Abstract
IL-4 is a pleiotropic immune cytokine secreted by activated TH2 cells that inhibits bone resorption both in vitro and in vivo. The cellular targets of IL-4 action as well as its intracellular mechanism of action remain to be determined. We show here that IL-4 inhibits receptor activator of NF-κB ligand-induced osteoclast differentiation through an action on osteoclast precursors that is independent of stromal cells. Interestingly, this inhibitory effect can be mimicked by both natural as well as synthetic peroxisome proliferator-activated receptor γ1 (PPARγ1) ligands and can be blocked by the irreversible PPARγ antagonist GW 9662. These findings suggest that the actions of IL-4 on osteoclast differentiation are mediated by PPARγ1, an interpretation strengthened by the observation that IL-4 can activate a PPARγ1-sensitive luciferase reporter gene in RAW264.7 cells. We also show that inhibitors of enzymes such as 12/15-lipoxygenase and the cyclooxygenases that produce known PPARγ1 ligands do not abrogate the IL-4 effect. These findings, together with the observation that bone marrow cells from 12/15-lipoxygenase-deficient mice retain sensitivity to IL-4, suggest that the cytokine may induce novel PPARγ1 ligands. Our results reveal that PPARγ1 plays an important role in the suppression of osteoclast formation by IL-4 and may explain the beneficial effects of the thiazolidinedione class of PPARγ1 ligands on bone loss in diabetic patients.
The skeleton is renewed throughout life as a result of the coupled actions of two cell types, the bone-resorbing osteoclast and the bone-forming osteoblast (1). Although resorption and formation are generally in balance, excessive osteoclast formation, activity, or survival is capable of overwhelming bone formation. These situations occur as a result of age, sex hormone status, or cancer or in conjunction with a variety of diseases associated with activation of the immune system and often lead to either local or systemic bone loss and eventually osteoporosis (2, 3).
Osteoclasts are derived from the monocyte-macrophage lineage under the influence of local factors that include granulocyte/macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), and receptor activator of NF-κB ligand (RANKL), as well as proinflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor (TNF) (4). The most important of these is RANKL, a TNF-like molecule that together with M-CSF is essential for osteoclast differentiation and function (5). The importance of RANKL in osteoclast differentiation is highlighted in RANKL-deficient mice, which reveal an absence of osteoclasts and the appearance of osteopetrosis (6). RANKL is produced predominantly as a membrane-bound protein by stromal cells, osteoblasts, and lymphoid cells in response to a variety of factors that include vitamin D, parathyroid hormone, and prostaglandin E2 (5). Although RANKL expression is essential for normal osteoclast differentiation, its production from activated T cells may be responsible for the osteolytic bone loss associated with arthritis and diseases of the immune system (7).
The process of osteoclastogenesis can be inhibited by systemic factors such as the sex steroids and local factors including cytokines, γ-interferon, and certain prostaglandins (8, 9). IL-4 is a pleiotropic immune cytokine secreted from activated TH2 lymphocytes that regulates the growth, activity, and survival of certain cells of the lymphoid lineage (10). IL-4 also modulates macrophage function, regulating the expression of proinflammatory cytokines such as IL-1, TNF-α, and IL-6 (11), as well as other genes integral to macrophage activity (12). Interestingly, IL-4 also inhibits bone resorption both in vitro and in vivo (13–15). This activity is likely manifested through its ability to inhibit the expression of inflammatory cytokines such as IL-1, TNF, and RANKL from adjacent cells that modulate osteoclast production, activity, and life span (15). A direct action by IL-4 on osteoclast precursors also has been hypothesized (14, 16). We show herein that IL-4 can suppress RANKL-induced osteoclast differentiation through direct action on monocyte/macrophage precursors that is independent of supportive cells. We also show that this effect is mediated via peroxisome proliferator-activated receptor γ1 (PPARγ1). The ability of PPARγ1 to suppress osteoclast differentiation may explain the antiresorptive effects of the thiazolidinedione class of PPARγ1 ligands on bone loss observed in diabetes.
Materials and Methods
Materials.
α-MEM and DMEM were purchased from Life Technologies (Grand Island, NY). Murine M-CSF was obtained from R & D Systems. Human RANKL (residues 137–316) cDNA was expressed and purified as described (17). Murine IL-4 was purchased from PharMingen. 15(S)-Hydroxyeicosatetraenoic acid [15(S)-HETE] and ibuprofen were obtained from Cayman Chemicals (Ann Arbor, MI). Ciglitazone was obtained from Biomol (Plymouth Meeting, PA). 15-Deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2) was acquired from Calbiochem. The PPARγ1 antagonist GW 9662 was provided by Glaxo Wellcome. Other chemicals were purchased from Sigma.
Cell Culture.
Bone marrow cells from normal (C57BL/6) and 12/15-lipoxygenase (12/15-LO) heterozygous and homozygous null female mice (18) were cultured for 24 h in α-MEM with 10% FBS. Nonadherent cells were isolated and enriched as described (17). The murine monocytic cell line RAW264.7 was cultured in phenol red-free α-MEM supplemented with 10% charcoal-stripped FBS.
