Abstract
The question of whether hypersensitivity to streptococcal antigens plays a role in the pathogenesis of the nonsuppurative sequelae of streptococcal infections remains at present unclear. As a first step in the approach to this question, the degree of cellular reactivity of peripheral blood leucocytes to streptococcal antigens was investigated in a number of rheumatic fever patients, patients with uncomplicated streptococcal infections, as well as normal healthy subjects.
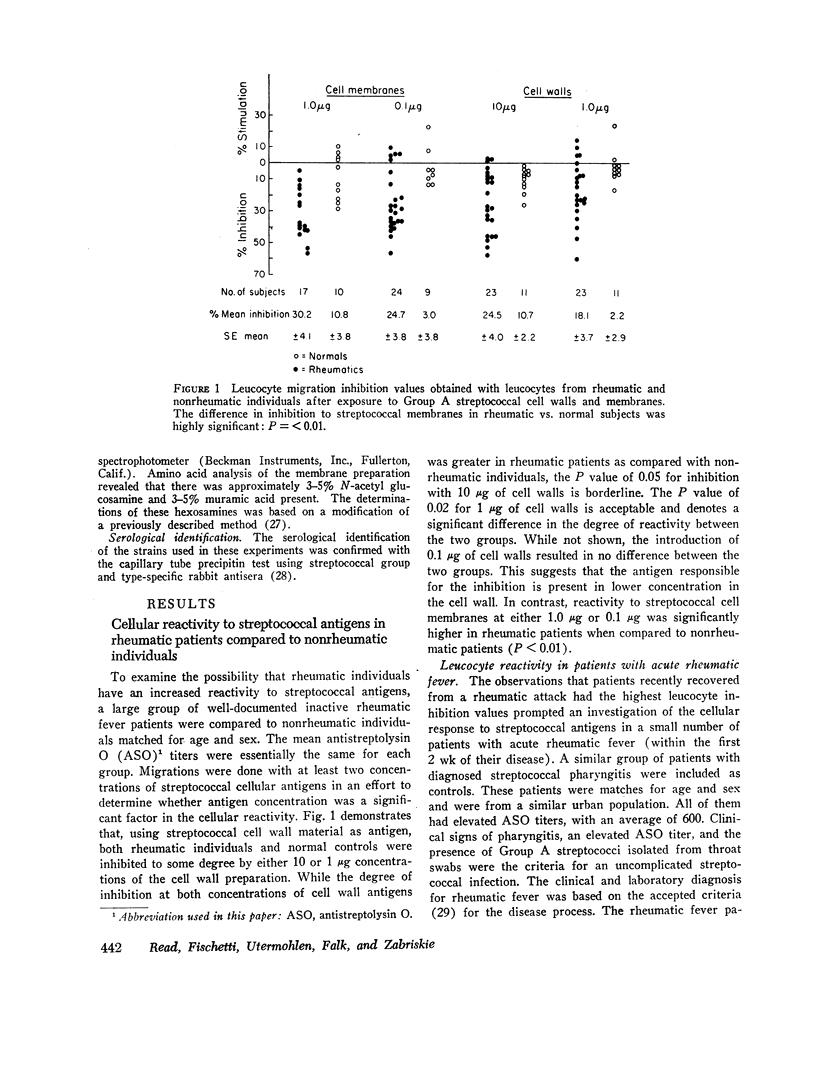
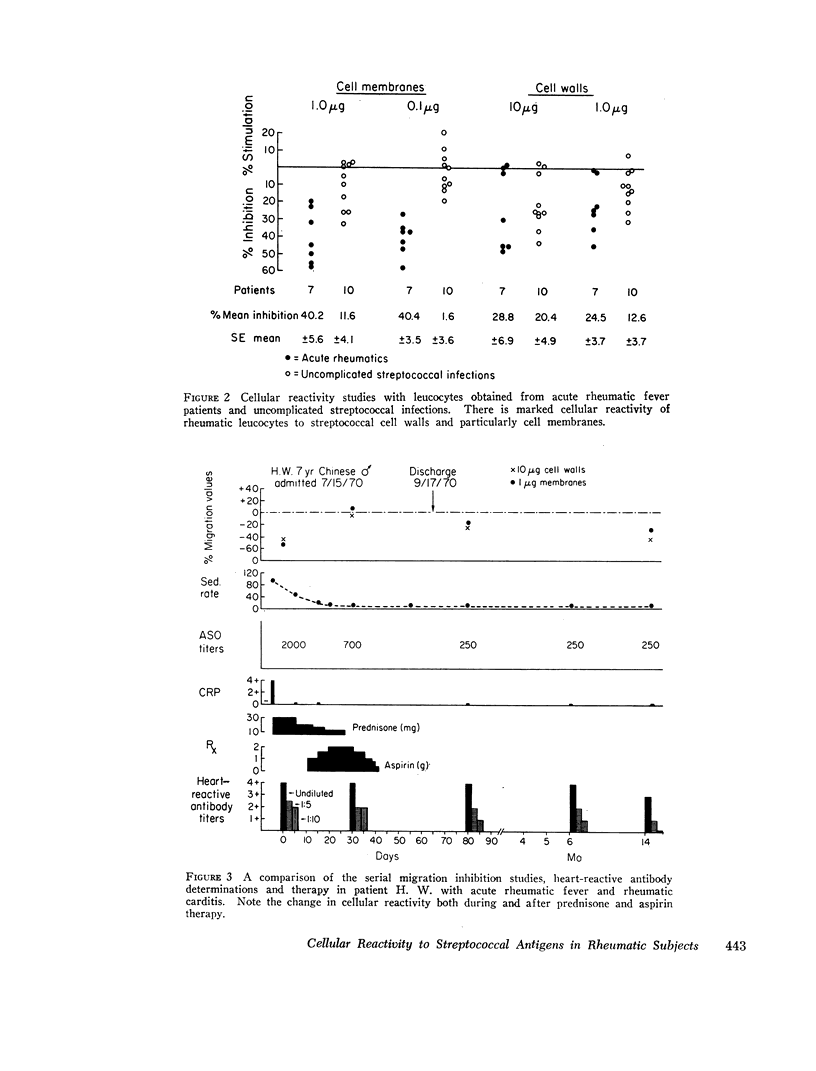
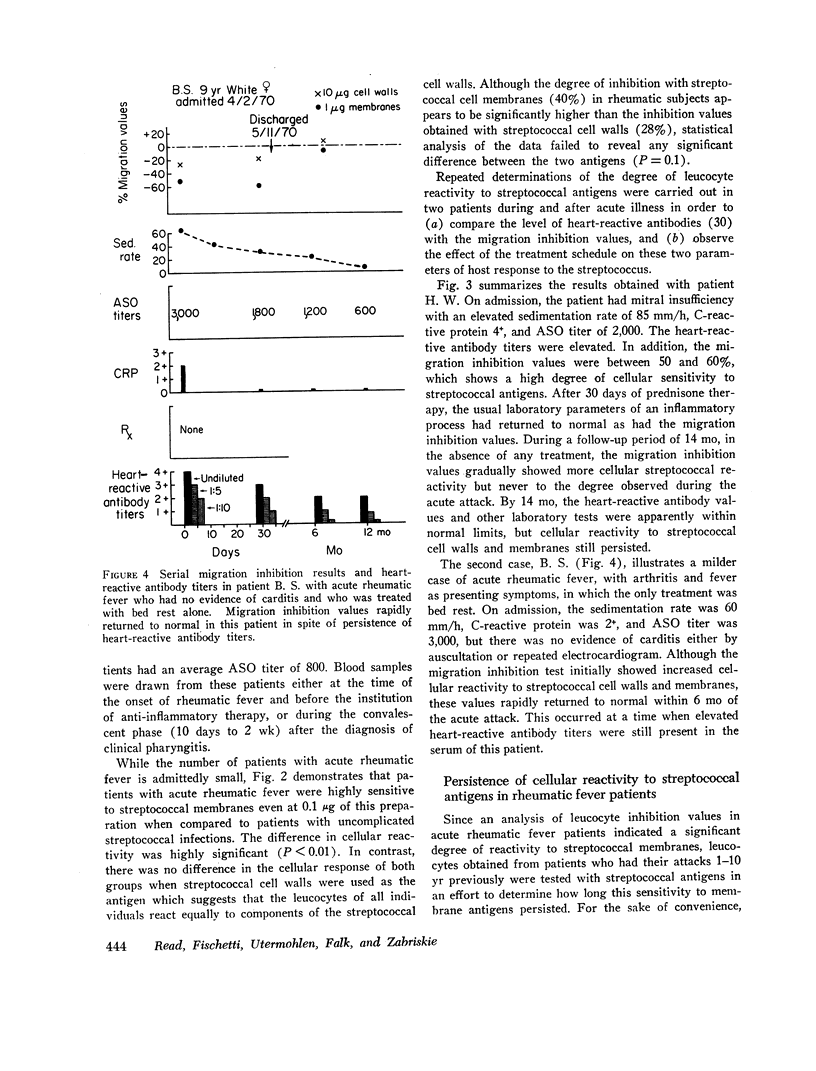
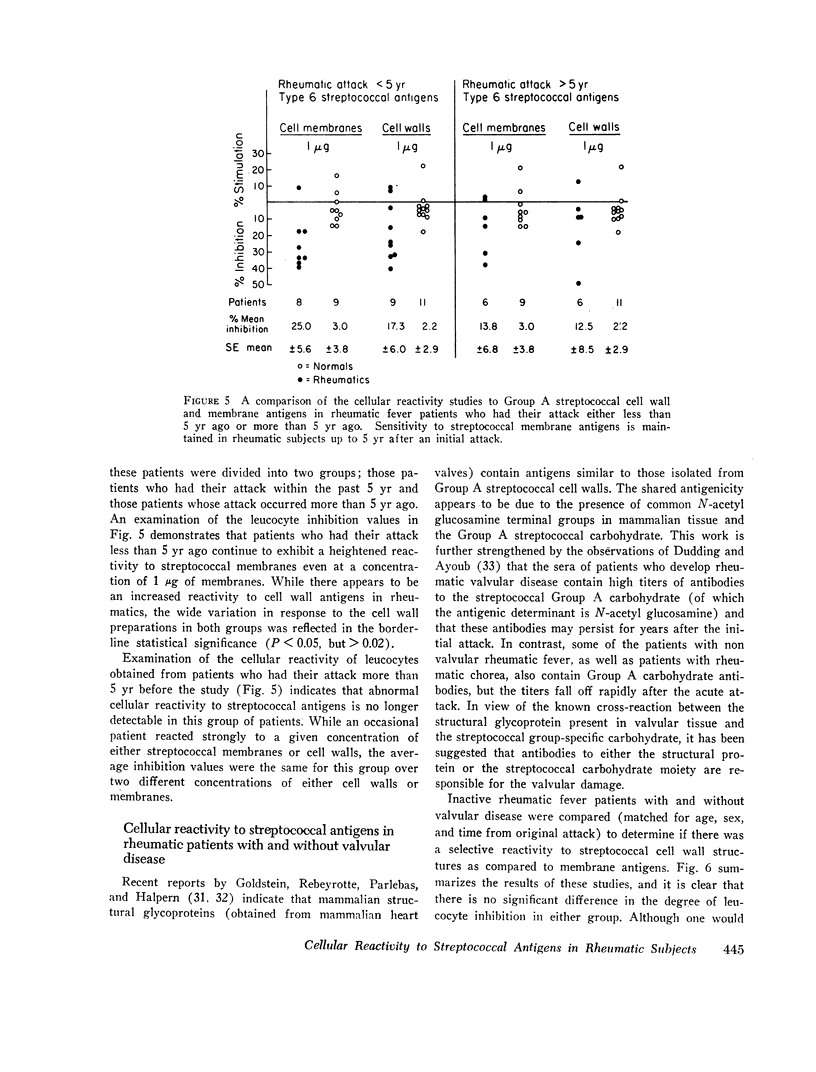
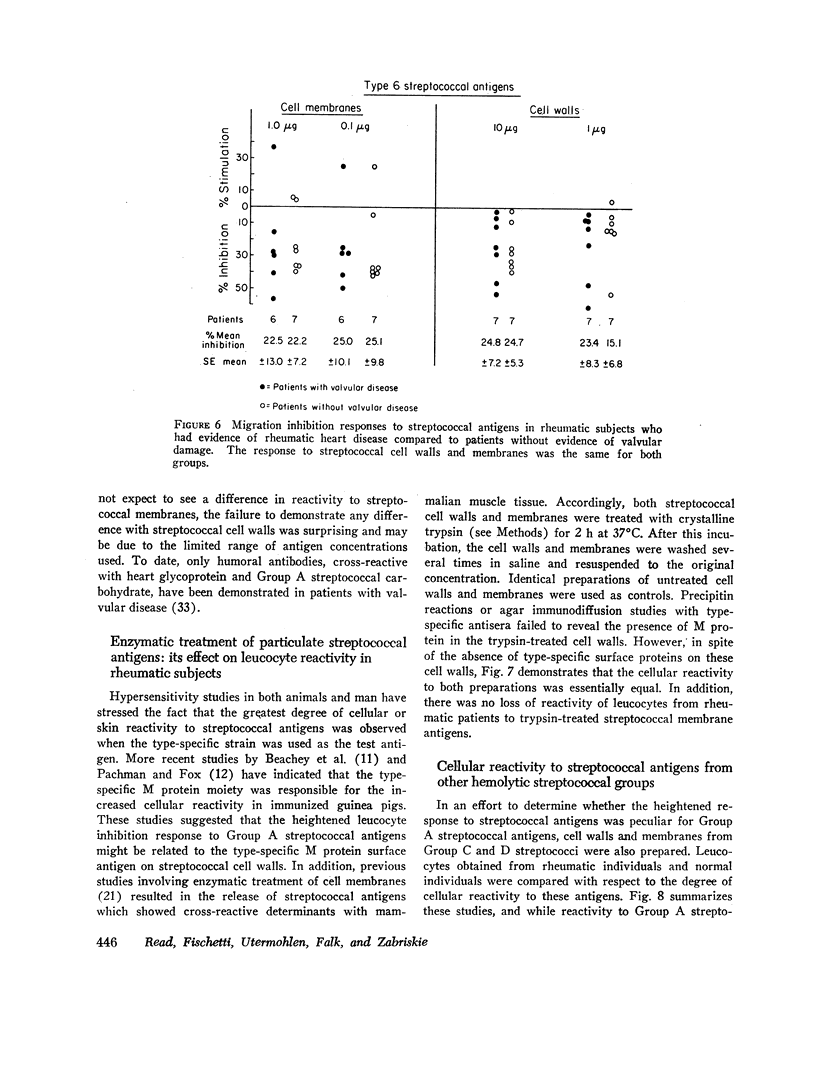
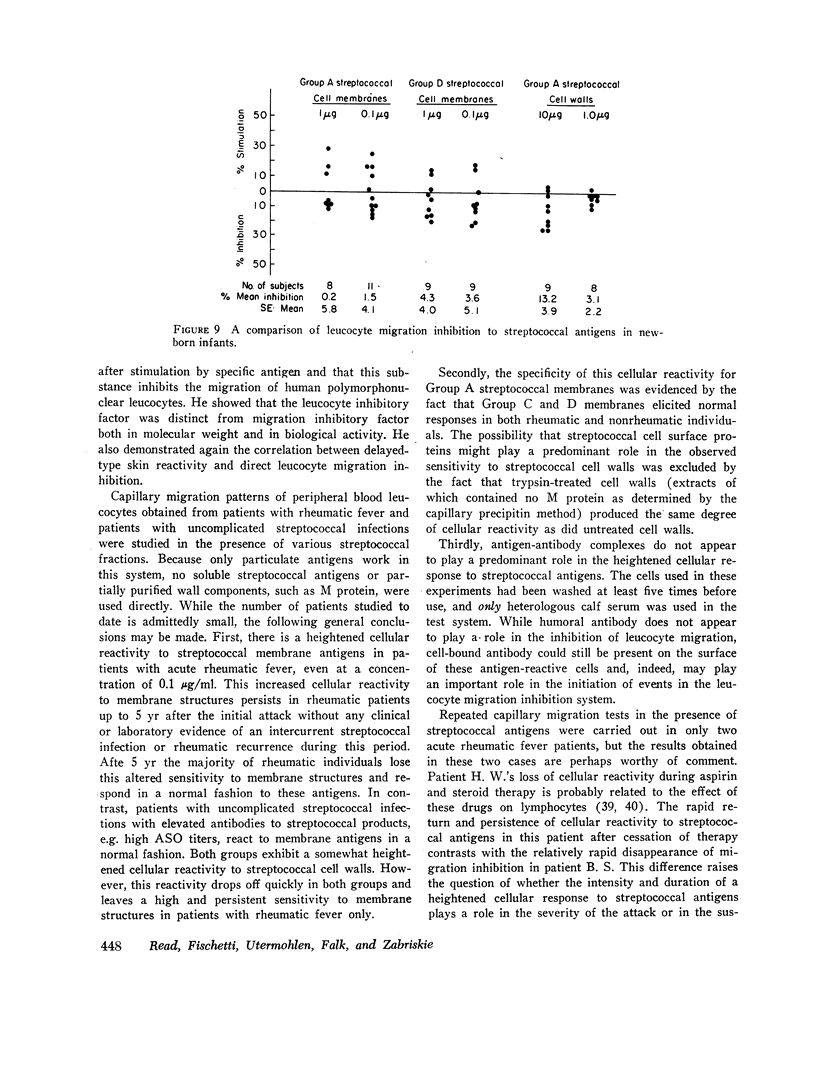
Using the in vitro technique for the inhibition of capillary migration of peripheral blood leucocytes as an index of the degree of sensitivity to streptococcal antigens, the results indicate that patients with acute rheumatic fever exhibit an exaggerated cellular reactivity to these antigens and in particular to streptococcal cell membrane antigens. This abnormal response to streptococcal membrane antigens appears to persist in rheumatic subjects for at least 5 yr after the initial attack of rheumatic fever. Only Group A streptococcal membrane antigens elicited this unusual response in rheumatic subjects, since the cellular reactivity to Group C and D streptococcal membranes was the same in all groups. Patients with evidence of valvular disease exhibited the same degree of cellular reactivity to these antigens as did patients without clinical evidence of rheumatic heart disease.
