Abstract
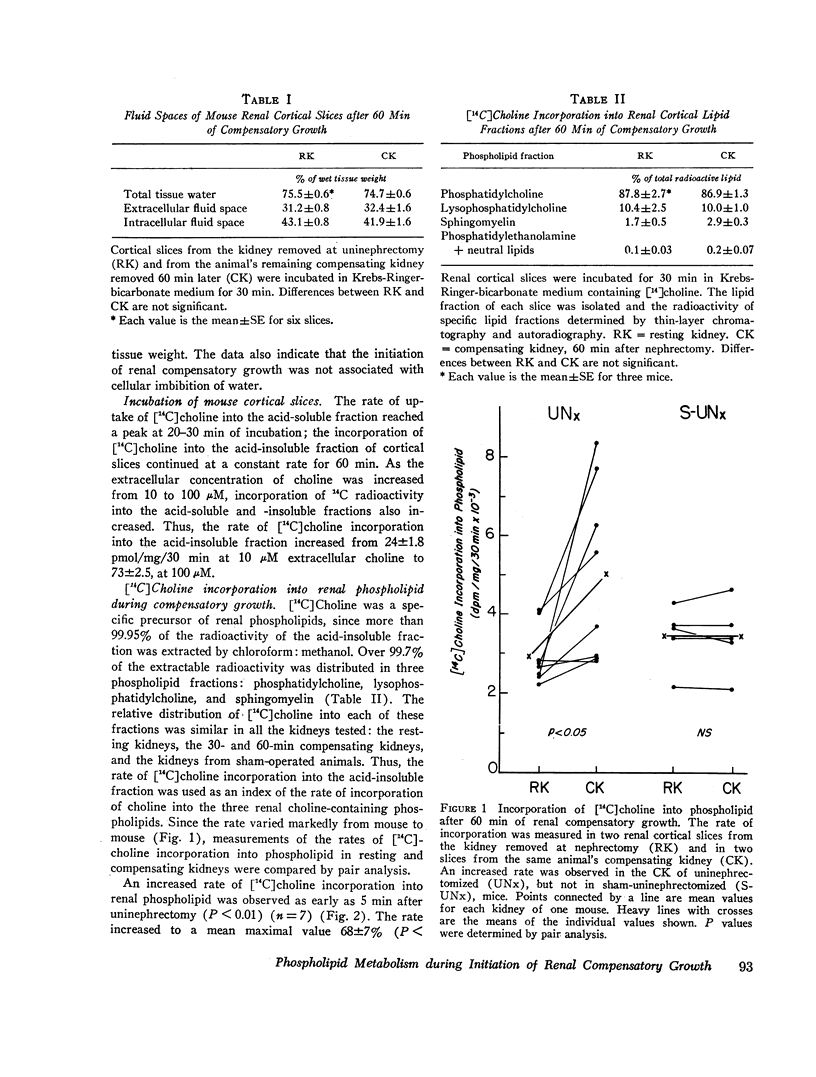
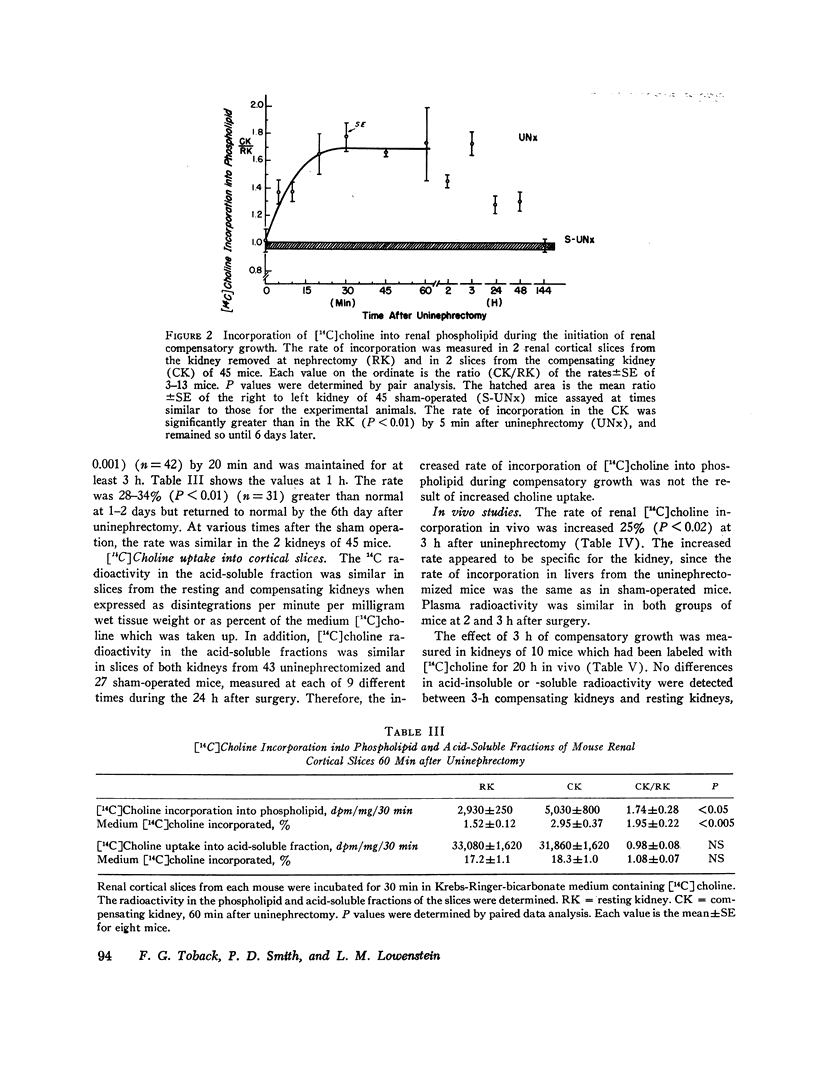
Membrane metabolism was studied during the initiation of compensatory growth after acute reduction in renal mass. The rate of [14C]choline incorporation into phospholipid in renal cortical slices was increased by 37% at 5 min of compensatory growth in mice. The rate increased to the maximal value of 68% by 20 min and remained there for 3 h. The rate then remained increased at 28-34% above normal for 2 days and returned to normal by the 6th day.