Characterization and Quantitation of Osteoclast-like Cells.
Primary bone marrow monocytes (BMs) (1 × 105 cells per well) or RAW 264.7 cells (2 × 103) were cultured in 48-well plates with the indicated factors added at day 0 and during a medium change on day 3. Osteoclast formation was assessed by counting multinucleated (>3 nuclei), tartrate-resistant acid phosphatase (TRAP)-positive cells present on day 10 (BMs) or day 5 (RAW264.7) (17).
Nonspecific Acid Esterase Staining for Macrophages.
Marrow cells were incubated with α-naphthylacetate in the presence of freshly formed diazonium salt (Sigma), fixed with a citrate-acetone-formaldehyde solution, and then counterstained with hematoxylin.
Detection of PPARγ1 Transcripts.
Total RNA was extracted from BMs and RAW264.7 cells with Tri Reagent (Molecular Research Center, Cincinnati) and used to prepare cDNA. cDNA was amplified with the use of specific primers for mouse PPARγ1 (19) or mouse glyceraldehyde-3-phosphate dehydrogenase (19). DNA fragments of 412 and 414 nt, respectively, were visualized with the use of ethidium bromide.
Western Blot Analysis of PPARγ1 Protein.
Enriched marrow cells were stimulated for 48 or 72 h with 10 or 100 ng/ml of M-CSF. Nuclear protein was evaluated by Western analysis with an anti-PPARγ1 antibody obtained from Santa Cruz Biotechnology (17).
Electrophoretic Mobility-Shift Assay.
Nuclear extracts were isolated from RAW 264.7 cells treated with factors as described (17). An electrophoretic mobility-shift assay was performed in 20 mM Tris⋅HCl (pH 7.6), 20% glycerol, 1 μg poly d(I-C), 1 mM DTT, and 1 ng of γ-32P-ATP-labeled NF-κB consensus sequence. Protein samples (10 μg) were incubated at 22°C for 20 min with or without anti-p65 antibody and subjected to standard electrophoretic mobility-shift assay procedures.
Transfections.
pCMV-PPARγ1 and the luciferase reporter genes pTK-luc and pAOx-TK-luc have been described (20). The latter contains three copies of the PPAR response element from the aryl CoA oxidase (AOx) gene promoter. RAW264.7 cells were seeded in 6-well plates at a density of 7.5 × 105 per well and transfected with a total of 2 μg of DNA with the use of Lipofectamine Plus (GIBCO). Cells then were cultured for 18 h in medium containing 0.5% charcoal-stripped serum and the indicated growth factors/cytokines and/or ligands. Cells were harvested, lysed, and evaluated for both luciferase and β-galactosidase activities. Transfection efficiency was normalized with the use of a pCMV-β-gactosidase expression vector.
Results
IL-4 Suppresses RANKL-Induced Osteoclast Formation from Murine Monocytes.
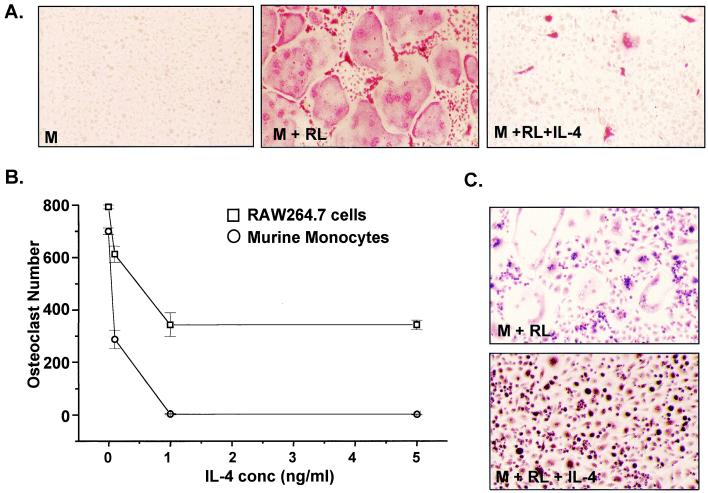
Treatment of isolated murine BMs with soluble human RANKL (30 ng/ml) and murine M-CSF (10 ng/ml) results in numerous multinucleated, TRAP-positive osteoclasts (Fig. 1A). These cells express both vitronectin (αVβ3) and calcitonin receptors and form resorption lacunae when plated on synthetic bone discs (17). We therefore treated cells with RANKL and M-CSF (RANKL/M-CSF), plus increasing concentrations of IL-4 and quantitated multinucleated (more than three nuclei), TRAP-positive osteoclasts on day 10. IL-4 significantly suppressed osteoclast formation at concentrations between 0.1 and 5 ng/ml (Fig. 1 A and B). Although IL-4 treatment did not inhibit cellular proliferation, it did appear to induce a more differentiated macrophage phenotype, as assessed both morphologically and enzymatically with the use of α-naphthylacetate staining (Fig. 1C). These results reveal that IL-4 can selectively inhibit RANKL-induced osteoclast formation through an action independent of supportive cells.
