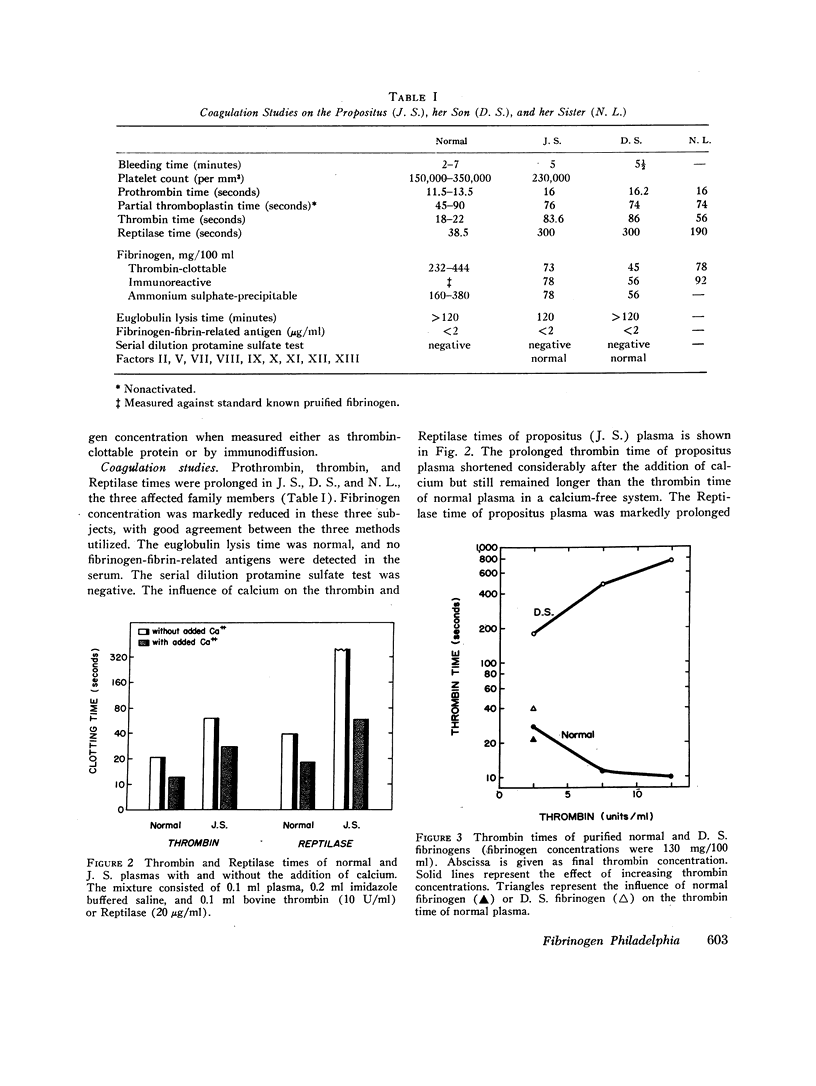
A new, autosomally inherited abnormal fibrinogen associated with hypofibrinogenemia has been described in several members of a family. Plasma fibrinogen measured either as thrombin-clottable protein or by immunodiffusion revealed a fibrinogen level ranging between 60 and 90 mg/100 ml. The thrombin time of plasma or purified fibrinogen was prolonged and only partially corrected by the addition of calcium. Purified fibrinogen prolonged the thrombin time of normal plasma. Fibrinopeptide release by thrombin was normal in rate and amount, but fibrin monomer aggregation was grossly disturbed, especially in a high ionic strength medium. We have designated this fibrinogen "fibrinogen Philadelphia." Acrylamide gel electrophoresis of mixtures of [121I]normal and [125I]abnormal fibrinogens revealed a slight increase in the anodal mobility of fibrinogen Philadelphia. Similarly, DEAE-cellulose chromatography showed slightly stronger binding of fibrinogen Philadelphia than normal. To elucidate the mechanism responsible for the low plasma fibrinogen concentration, simultaneous metabolic studies of autologous (patient) and homologous (normal) fibrinogen, labeled with 125I and 121I, respectively, were performed in two affected subjects. Autologous fibrinogen half-life was short and the fractional catabolic rate was markedly increased in both family members. In contrast, homologous fibrinogen half-life and fractional catabolic rate were normal. These metabolic studies demonstrate that rapid degradation of fibrinogen Philadelphia is largely responsible for the depressed levels of a plasma fibrinogen. This represents the first example of a mutant plasma protein in which the molecular defect is associated with an altered catabolism.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABILDGAARD U., GODAL H. C. AGGREGATION OF FIBRIN AND FIBRINOGEN BY TAME. Scand J Clin Lab Invest. 1964;16:531–539. doi: 10.3109/00365516409060551. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Studies in vivo and in vitro on an abnormality in the metabolism of C3 in a patient with increased susceptibility to infection. J Clin Invest. 1970 Nov;49(11):1975–1985. doi: 10.1172/JCI106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Bloch K. J., Rosen F. S. Increased susceptibility to infection in a patient with type II essential hypercatabolism of C3. N Engl J Med. 1973 Mar 22;288(12):601–606. doi: 10.1056/NEJM197303222881204. [DOI] [PubMed] [Google Scholar]
- BRECHER G., CRONKITE E. P. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950 Dec;3(6):365–377. doi: 10.1152/jappl.1950.3.6.365. [DOI] [PubMed] [Google Scholar]
- Baker L. R., Rubenberg M. L., Dacie J. V., Brain M. C. Fibrinogen catabolism in microangiopathic haemolytic anaemia. Br J Haematol. 1968 Jun;14(6):617–625. doi: 10.1111/j.1365-2141.1968.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Beck E. A., Charache P., Jackson D. P. A new inherited coagulation disorder caused by an abnormal fibrinogen ('fibrinogen Baltimore'). Nature. 1965 Oct 9;208(5006):143–145. doi: 10.1038/208143a0. [DOI] [PubMed] [Google Scholar]
- Beck E. A., Shainoff J. R., Vogel A., Jackson D. P. Functional evaluation of an inherited abnormal fibrinogen: fibrinogen "Baltimore". J Clin Invest. 1971 Sep;50(9):1874–1884. doi: 10.1172/JCI106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blombäck M., Blombäck B., Mammen E. F., Prasad A. S. Fibrinogen Detroit--a molecular defect in the N-terminal disulphide knot of human fibrinogen? Nature. 1968 Apr 13;218(5137):134–137. doi: 10.1038/218134a0. [DOI] [PubMed] [Google Scholar]