The increase in rate of choline incorporation into renal phospholipid was independent of choline uptake. [14C]Choline was found to be a specific precursor of the three renal phospholipids, phosphatidylcholine, lysophosphatidylcholine, and sphingomyelin, which comprise over half the amount of the phospholipids. The relative distribution of the label in each of the three phospholipid classes did not change with compensatory growth. An increased rate of choline incorporation was also observed in kidneys of rats during compensatory growth and in the compensating kidneys of mice treated with indomethacin before uninephrectomy. The rate was increased 24% at 3 h after uninephrectomy in vivo. The increase appeared to be specific for the kidney, since it did not occur in the livers of these mice.
The results indicate that the onset of renal compensatory growth is associated with a specific enhancement of the synthesis of renal choline-containing phospholipids. Since the phospholipids largely occur in the cell membrane, early alterations in cell membrane metabolism may thus play a role in the initiation of cell growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. A. The fine structure of compensatory growth in the rat kidney after unilateral nephrectomy. Am J Anat. 1967 Sep;121(2):217–247. doi: 10.1002/aja.1001210204. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Warmsley A. M., Pasternak C. A. Phospholipid synthesis and degradation during the life-cycle of P815Y mast cells synchronized with excess of thymidine. Biochem J. 1970 Sep;119(3):489–492. doi: 10.1042/bj1190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe F. L., Korty P. R. Protein synthesis during compensatory renal hypertrophy. Am J Physiol. 1967 Dec;213(6):1585–1589. doi: 10.1152/ajplegacy.1967.213.6.1585. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppage F. E., Chiga M., Tate A. Mitochondrial proliferation within the nephron. I. Comparison of mitochondrial hyperplasia of tubular regeneration with compensatory hypertrophy. Am J Pathol. 1973 Jan;70(1):119–130. [PMC free article] [PubMed] [Google Scholar]
- Eibl H., Hill E. E., Lands W. E. The subcellular distribution of acyltransferases which catalyze the synthesis of phosphoglycerides. Eur J Biochem. 1969 Jun;9(2):250–258. doi: 10.1111/j.1432-1033.1969.tb00602.x. [DOI] [PubMed] [Google Scholar]
- GETZ G. S., BARTLEY W., STRIPE F., NOTTON B. M., RENSHAW A., ROBINSON D. S. The lipid composition of rat-liver cell sap. Biochem J. 1961 Oct;81:214–220. doi: 10.1042/bj0810214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. Chemical aspects of compensatory renal hypertrophy. Cancer Res. 1965 Dec;25(11):1882–1887. [PubMed] [Google Scholar]
- Hillyard L. A., Abraham S. Membrane proliferation and phosphatidylcholine synthesis in normal, preneoplastic, and neoplastic mammary gland tissues in C3H mice. Cancer Res. 1972 Dec;32(12):2834–2841. [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe B. M., Parker C. W., Marshall G. R., Needleman P. Renal concentrations of prostaglandin E in acute and chronic renal ischemia. Biochem Biophys Res Commun. 1972 Nov 1;49(3):799–805. doi: 10.1016/0006-291x(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Johnson H. A., Amendola F. Mitochondrial proliferation in compensatory growth of the kidney. Am J Pathol. 1969 Jan;54(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- Lands W. E., Samuelsson B. Phospholipid precursors of prostaglandins. Biochim Biophys Acta. 1968 Oct 22;164(2):426–429. doi: 10.1016/0005-2760(68)90168-9. [DOI] [PubMed] [Google Scholar]
- Leak L. V., Rosen V. J., Jr Early ultrastructural alterations in proximal tubular cells after unilateral nephrectomy and x-irradiation. J Ultrastruct Res. 1966 Jun;15(3):326–348. doi: 10.1016/s0022-5320(66)80112-0. [DOI] [PubMed] [Google Scholar]
- Lowenstein L. M., Smith I., Segal S. Amino acid transport in the rat renal papilla. Biochim Biophys Acta. 1968 Jan 3;150(1):73–81. doi: 10.1016/0005-2736(68)90010-2. [DOI] [PubMed] [Google Scholar]