Figure 1.
IL-4 suppresses M-CSF/RANKL-induced osteoclast formation in both murine BMs and RAW264.7 cells. (A) BMs were treated with M-CSF (M) (10 ng/ml) or M-CSF plus RANKL (RL) (30 ng/ml) in the absence or presence of IL-4 (1 ng/ml) for 10 days, stained for TRAP, and photographed at ×20. (B) BMs (1 × 105 cells per well) and RAW264.7 cells (2 × 103 cells per well) were plated in triplicate and induced with M-CSF/RANKL in the absence or presence of increasing amounts of IL-4. Multinucleated (more than three nuclei), TRAP-positive osteoclasts were quantitated after 10 days (BMs) or 5 days (RAW264.7). Mean ± SE, n = 3. (C) IL-4 treatment suppresses osteoclastogenesis and results in enhanced macrophage formation in BMs. BMs were treated with the indicated factors for 10 days and then fixed and stained for α-naphthylacetate esterase.
IL-4 Suppresses RANKL-Induced Osteoclast Formation from RAW264.7 Cells.
We also investigated the effects of IL-4 in the murine macrophagic cell line RAW264.7, a previously established model of osteoclast differentiation (17). IL-4 also suppressed RANKL/M-CSF-induced osteoclast formation in this line (Fig. 1B). The efficiency of suppression was somewhat less than that observed with BMs, however, even at concentrations as high as 5 ng/ml. This lower efficiency of suppression suggests a possible deficiency in the IL-4 signaling pathway in RAW264.7 cells relative to BMs. The clonal nature of this line, however, establishes unequivocally that osteoclast precursors are direct cellular targets of IL-4.
Osteoclast Formation Is Suppressed by PPARγ1 Agonists and Reversed in the Presence of GW 9662.
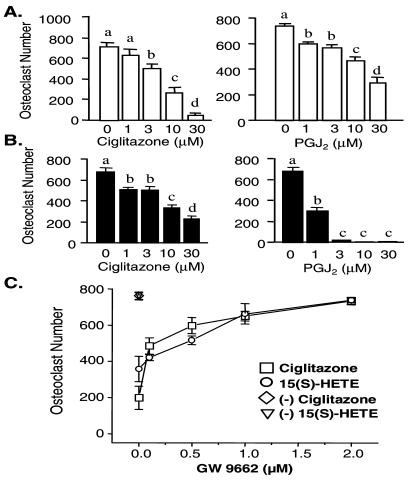
Recent studies suggest that IL-4 may regulate cellular function in macrophages by stimulating the production of PPARγ1 ligands (17, 21). Because osteoclasts are derived from monocyte-macrophage precursors, the above observations raise the possibility that IL-4 might function through PPARγ1. We tested this hypothesis by first determining whether known PPARγ1 ligands could suppress RANKL-induced osteoclast formation. Whereas differentiation was strongly induced by RANKL/M-CSF in BMs and in RAW264.7 cells, both the PPARγ1 ligand 15d-PGJ2 (22) and the thiazolidinedione ciglitazone (23) exerted a dose-dependent inhibition (Fig. 2 A and B). An additional natural PPARγ1 ligand, 15(S)-HETE (12), also suppressed osteoclast formation in both cell types, whereas WY-14643, a PPARα-activating ligand (24), had no effect (data not shown). Importantly, suppression by these ligands was reversed in a concentration-dependent fashion with GW 9662, a selective and irreversible inhibitor of PPARγ1 (12, 25) [Fig. 2C; 15(S)-HETE and ciglitazone shown]. Osteoclasts formed in the presence of GW 9662 were morphologically indistinguishable from those induced by RANKL/M-CSF alone. Identical results were observed when RAW264.7 cells were used as precursors (data not shown). Interestingly, the PPARγ1 agonists blocked RANKL/M-CSF-induced osteoclast formation in RAW264.7 cells more efficiently than did IL-4. These experiments demonstrate that ligand-activated PPARγ1 can efficiently mimic the effects of IL-4 in both BMs and RAW264.7 cells.
Figure 2.
Ciglitazone and 15d-PGJ2 act via PPARγ to suppress M-CSF/RANKL-induced osteoclast formation in primary murine myeloid (BMs) and RAW264.7 cells. (A) BMs were plated in triplicate at 105 cells per well and treated with M-CSF/RANKL in the presence of vehicle (ethanol or DMSO), ciglitazone (1–30 μM), or 15d-PGJ2 (PGJ2) (1–30 μM) for a period of 7–10 days, and then multinucleated (more than three), TRAP-positive osteoclasts were quantitated. (B) RAW264.7 cells were plated in triplicate at 2 × 103 cells per well and treated as in A. Multinucleated, TRAP-positive osteoclasts were quantitated after 5 days. (C) The PPARγ1 antagonist GW 9662 prevents ciglitazone- and 15(S)-HETE-induced suppression of osteoclast formation in BMs. Cells were incubated with M-CSF/RANKL and either vehicle, ciglitazone (30 μM) or 15(S)-HETE (30 μM), in the presence of increasing concentrations of GW 9662. Mean ± SE, n = 3 (b, c, and d are significant vs. a at P < 0.05).
