Abstract
Groups of dogs were immunized with sheep erythrocytes administered either directly into the lower respiratory tract (bronchoalveolar spaces) or intravenously. The hemolytic plaque-forming response (Jerne plaque assay) was studied in various canine lymphoid populations (bronchoalveolar cells, hilar lymph nodes, peripheral lymph nodes, and splenic and peripheral blood leukocytes) as a function of time after immunization and as a function of the dose of antigen administered. Serum hemagglutinating antibody titers against sheep erythrocytes were also measured.
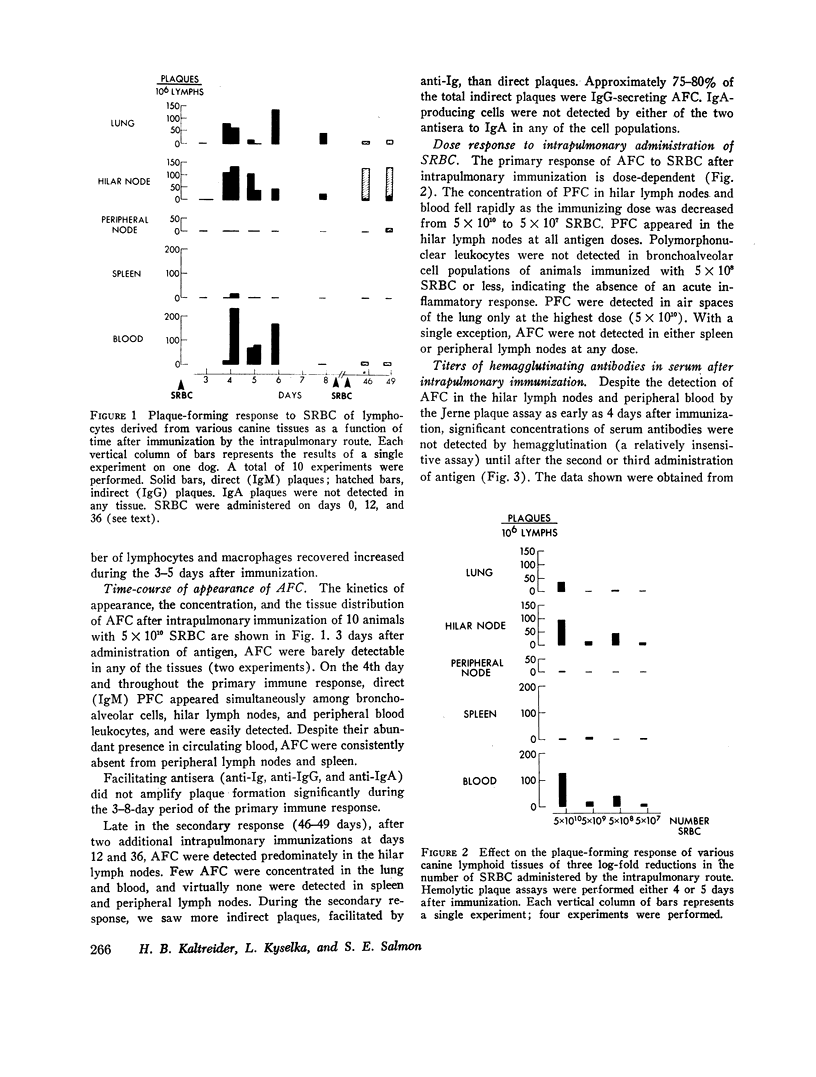
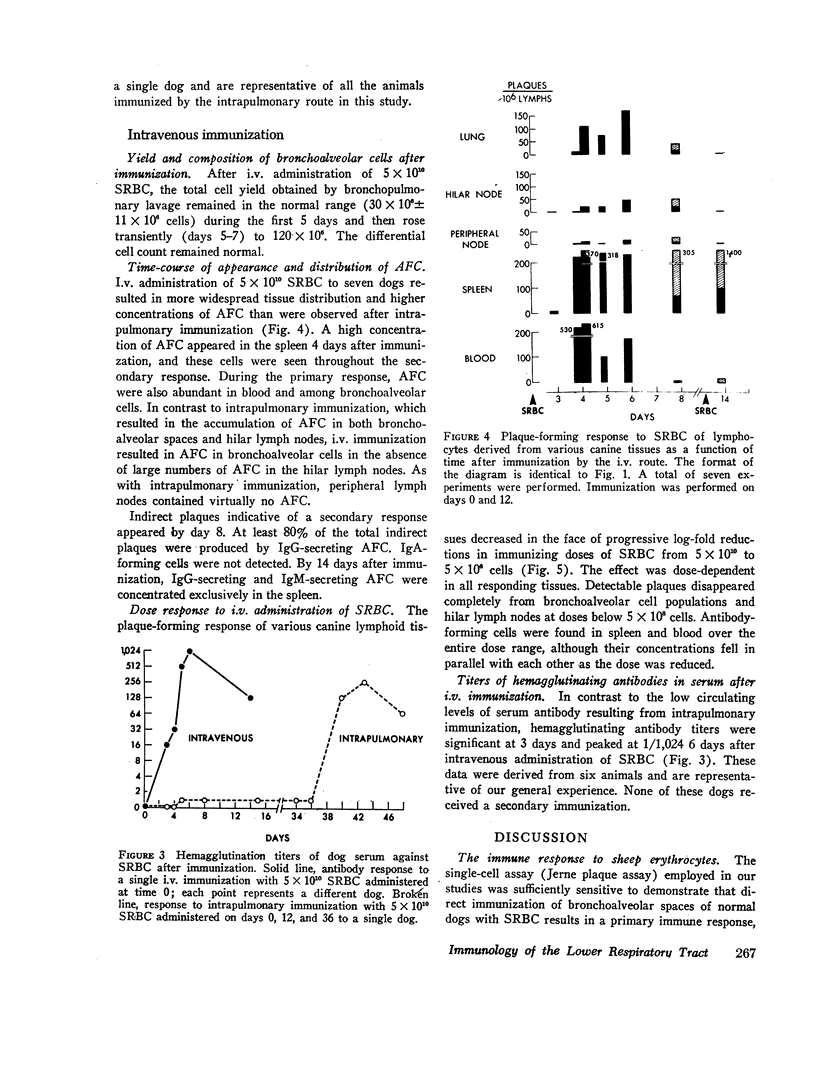
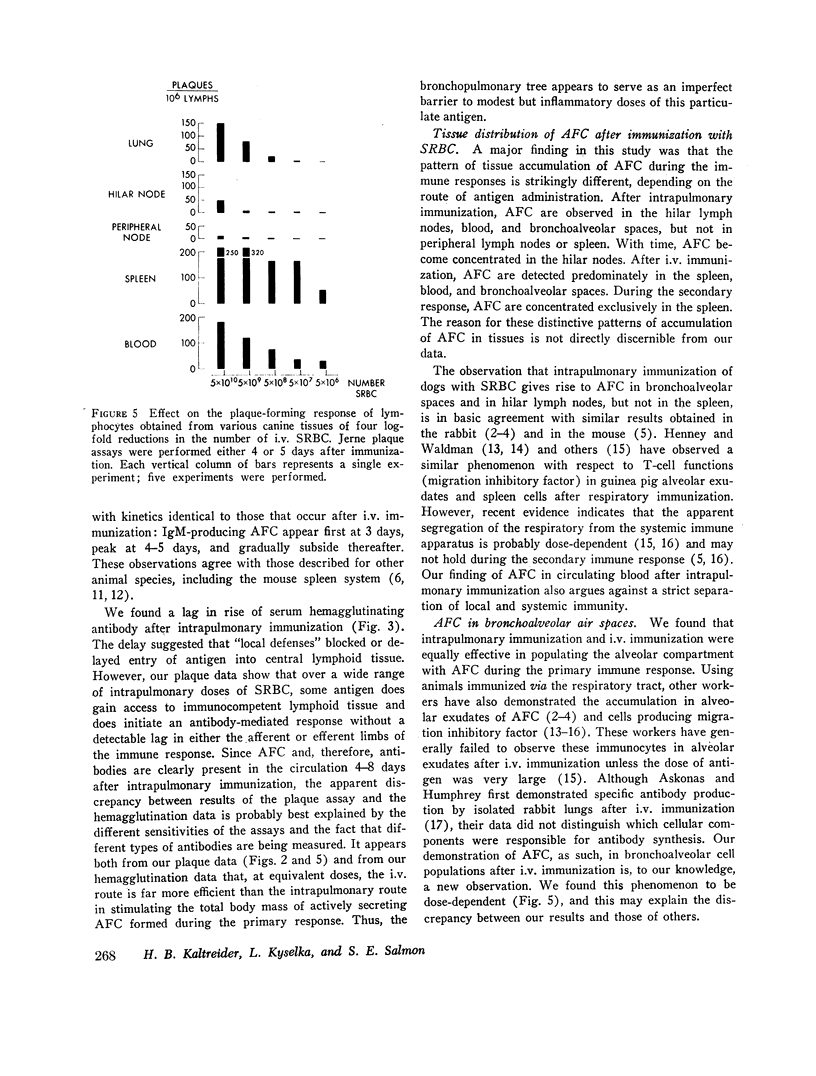
Intrapulmonary and intravenous administration of sheep erythrocytes to dogs both result in an immune response, the kinetics of which are identical to those observed in other animal species. At equivalent doses, the intravenous route is more efficient than the intrapulmonary route in generating serum hemagglutinating antibodies and antibody-forming cells. Both routes give rise transiently to circulating antibody-forming cells during the primary response; the distribution in tissues of antibody-forming cells is distinctive and unique, depending on the route of immunization. After i.v. immunization, antibody-forming cells are found predominately in spleen, blood, and bronchoalveolar spaces; after intrapulmonary immunization, they are located predominately in hilar lymph nodes, blood, and bronchoalveolar spaces. The reasons for this pattern of distribution are not known. Both routes of immunization are equally effective in populating bronchoalveolar air spaces with antibody-forming cells, which are predominately IgM-secreting and IgG-secreting cells. IgA-secreting cells were not detected.