- Malt R. A. Compensatory growth of the kidney. N Engl J Med. 1969 Jun 26;280(26):1446–1459. doi: 10.1056/NEJM196906262802606. [DOI] [PubMed] [Google Scholar]
- McMurray W. C., Dawson R. M. Phospholipid exchange reactions within the liver cell. Biochem J. 1969 Mar;112(1):91–108. doi: 10.1042/bj1120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagley P., Hallinan T. The use of radioactive choline as a label for microsomal membranes. I. Selectivity of label for endoplasmic reticulum and specificity for lecithin. Biochim Biophys Acta. 1968 Sep 17;163(2):218–225. doi: 10.1016/0005-2736(68)90100-4. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B. CELL DIVISION AND A HYPOTHESIS OF CANCER. Natl Cancer Inst Monogr. 1964 May;14:7–20. [PubMed] [Google Scholar]
- Pasternak C. A., Friedrichs B. Turnover of mammalian phospholipids. Rates of turnover and metabolic heterogeneity in cultured human lymphocytes and in tissues of healthy, starved and vitamin A-deficient rats. Biochem J. 1970 Sep;119(3):481–488. doi: 10.1042/bj1190481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G. Choline metabolism and membrane formation in rat hepatoma cells grown in suspension culture. 3. Choline transport and uptake by simple diffusion and lack of direct exchange with phosphatidylcholine. J Lipid Res. 1971 Nov;12(6):715–724. [PubMed] [Google Scholar]
- Plagemann P. G. Choline metabolism and membrane formation in rat hepatoma cells grown in suspension culture. I. Incorporation of choline into phosphatidylcholine of mitochondria and other membranous structures and effect of metabolic inhibitors. Arch Biochem Biophys. 1968 Oct;128(1):70–87. doi: 10.1016/0003-9861(68)90009-x. [DOI] [PubMed] [Google Scholar]
- Rouser G., Simon G., Kritchevsky G. Species variations in phospholipid class distribution of organs. I. Kidney, liver and spleen. Lipids. 1969 Nov;4(6):599–606. doi: 10.1007/BF02531047. [DOI] [PubMed] [Google Scholar]
- Sarzala M. G., Van Golde L. M., De Kruyff B., Van Deenen L. L. The intramitochondrial distribution of some enzymes involved in the biosynthesis of rat-liver phospholipids. Biochim Biophys Acta. 1970 Feb 10;202(1):106–119. doi: 10.1016/0005-2760(70)90222-5. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Moddox I. S. Prostaglandin production by experimental tumours and effects of anti-inflammatory compounds. Nat New Biol. 1972 May 10;237(71):59–61. doi: 10.1038/newbio237059a0. [DOI] [PubMed] [Google Scholar]
- Tinker D. O., Hanahan D. J. Phospholipid metabolism in kidney. 3. Biosynthesis of phospholipids from radioactive precursors in rabbit renal cortex slices. Biochemistry. 1966 Feb;5(2):423–435. doi: 10.1021/bi00866a006. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Lowenstein L. M. Uridine metabolism during normal and compensatory renal growth. Growth. 1974 Mar;38(1):17–34. [PubMed] [Google Scholar]
- Vonkeman H., van Dorp D. A. The action of prostaglandin synthetase on 2-arachidonyl-lecithin. Biochim Biophys Acta. 1968 Oct 22;164(2):430–432. doi: 10.1016/0005-2760(68)90169-0. [DOI] [PubMed] [Google Scholar]
- WILGRAM G. F., KENNEDY E. P. INTRACELLULAR DISTRIBUTION OF SOME ENZYMES CATALYZING REACTIONS IN THE BIOSYNTHESIS OF COMPLEX LIPIDS. J Biol Chem. 1963 Aug;238:2615–2619. [PubMed] [Google Scholar]
- Wheldrake J. F. Kidney phospholipid synthesis. Effect of adrenalectomy and of aldosterone and other adrenocortical hormones in adrenalectomised rats. Biochim Biophys Acta. 1972 Apr 18;260(4):583–592. [PubMed] [Google Scholar]
- Willems M., Musilova H. A., Malt R. A. Giant nucleoplasmic RNA in the switch-on of compensatory renal growth. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1189–1194. doi: 10.1073/pnas.62.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz K. W., Zilversmit D. B. Participation of soluble liver proteins in the exchange of membrane phospholipids. Biochim Biophys Acta. 1969 Oct 14;193(1):105–116. doi: 10.1016/0005-2736(69)90063-7. [DOI] [PubMed] [Google Scholar]