PPARγ1 Is Expressed in Both BMs and RAW264.7 Cells.
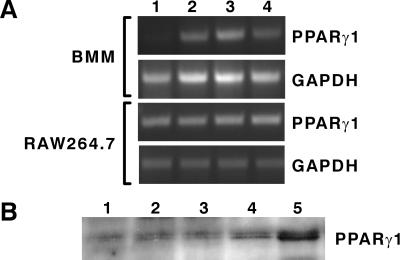
The ability of PPARγ1 ligands to elicit a biological response reversible by GW 9662 suggests the involvement of PPARγ1. We confirmed the expression of PPARγ1 in these cells with the use of both reverse transcription–PCR and Western blot analyses (Fig. 3). Interestingly, although PPARγ1 transcripts were induced in BMs after treatment with IL-4, M-CSF, and GM-CSF as reported (12, 26), these factors had no effect in RAW264.7 cells (Fig. 3A). M-CSF increased not only the level of PPARγ1 transcripts in BMs, but the protein level as well (Fig. 3B). These results, together with those obtained with the PPARγ1 agonists and GW 9662, suggest that PPARγ1 is the mediator of osteoclast suppression.
Figure 3.
Detection of PPARγ in BM and RAW264.7 cells. (A) Regulation of PPARγ1 mRNA by GM-CSF, M-CSF, and IL-4 in BMs but not in RAW264.7 cells. BMs and RAW264.7 cells were cultured in the absence or presence of one of the following cytokines for 24 h: vehicle (lane 1); mGM-CSF (10 ng/ml, lane 2), M-CSF (10 ng/ml, lane 3), or IL-4 (10 ng/ml, lane 4). Total RNA was isolated, treated with DNase, and subjected to reverse transcription–PCR analysis. (B) Detection of PPARγ1 protein. BMs were incubated untreated (lane 1) or were treated with 10 ng/ml M-CSF for 48 h (lane 2) or 72 h (lane 3) or with 100 ng/ml M-CSF for 48 h (lane 4) or 72 h (lane 5), and nuclear extracts (75 μg) were evaluated by Western analysis.
GW 9662 Blocks IL-4 Suppression of Osteoclast Formation.
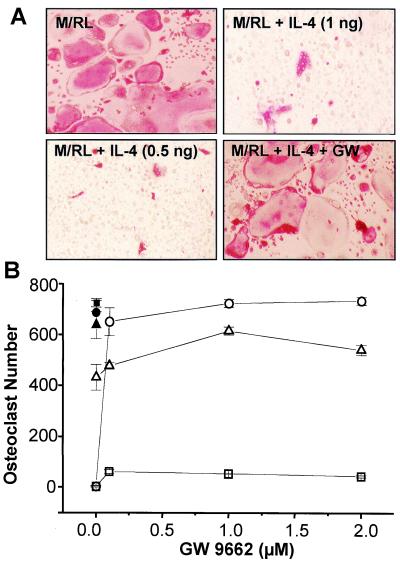
To test the hypothesis that IL-4 might function through PPARγ1, we treated both BMs and RAW264.7 cells with RANKL/M-CSF and IL-4 in the presence of the PPARγ1 antagonist GW 9662 and assessed osteoclast number on day 10. GW 9662 clearly blocked the ability of IL-4 to suppress RANKL/M-CSF-induced osteoclastogenesis in BMs in a dose-dependent fashion (Fig. 4). Identical results were obtained with RAW264.7 cells (data not shown). The concentrations of GW 9662 required for inhibition (<1 μM) were well within the range of isoform selectivity for the PPARγ1 (12, 25). Interestingly, GW 9662 was unable to reverse the IL-4 activity evident at 1 ng/ml. This failure to reverse the IL-4 activity suggests an additional complexity to the ability of IL-4 to suppress osteoclast formation at the higher concentrations. Alternatively, dissimilar kinetic and/or clearance rates of IL-4 and the antagonist could be involved, inasmuch as the stage at which IL-4 exerts its effect on these cells during differentiation is unclear. Nevertheless, the ability of GW 9662 to block inhibition of osteoclast formation by IL-4 suggests that the latter functions, at least in part, via PPARγ1.
Figure 4.
IL-4 suppresses M-CSF/RANKL-induced osteoclast formation in BMs. (A) BMs were treated with M-CSF (10 ng/ml) and RANKL (30 ng/ml) in the absence or presence of IL-4 (1 ng/ml) for 10 days, stained for TRAP, and photographed at ×20. (Upper) Cells that have been cultured in the absence (Left) or presence (Right) of 1 ng/ml IL-4. (Lower) Cells that have been cultured in the presence of 0.5 ng/ml IL-4 in the absence (Left) or presence (Right) of the PPARγ1 antagonist GW 9662 (2 μM). (B) BMs (1 × 105 cells per well) were plated in triplicate and induced with M-CSF/RANKL in the absence (●, ■, ▴) or presence of IL-4 (□, 1 ng/ml; ○, 0.5 ng/ml; ▵, 0.1 ng/ml) and increasing amounts of GW 9662 (0, 0.1, 1 and 2 μM). Multinucleated (more than three nuclei), TRAP-positive osteoclasts were quantitated after 10 days. Mean ± SE, n = 3.