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., HUMPHREY J. H. Formation of antibody by isolated perfused lungs of immunized rabbits: the use of [14C]amino acids to study the dynamics of antibody secretion. Biochem J. 1958 Oct;70(2):212–222. doi: 10.1042/bj0700212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann A. J., Pelger R. J. Determination of antibody to pneumococcal polysaccharides with chromic chloride-treated human red blood cells and indirect hemagglutination. Appl Microbiol. 1972 Nov;24(5):679–683. doi: 10.1128/am.24.5.679-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain D. W., Nopajaroonsri C., Simon G. T. Ultrastructure of the pulmonary lymphoid tissue. Am Rev Respir Dis. 1973 Sep;108(3):621–631. doi: 10.1164/arrd.1973.108.3.621. [DOI] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Finley T. N., Swenson E. W., Curran W. S., Huber G. L., Ladman A. J. Bronchopulmonary lavage in normal subjects and patients with obstructive lung disease. Ann Intern Med. 1967 Apr;66(4):651–658. doi: 10.7326/0003-4819-66-4-651. [DOI] [PubMed] [Google Scholar]
- Ford R. J., Jr, Kuhn C. Immunologic competence of alveolar cells. I. The plaque-forming response to particulate and soluble antigens. Am Rev Respir Dis. 1973 May;107(5):763–770. doi: 10.1164/arrd.1973.107.5.763. [DOI] [PubMed] [Google Scholar]
- Ford R. J., Jr, Kuhn C. Immunologic competence of alveolar cells. II. Modification of the plaque-forming response by inhibitors, tolerance, and chronic stimulation. Am Rev Respir Dis. 1973 May;107(5):771–775. doi: 10.1164/arrd.1973.107.5.771. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Hand W. L., Cantey J. R. Antibacterial mechanisms of the lower respiratory tract. I. Immunoglobulin synthesis and secretion. J Clin Invest. 1974 Feb;53(2):354–362. doi: 10.1172/JCI107567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Waldman R. H. Cell-mediated immunity shown by lymphocytes from the respiratory tract. Science. 1970 Aug 14;169(3946):696–697. doi: 10.1126/science.169.3946.696. [DOI] [PubMed] [Google Scholar]