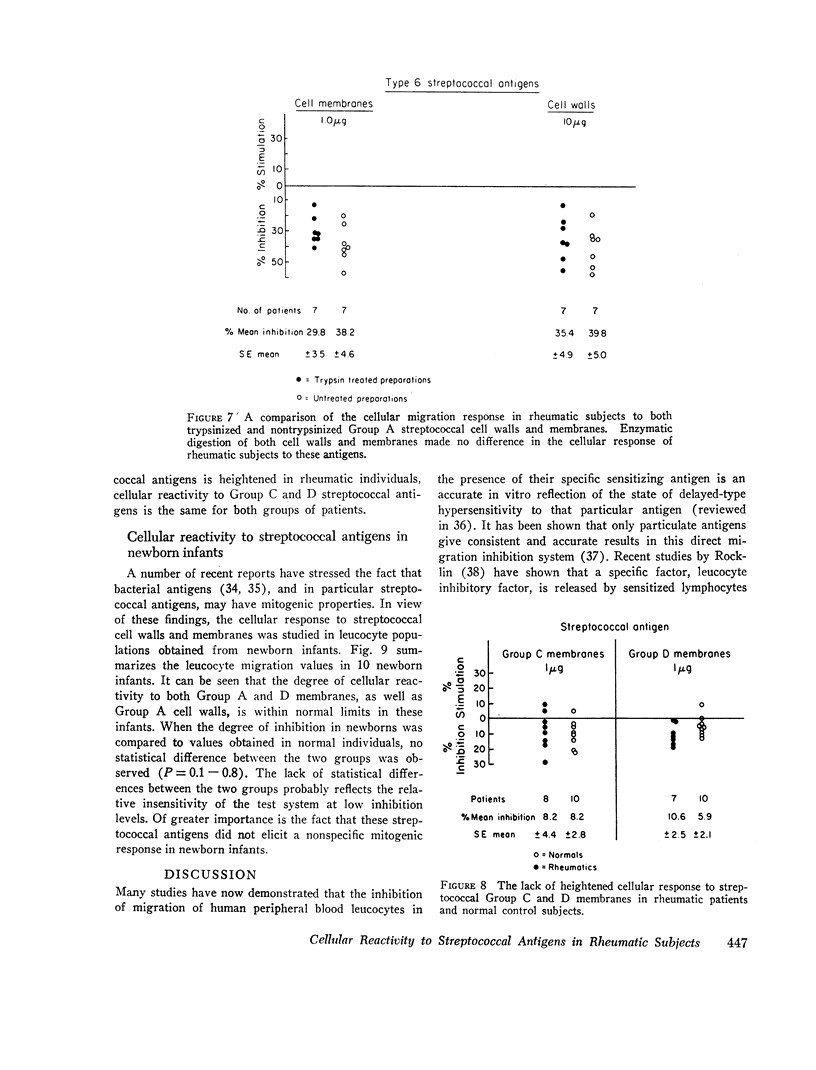
The nature of the antigens responsible for the observed cellular response remains unknown. Enzymatic treatment of streptococcal cell walls and membranes designed to remove type-specific M proteins did not alter the observed cellular reactivity to the streptococcal antigens. The finding that an abnormal cellular response to certain streptococcal antigens is present only in rheumatic patients suggests that cell-mediated factors may play an important role in the disease process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLEIWEIS A. S., KARAKAWA W. W., KRAUSE R. M. IMPROVED TECHNIQUE FOR THE PREPARATION OF STREPTOCOCCAL CELL WALLS. J Bacteriol. 1964 Oct;88:1198–1200. doi: 10.1128/jb.88.4.1198-1200.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Alberti H., Stollerman G. H. Delayed hypersensitivity to purified streptococcal m protein in guinea pigs and in man. J Immunol. 1969 Jan;102(1):42–52. [PubMed] [Google Scholar]
- Dudding B. A., Ayoub E. M. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J Exp Med. 1968 Nov 1;128(5):1081–1098. doi: 10.1084/jem.128.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER R. J., THOMAS W. A., MORSE S. I., DARNELL J. E., Jr The incidence and pathogenesis of myocarditis in rabbits after group A streptococcal pharyngeal infections. J Exp Med. 1956 Jan 1;103(1):173–188. doi: 10.1084/jem.103.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Rebeyrotte P., Parlebas J., Halpern B. Isolation from heart valves of glycopeptides which share immunological properties with Streptococcus haemolyticus group A polysaccharides. Nature. 1968 Aug 24;219(5156):866–868. doi: 10.1038/219866a0. [DOI] [PubMed] [Google Scholar]
- Green C. A. Haemolytic Streptococcal Infections and Acute Rheumatism. Ann Rheum Dis. 1942 May;3(1):4–41. doi: 10.1136/ard.3.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., PAGEL W. The tissue response to heat-killed streptococci in the skin of normal subjects, and in persons with rheumatic fever, rheumatoid arthritis, subacute bacterial endocarditis and erythema nodosum. Br J Exp Pathol. 1949 Aug;30(4):282-8, 2 pl. [PMC free article] [PubMed] [Google Scholar]
- Halpern B., Parlebas J., Goldstein I. Isolement à partir du cytoplasme du streptocoque A d'une glycoprotéine qui présente un parenté immunologique avec des glycoprotéines des valvules cardiaques. C R Acad Sci Hebd Seances Acad Sci D. 1971 Sep 13;273(11):995–998. [PubMed] [Google Scholar]