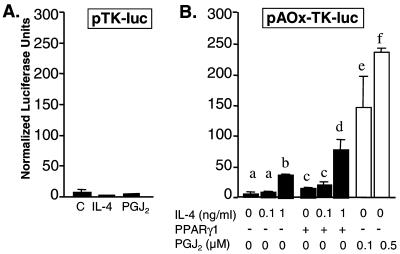
IL-4 and 15d-PGJ2 Activate a PPARγ1-Responsive Luciferase Reporter.
Based on the above result, we assessed the capacity of IL-4 to stimulate transcription of a PPARγ1-sensitive reporter gene (pAOx-TK-luc) (12) in RAW264.7 cells. IL-4 induced a significant concentration-dependent 5-fold increase in the activity of pAOx-TK-luc (Fig. 5B), an activity that was not evident in the pTK-luc control plasmid (Fig. 5A). 15d-PGJ2 also stimulated reporter gene activity as expected (Fig. 5B). Furthermore, cointroduction of a PPARγ1 expression vector increased the magnitude of the pAOx-TK-luc reporter gene response to IL-4 (Fig. 5B). These results are consistent with a PPARγ1-mediated action of IL-4 on osteoclast differentiation.
Figure 5.
IL-4 induces activation of PPARγ1-mediated transcription in RAW264.7 cells. (A) RAW264.7 cells were transfected with pTK-luc and stimulated with either vehicle, IL-4 (1 ng/ml) or 15d-PGJ2 (PGJ2) (0.5 μM) in the presence of 0.5% serum. (B) RAW264.7 cells were transfected with pAOx-TK-luc without or with pCMX-PPARγ1 (10 ng) and stimulated with IL-4 or 15d-PGJ2 (PGJ2) as indicated. Mean ± SE, n = 3 (b, e, and f are significant vs. a, and d is significant vs. c at P < 0.05).
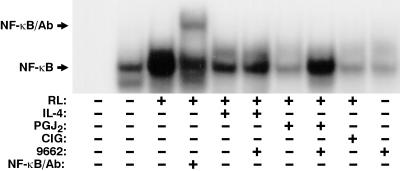
IL-4 Inhibits RANKL Activation of NF-κB.
Osteoclast differentiation involves activation of NF-κB (5). This requirement is highlighted in mice deficient for both the p50 and p52 subunits of NF-κB (27). Because RANKL is a strong inducer of NF-κB, we examined the possibility that IL-4 might function to block RANKL activation of NF-κB and thus prevent osteoclast formation. Treatment of RAW264.7 cells with RANKL for 30 min led to a clear activation of NF-κB, as assessed by DNA binding (Fig. 6). PPARγ1 ligands 15d-PGJ2 and ciglitazone also efficiently suppressed NF-κB activation by RANKL, an effect in the case of 15d-PGJ2 that was reversible with GW 9662 (Fig. 6). Importantly, IL-4 also suppressed RANKL-induced activation of NF-κB (Fig. 6). This suppression was not blocked, however, with GW 9662. This lack of effect of GW 9662 supports the idea that IL-4 might function in part to induce the synthesis of PPARγ1-activating ligands, an action unlikely during the 30-min stimulation period. These results support an obligate linkage between IL-4 action and PPARγ1, but also suggest additional PPARγ1-independent complexity in the action of IL-4.
Figure 6.
IL-4 and PPARγ ligands suppress RANKL-dependent activation of NF-κB. RAW264.7 cells (2.5 × 106 cells per plate) were pretreated for 30 min with IL-4 (1 ng/ml), 15d-PGJ2 (PGJ2) (0.5 μM), ciglitazone (CIG) (10 μM), and/or GW 9662 (1 μM) as indicated and then treated for 30 min with RANKL (100 ng/ml). Electrophoretic mobility-shift assay was carried out as indicated in Materials and Methods. Lane 1 represents the free probe. The NF-κB specific DNA complexes and supershifted NF-κB are indicated by the arrows.
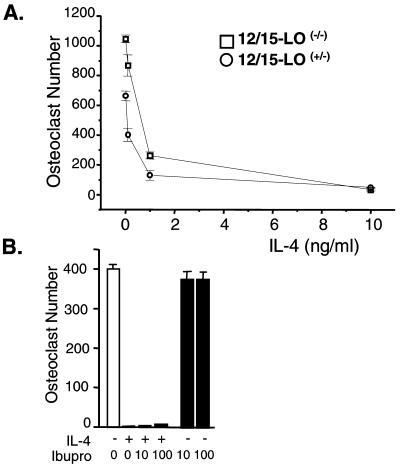
BMs from 12/15-LO Null Mice Retain Sensitivity to IL-4.