- Boyer M. H., Shainoff J. R., Ratnoff O. D. Acceleration of fibrin polymerization by calcium ions. Blood. 1972 Mar;39(3):382–387. [PubMed] [Google Scholar]
- CAMPBELL R. M., CUTHBERTSON D. P., MATTHEWS C. M., MCFARLANE A. S. Behaviour of 14C- and 131I-labelled plasma proteins in the rat. Int J Appl Radiat Isot. 1956 Jul;1(1-2):66–84. doi: 10.1016/0020-708x(56)90020-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DENSON K. W. The specific assay of Prower-Stuart factor and factor VII. Acta Haematol. 1961 Feb;25:105–120. doi: 10.1159/000206523. [DOI] [PubMed] [Google Scholar]
- ELLIS B. C., STRANSKY A. A quick and accurate method for the determination of fibronogen in plasma. J Lab Clin Med. 1961 Sep;58:477–488. [PubMed] [Google Scholar]
- Egeberg O. Inherited fibrinogen abnormality causing thrombophilia. Thromb Diath Haemorrh. 1967 Feb 28;17(1-2):176–187. [PubMed] [Google Scholar]
- Forman W. B., Ratnoff O. D., Boyer M. H. An inherited qualitative abnormality in plasma fibrinogen: fibrinogen Cleveland. J Lab Clin Med. 1968 Sep;72(3):455–472. [PubMed] [Google Scholar]
- GITLIN D., BORGES W. H. Studies on the metabolism of fibrinogen in two patients with congenital afibrinogenemia. Blood. 1953 Aug;8(8):679–686. [PubMed] [Google Scholar]
- Gralnick H. R., Finlayson J. S. Congenital dysfibrinogenemias. Ann Intern Med. 1972 Sep;77(3):471–473. doi: 10.7326/0003-4819-77-3-471. [DOI] [PubMed] [Google Scholar]
- Gralnick H. R., Givelber H. M., Finlayson J. S. A new congenital abnormality of human fibrinogen. Fibrinogen Bethesda II. Thromb Diath Haemorrh. 1973 Jun 28;29(3):562–571. [PubMed] [Google Scholar]
- Gralnick H. R., Givelber H. M., Shainoff J. R., Finlayson J. S. Fibrinogen Bethesda: a congenital dysfibrinogenemia with delayed fibrinopeptide release. J Clin Invest. 1971 Sep;50(9):1819–1830. doi: 10.1172/JCI106673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDISTY R. M., MACPHERSON J. C. A one-stage factor VIII (antihaemophilic globulin) assay and its use on venous and capillary plasma. Thromb Diath Haemorrh. 1962 May 15;7:215–228. [PubMed] [Google Scholar]
- HARDISTY R. M., PINNIGER J. L. Congenital afibrinogenaemia; further observations on the blood coagulation mechanism. Br J Haematol. 1956 Apr;2(2):139–152. doi: 10.1111/j.1365-2141.1956.tb06822.x. [DOI] [PubMed] [Google Scholar]
- HASSELBACK R., MARION R. B., THOMAS J. W. Congenital hypofibrinogenemia in five members of a family. Can Med Assoc J. 1963 Jan 5;88:19–22. [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ H. I., WILCOX W. P., FUJIMOTO M. M. Assay of plasma thromboplastin antecedent (PTA) with artificially depleted normal plasma. Blood. 1963 Jul;22:35–43. [PubMed] [Google Scholar]
- Hampton J. W., Garrison D. Fibrinogen and fibrin-stabilizing factor. Med Clin North Am. 1972 Jan;56(1):133–143. doi: 10.1016/s0025-7125(16)32429-4. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J. Platelet and fibrinogen consumption in man. N Engl J Med. 1972 Nov 16;287(20):999–1005. doi: 10.1056/NEJM197211162872001. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Mosesson M. W. Degradation of human fibrinogen by plasms alpha2-macroglobulin-enzyme complexes. J Clin Invest. 1973 Sep;52(9):2175–2184. doi: 10.1172/JCI107402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMPERATO C., DETTORI A. G. Ipofibrinogenemia congenita con fibrinoastenia. Helv Paediatr Acta. 1958 Oct;13(4):380–399. [PubMed] [Google Scholar]