- Holub M., Hauser R. E. Lung alveolar histiocytes engaged in antibody production. Immunology. 1969 Aug;17(2):207–226. [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., Vaughan J. H., Swisher S. N. Canine immunoglobulins. II. Antibody activities in six immunoglobulin classes. J Immunol. 1967 May;98(5):935–940. [PubMed] [Google Scholar]
- Kaltreider H. B., Johnson J. S. Porcine immunoglobulins. I. Identification of classes and preparation of specific antisera. J Immunol. 1972 Nov;109(5):992–998. [PubMed] [Google Scholar]
- Kaltreider H. B., Salmon S. E. Immunology of the lower respiratory tract. Functional properties of bronchoalveolar lymphocytes obtained from the normal canine lung. J Clin Invest. 1973 Sep;52(9):2211–2217. doi: 10.1172/JCI107406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoulas A. O., Moroz L. A. The direct hemolytic plaque response to hemocyanin in the rabbit. J Immunol. 1971 Jun;106(6):1641–1646. [PubMed] [Google Scholar]
- Lieberman P., Patterson R., Petersen V., Chakrin L. W., Wardell J. R., Jr, Ricks J. Effect of antigen variation on production of antibody in canine tracheal secretions. J Immunol. 1971 Nov;107(5):1349–1356. [PubMed] [Google Scholar]
- Nash D. R. Direct and indirect plaque forming cells in extrapulmonary lymphoid tissue following local vs systemic injection of soluble antigen. Cell Immunol. 1973 Nov;9(2):234–241. doi: 10.1016/0008-8749(73)90074-9. [DOI] [PubMed] [Google Scholar]
- Nash D. R., Holle B. Local and systemic cellular immune responses in guinea-pigs given antigen parenterally or directly into the lower respiratory tract. Clin Exp Immunol. 1973 Apr;13(4):573–583. [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Johnson J. S. Canine immunoglobulins. 3. Distribution of immunoglobulins in colostrum and isolation of secretory IgA 7S gamma 1. J Immunol. 1970 Apr;104(4):1000–1008. [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]