The ability of IL-4 to regulate macrophage function involves the stimulation of PPARγ1 expression and up-regulation of natural PPARγ1 ligands such as 15(S)-HETE (12) or perhaps the cyclopentenone prostaglandin PGD2 (22). The former is derived from arachidonic acid through the synthetic activity of 12/15-LO, a lipid-peroxidating enzyme induced by IL-4 in monocytes and macrophages (18, 21). PGD2 is produced in turn via cyclooxygenase activation (22). To assess the contribution of the 12/15-LO pathway in this system, we examined the ability of IL-4 to suppress osteoclast formation in BMs derived from 12/15-LO heterozygous and homozygous null mice (18). As observed in Fig. 7A, whereas the 12/15-LO null mice exhibited a significant increase in the number of osteoclasts over heterozygous controls, osteoclast formation in BMs from both mice appeared to be equally sensitive to suppression by IL-4, particularly at concentrations below 1 ng/ml. In addition, neither nordihydroguaiaretic acid nor caffeic acid, inhibitors of 12/15-LO and 5-LO, respectively, was able to reverse the effects of IL-4 (data not shown). Finally, the cyclooxygenase inhibitor ibuprofen also had no effect on IL-4 action (Fig. 7B). Importantly, although ibuprofen may function as a PPARγ1 agonist, no such activity was observed in this experiment. These data suggest that the PPARγ1 ligand(s) responsible for inhibition of osteoclast formation is not synthesized by 5-LO, 12/15-LO, or the cyclooxygenases.
Figure 7.
12/15-LO and COX-1/COX-2 are not required for IL-4 action. (A) BMs were isolated from mice heterozygous (+/−) or homozygous (−/−) for the 12/15-lipoxygenase null allele, plated in triplicate (1 × 105 cells per well), and induced with M-CSF/RANKL in the absence or presence of increasing amounts of IL-4 (0.1, 1, and 10 ng/ml). Multinucleated (more than three nuclei), TRAP-positive osteoclasts were quantitated after 10 days. (B) BMs from normal mice were treated for 10 days with M-CSF/RANKL, IL-4 (0.5 ng/ml), or ibuprofen (10 or 100 μM) as indicated. Mean ± SE, n = 3.
Discussion
IL-4 is a pleiotropic TH2 lymphocyte-derived cytokine (10). In addition to the activity of IL-4 on lymphoid cells and its ability to regulate macrophage differentiation and function, IL-4 also functions to block bone resorption (13–15). This effect likely results from the cytokine's dual ability to suppress the production of osteoclastogenic cytokines such as IL-1, TNF-α, and IL-6 from regulatory cells and to limit the production of functional osteoclasts, as observed here. Recent experiments suggest that the latter activity may be due to a direct action on osteoclast precursors (16). Our studies unequivocally confirm this hypothesis both in monocytes and particularly in RAW264.7 cells, a clonal cell line free of potential stromal cell contaminants. We also show, with the use of both selective agonists and the PPARγ1-specific antagonist GW 9962, that the inhibitory actions of IL-4 are mediated, at least in part, via PPARγ1. These studies support the idea that inhibition of bone resorption by IL-4 may be due to the cytokine's capacity to inhibit osteoclast formation directly through the activation of PPARγ1.
IL-4 is known to regulate cellular functions through stimulation of the IL-4 receptor complex and activation of Stat6 (28). Thus the finding that IL-4 also activates PPARγ1 and that this factor regulates osteoclast formation as well is surprising. The mechanism through which PPARγ1 mediates this activity is unknown. However, PPARγ1 regulates gene expression as a retinoid X receptor heterodimer through direct binding to PPAR response elements located within the promoter region of PPARγ1-sensitive genes such as CD36 (29). Under these circumstances, synthetic retinoid X receptor-selective ligands such as LG268 can potentiate the biologic activities of activated PPARγ1 (30). LG268 did not potentiate the suppressive effects evident here with either IL-4 or the PPARγ1 ligands (data not shown). This observation raises the possibility that the inhibitory actions of PPARγ1 on osteoclast differentiation do not involve direct DNA binding, but rather occur through the ability of PPARγ1 to interfere with the activation or downstream activity of transcription factors essential for RANKL-mediated osteoclastogenic events.