- Jackson D. P., Beck E. A., Charache P. Congenital disorders of fibrinogen. Fed Proc. 1965 Jul-Aug;24(4):816–821. [PubMed] [Google Scholar]
- Janssen C. L., Vreeken J. Fibrinogen Amsterdam, another hereditary abnormality of fibrinogen. Br J Haematol. 1971 Mar;20(3):287–298. doi: 10.1111/j.1365-2141.1971.tb07039.x. [DOI] [PubMed] [Google Scholar]
- KAZAL L. A., AMSEL S., MILLER O. P., TOCANTINS L. M. THE PREPARATION AND SOME PROPERTIES OF FIBRINOGEN PRECIPITATED FROM HUMAN PLASMA BY GLYCINE. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:989–994. doi: 10.3181/00379727-113-28553. [DOI] [PubMed] [Google Scholar]
- Kohler P. F., Müller-Eberhard H. J. Metabolism of human C1q. Studies in hypogammaglobulinemia, myeloma, and systemic lupus erythematosus. J Clin Invest. 1972 Apr;51(4):868–875. doi: 10.1172/JCI106881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATALLO Z. S., FLETCHER A. P., ALKJAERSIG N., SHERRY S. Influence of pH, ionic strength, neutral ions, and thrombin on fibrin polymerization. Am J Physiol. 1962 Apr;202:675–680. doi: 10.1152/ajplegacy.1962.202.4.675. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mammen E. F., Prasad A. S., Barnhart M. I., Au C. C. Congenital dysfibrinogenemia: fibrinogen Detroit. J Clin Invest. 1969 Feb;48(2):235–249. doi: 10.1172/JCI105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Shapiro S. S., Holburn R. R., Carabasi R. A. Hypofibrinogenemia associated with hemangioma of the liver. Am J Clin Pathol. 1973 Aug;60(2):192–197. doi: 10.1093/ajcp/60.2.192. [DOI] [PubMed] [Google Scholar]
- Martinez J., Shapiro S. S., Holburn R. R. Metabolism of human prothrombin and fibrinogen in patients with thrombocytosis secondary to myeloproliferative states. Blood. 1973 Jul;42(1):35–46. [PubMed] [Google Scholar]
- McDonagh J., Messel H., McDonagh R. P., Jr, Murano G., Blombäck B. Molecular weight analysis of fibrinogen and fibrin chains by an improved sodium dodecyl sulfate gel electrophoresis method. Biochim Biophys Acta. 1972 Jan 26;257(1):135–142. doi: 10.1016/0005-2795(72)90262-0. [DOI] [PubMed] [Google Scholar]
- Melliger E. J. Detection of fibrinogen degradation products by use of antibody coated latex particles. The possibilities and limits of the method. Thromb Diath Haemorrh. 1970 May 31;23(2):211–227. [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Mosesson M. W., Beck E. A. Chromatographic, ultracentrifugal, and related studies of fibrinogen "Baltimore". J Clin Invest. 1969 Sep;48(9):1656–1662. doi: 10.1172/JCI106130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Umfleet R. A., Galanakis D. Human fibrinogen heterogeneities. I. Structural and related studies of plasma fibrinogens which are high solubility catabolic intermediates. J Biol Chem. 1972 Aug 25;247(16):5210–5219. [PubMed] [Google Scholar]
- Niewiarowski S., Gurewich V. Laboratory identification of intravascular coagulation. The serial dilution protamine sulfate test for the detection of fibrin monomer and fibrin degradation products. J Lab Clin Med. 1971 Apr;77(4):665–676. [PubMed] [Google Scholar]
- RAUSEN A. R., CRUCHAUD A., McMILLAN C. W., GITLIN D. A study of fibrinogen turnover in classical hemophilia and congenital afibrinogenemia. Blood. 1961 Dec;18:710–716. [PubMed] [Google Scholar]
- REGOECZI E., REGOECZI G. E., MCFARLANE A. S. RELATION BETWEEN RATE OF CATABOLISM, PLASMA CONCENTRATION AND POOL SIZE OF FIBRINOGEN. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Mar 12;279:17–25. doi: 10.1007/BF00363316. [DOI] [PubMed] [Google Scholar]