Our studies demonstrate that both IL-4 and PPARγ1 ligands such as 15d-PGJ2 and ciglitazone suppress RANKL-induced NF-κB DNA binding. Activation of this transcription factor is essential to osteoclast formation, based on the complete loss of osteoclast production and concomitant osteopetrosis observed in p50/p52 null mice (27). The findings herein are consistent with a previous observation made with 15d-PGJ2 alone (16). Only the present work with selective PPARγ1 agonists and the antagonist GW 9662 demonstrates an unequivocal involvement of PPARγ1, however, because 15d-PGJ2 can directly inhibit IκB kinase through an action independent of PPARγ1 (31). Recent studies using macrophages derived from PPARγ-deficient embryonic stem cells suggest that the thiazolidinedione class of compounds exhibits PPARγ-independent actions as well (32, 33). Interestingly, although RANKL-induced activation of NF-κB was also suppressed by IL-4, GW 9662 was unable to reverse this effect. This result is perhaps not surprising because 30 min of acute treatment with IL-4 is unlikely to be a sufficient period of time to induce the synthesis of an enzyme(s) responsible for the production of PPARγ1-activating ligands. More importantly, the ability of IL-4 to suppress RANKL-induced NF-κB activation in the absence of PPARγ1 clearly suggests that factors other than the latter protein regulator may be involved. Regardless, these experiments indicated that suppression of RANKL-induced, NF-κB-mediated events essential for osteoclast differentiation may be central to the mechanism of action of IL-4.
Stat6 likely represents an additional factor integral to IL-4 action. Indeed, recent preliminary studies indicate that the activity of Stat6 is essential for IL-4-mediated suppression of RANKL-induced osteoclast formation.¶ The exact role of Stat6 remains to be determined, however. Stat6 could inhibit either the activation or transactivation capabilities of NF-κB or both. Stat6 also might function to induce the synthesis of PPARγ1-activating ligands. The ability of PPARγ1 ligands to suppress NF-κB activation as well as osteoclast formation in the absence of IL-4 supports both possibilities, although activation of Stat6 is clearly not a prerequisite. Elucidation of the exact role of NF-κB in RANKL-induced osteoclast formation will be required to ascertain the importance of its suppression by IL-4.
Because the PPARγ1 gene is not up-regulated by IL-4 in RAW264.7 cells, this action is not central to IL-4 function and focuses attention on the identity of PPARγ1 ligands induced in osteoclast precursors. Numerous natural PPARγ1 ligands have been identified, including oxygenated products of the 12/15-LO pathway such as 15(S)-HETE (12) and products of the cyclooxygenase pathways such as the cyclopentenone prostaglandin PGD2 (22). Inhibitors of these pathways, including caffeic acid, nordihydroguaiaretic acid, and ibuprofen, were unable to block the effects of IL-4, however. These findings, together with the observation that the 12/15-LO-deficient mouse retained responsiveness to IL-4, suggest that PPARγ1 activation may involve as yet undescribed novel ligands. Interestingly, recent studies suggest that PPARγ1 also can be regulated through phosphorylation via the mitogen-activated protein kinase (34) and protein kinase A pathways (35).
PPARγ1 appears to play a reciprocal role in the production of macrophages and osteoclasts. The ability of IL-4 as well as other factors to influence RANKL-mediated osteoclast formation highlights the critical role of the environment in precursor commitment to a particular differentiation program. The ability of PPARγ1 to regulate this process is reminiscent of the role of PPARγ1 role in adipogenesis, wherein this nuclear receptor stimulates adipocyte differentiation and suppresses osteoblast differentiation from common mesenchymal precursors (36). The actions of PPARγ1 in reducing the formation of both osteoblasts and osteoclasts suggest an additional role for this receptor in the modulation of bone remodeling. They may also explain why treatment with the thiazolidinedione class of insulin sensitizers appears to initiate the reversal of bone loss associated with diabetes (37, 38).
Acknowledgments
We thank Glenn Doerman for help in preparing both the figures and the manuscript and Dr. Chris Glass and Mercedes Ricote for providing pCMX-PPARγ1 and pAOx-TK-luc plasmids and for helpful discussions. This work was supported by The National Institutes of Health (DK-56059 to J.W.P. and N.K.S.).
Abbreviations
- BMs
bone marrow monocytes
- M-CSF
macrophage colony-stimulating factor
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- TNF
tumor necrosis factor
- RANKL
receptor activator of NF-κB ligand
- PPARγ1
peroxisome proliferator activated receptor γ1
- 12/15-LO
12/15-lipoxygenase
- 15d-PGJ2
15-deoxy-Δ12,14 prostaglandin J2
- AOx
aryl CoA oxidase
- 15(S)-HETE
15(S)-hydroxyeicosatetraenoic acid
- TRAP
tartrate-resistant acid phosphatase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Riechers, C., Huelsmann, A. & Abu-Amer, Y. (2000) J. Bone Miner. Res. 15, Suppl. 1, S182 (Abstr.).