- REVOL L. [The great hemorrhagic constitutional hypofibrinemias. Clinical, hematological, genetic and therapeutic study]. Hemostase. 1962 Sep;2:243–254. [PubMed] [Google Scholar]
- Regoeczi E. Iodine-labelled fibrinogen: a review. Br J Haematol. 1971 Jun;20(6):649–663. doi: 10.1111/j.1365-2141.1971.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Regoeczi E. Measuring the coagulability of fibrinogen in plasma by isotopic means. Method and principles of its use for in vivo studies. Thromb Diath Haemorrh. 1967 Aug 15;18(1-2):276–285. [PubMed] [Google Scholar]
- Rivero I., Ritz N. D. Abnormal fibrinogen in thrombotic thrombocytopenic purpura. Blood. 1968 Jul;32(1):140–147. [PubMed] [Google Scholar]
- Samama M., Soria J., Soria C., Bousser J. Dysfibrinogénémie congénitale et familiale sans tendance hémorragique. Nouv Rev Fr Hematol. 1969 Nov-Dec;9(6):817–832. [PubMed] [Google Scholar]
- Shapiro S. S., Martinez J. Human prothrombin metabolism in normal man and in hypocoagulable subjects. J Clin Invest. 1969 Jul;48(7):1292–1298. doi: 10.1172/JCI106095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A. Fibrinogen turnover: demonstration of multiple pathways of catabolism. J Lab Clin Med. 1972 May;79(5):710–723. [PubMed] [Google Scholar]
- Sherman L. A., Gaston L. W., Kaplan M. E., Spivack A. R. Fibrinogen St. Louis: a new inherited fibrinogen variant, coincidentally associated with hemophilia A. J Clin Invest. 1972 Mar;51(3):590–597. doi: 10.1172/JCI106848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria J., Soria C., Samama M., Poirot E., Kling C. Fibrinogen Troyes--fibrinogen Metz. Two new cases of congenital dysfibrinogenemia. Thromb Diath Haemorrh. 1972 Jul 31;27(3):619–633. [PubMed] [Google Scholar]
- Streiff F., Alexandre P., Vigneron C., Soria J., Soria C., Mester L. Un nouveau Ccas d'anomalie constitutionnelle et familiale du Fibrinogène sans Diathese hémorragique. Thromb Diath Haemorrh. 1971 Dec 31;26(3):565–576. [PubMed] [Google Scholar]
- Takeda Y. Studies of the metabolism and distribution of fibrinogen in healthy men with autologous 125-I-labeled fibrinogen. J Clin Invest. 1966 Jan;45(1):103–111. doi: 10.1172/JCI105314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Felten A., Duckert F., Frick P. G. Familial disturbance of fibrin monomer aggregation. Br J Haematol. 1966 Nov;12(6):667–677. doi: 10.1111/j.1365-2141.1966.tb00152.x. [DOI] [PubMed] [Google Scholar]
- WERDER E. KONGENITALE AFIBRINOGENAEMIE. Helv Paediatr Acta. 1963 Sep;18:208–229. [PubMed] [Google Scholar]
- Wochner R. D., Drews G., Strober W., Waldmann T. A. Accelerated breakdown of immunoglobulin G (IgG) in myotonic dystrophy: a hereditary error of immunoglobulin catabolism. J Clin Invest. 1966 Mar;45(3):321–329. doi: 10.1172/JCI105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Felten A., Frick P. G., Straub P. W. Studies on fibrin monomer aggregation in congenital dysfibrinogenaemia (fibrinogen "Zürich"): separation of a pathological from a normal fibrin fraction. Br J Haematol. 1969 Apr;16(4):353–361. doi: 10.1111/j.1365-2141.1969.tb00412.x. [DOI] [PubMed] [Google Scholar]
- von Felten A., Straub P. W., Frick P. G. Dysfibrinogenemia in a patient with primary hepatoma. First observation of an acquired abnormality of fibrin monomer aggregation. N Engl J Med. 1969 Feb 20;280(8):405–409. doi: 10.1056/NEJM196902202800802. [DOI] [PubMed] [Google Scholar]