References
- 1.Manolagas S C, Jilka R L. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 2.Turner R T, Riggs B L, Spelsberg T C. Endocr Rev. 1994;15:275–300. doi: 10.1210/edrv-15-3-275. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego: Academic; 1996. [Google Scholar]
- 4.Roodman G D. Exp Hematol. 1999;27:1229–1241. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 5.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie M T, Martin T J. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 6.Kong Y-Y, Yoshide H, Sarosi I, Tan H-L, Timms E, Capparelli C, Morony S, Olivera-Dos-Santos A J, Van G, Itie A, et al. Nature (London) 1999;397:315–322. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 7.Wong B R, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett F S, Frankel W N, et al. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 8.Pacifici R. J Bone Miner Res. 1996;8:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 9.Mundy G R, Boyce B F, Yoneda T, Bonewald L F, Roodman G D. In: Osteoporosis. Marcus R, Feldman D, Kelsey J, editors. San Diego: Academic; 1996. pp. 302–313. [Google Scholar]
- 10.Banchereau J, Rybak M E. In: The Cytokine Handbook. Thomson A, editor. San Diego: Academic; 1994. pp. 99–126. [Google Scholar]
- 11.Miossec P, Briolay J, Dechanet J, Wijdenes J, Martinez-Valdez H, Banchereau J. Arthritis Rheum. 1992;35:874–883. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]
- 12.Huang J T, Welch J S, Ricote M, Binder C J, Willson T M, Kelly C, Witztum J L, Funk C D, Conrad D, Glass C K. Nature (London) 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 13.Miossec P, Chomarat P, Dechanet J, Moreau J F, Roux J P, Delmas P, Banchereau J. Arthritis Rheum. 1992;37:1715–1722. doi: 10.1002/art.1780371202. [DOI] [PubMed] [Google Scholar]
- 14.Lacey D L, Erdmann J M, Teitelbaum S L, Tan H L, Ohara J, Shioi A. Endocrinology. 1994;136:2367–2376. doi: 10.1210/endo.136.6.7750457. [DOI] [PubMed] [Google Scholar]
- 15.Lubbers E, Joosten L A B, Chabaud M, van den Bersselaar L, Oppers B, Coenen-de Roo C J J, Richards C D, Miossec P, van den Berg W B. J Clin Invest. 2000;105:1689–1710. doi: 10.1172/JCI7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mbalaviele G, Abu-Amer Y, Meng A, Jaiswal R, Beck S, Pittenger M F, Thiede M A, Marshak D R. J Biol Chem. 2000;275:14388–14393. doi: 10.1074/jbc.275.19.14388. [DOI] [PubMed] [Google Scholar]
- 17.Shevde N K, Bendixen A C, Dienger K M, Pike J W. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. . (First Published June 27, 2000; 10.1073/pnas. 130200197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun D, Funk C D. J Biol Chem. 1996;271:24055–24062. [PubMed] [Google Scholar]
- 19.Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. FEBS Lett. 2000;467:259–262. doi: 10.1016/s0014-5793(00)01169-8. [DOI] [PubMed] [Google Scholar]
- 20.Kliewer S A, Forman B M, Blumberg B, Ono E S, Borgmeyer U, Mangelsdorf D J, Umesono K, Evans R M. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conrad D J, Kuhn H, Mulkins M, Highland E, Sigal E. Proc Natl Acad Sci USA. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 23.Willson T M, Cobb J E, Cowan D J, Wiethe R W, Correa I D, Prakash S R, Beck K D, Moore L B, Kliewer S A, Lehmann J M. J Med Chem. 1996;39:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 24.Tugwood J D, Aldridge T C, Lambe K G, Macdonald N, Woodyatt H J. Ann NY Acad Sci. 1996;804:252–265. doi: 10.1111/j.1749-6632.1996.tb18620.x. [DOI] [PubMed] [Google Scholar]
- 25.Miyahara T, Schrum L, Rippe R, Xiong S, Yee H F, Motomura K, Anania F A, Willson T M, Tsukamoto H. J Biol Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 26.Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, Witztum J L, Auwerx J, Falinski W, Glass C K. Proc Natl Acad Sci USA. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franzoso G, Carlson L, Xing L, Poljak L, Shores E W, Brown K D, Leonardi A, Tran T, Boyce B F, Siebenlist U. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou J, Schindler U, Henzel W J, Ho T C, Brasseur M, McKnight S L. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 29.Tontonoz P, Nagy L, Alvarez G A, Thomazy V A, Evans R M. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee R, Davies P J, Crombie D L, Bischoff E D, Cesario R M, Jow L, Haman L G, Boehm M F, Mondon C E, Nadzan A M, et al. Nature (London) 1997;386:407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 31.Rossi A, Kapahi P, Natolli G, Takahashi T, Chen Y, Karin M, Santoro M G. Nature (London) 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 32.Moore K J, Rosen E D, Fitzgerald M L, Randow F, Anderson L P, Altshuler D, Milstone D S, Mortensen R M, Spiegelman B M, Freeman M W. Nat Med. 2001;7:41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 33.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans R M. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 34.Camp H S, Tafuri S R. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 35.Lazennec G, Canaple L, Saugy D, Wahli W. Mol Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecka-Czernik B, Gubrij I, Moerman E J, Kajkenova O, Lipschitz D A, Manolagas S C, Jilka R L. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 37.Okazaki R, Totsuka Y, Hamano K, Ajima M, Miura M, Hiroto Y, Hata K, Fukumoto S, Matsumoto T. Endocrinology. 1999;140:5060–5065. doi: 10.1210/jcem.82.9.4258. [DOI] [PubMed] [Google Scholar]
- 38.Okazaki R. Nippon Rinsho. 2000;58:456–460. [PubMed] [Google Scholar]