- KAPLAN M. H. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. I. PROPERTIES OF AN ANTIGEN IN CERTAIN STRAINS OF GROUP A STREPTOCOCCI EXHIBITING AN IMMUNOLOGIC CROSS-REACTION WITH HUMAN HEART TISSUE. J Immunol. 1963 Apr;90:595–606. [PubMed] [Google Scholar]
- Karakawa W. W., Krause R. M. Studies on the immunochemistry of streptococcal mucopeptide. J Exp Med. 1966 Aug 1;124(2):155–171. doi: 10.1084/jem.124.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser H., Kushner I., Kaplan M. H. "Nonspecific" stimulation of lymphocyte transformation by cellular fractions and acid extracts of group A streptococci. J Immunol. 1971 Jun;106(6):1593–1601. [PubMed] [Google Scholar]
- Pachman L. M., Esterly N. B., Peterson R. D. The effect of salicylate on the metabolism of normal and stimulated human lymphocytes in vitro. J Clin Invest. 1971 Jan;50(1):226–230. doi: 10.1172/JCI106478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman L. M., Fox E. N. Cellular and antibody reactions to streptococcal M protein types 1, 3, 6 and 12. J Immunol. 1970 Oct;105(4):898–907. [PubMed] [Google Scholar]
- RANTZ L. A., MARONEY M., DI CAPRIO J. M. Antistreptolysin O response following hemolytic streptococcus infection in early childhood. AMA Arch Intern Med. 1951 Mar;87(3):360–371. doi: 10.1001/archinte.1951.03810030033003. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., MARONEY M., DI CAPRIO J. M. Hemolytic streptococcal infection in childhood. Pediatrics. 1953 Nov;12(5):498–515. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soborg M., Bendixen G. Human lymphocyte migration as a parameter of hypersensitivity. Acta Med Scand. 1967 Feb;181(2):247–256. doi: 10.1111/j.0954-6820.1967.tb07255.x. [DOI] [PubMed] [Google Scholar]
- Taranta A., Cuppari G., Quagliata F. Dissociation of hemolytic and lymphocyte-transforming activities of streptolysin S preparations. J Exp Med. 1969 Apr 1;129(4):605–622. doi: 10.1084/jem.129.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Falk R. E. In vitro reactivity of lymphocytes to particulate and soluble antigens. Nature. 1970 Jun 6;226(5249):943–945. doi: 10.1038/226943a0. [DOI] [PubMed] [Google Scholar]
- Zabriskie J. B., Freimer E. H. An immunological relationship between the group. A streptococcus and mammalian muscle. J Exp Med. 1966 Oct 1;124(4):661–678. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Hsu K. C., Seegal B. C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970 Aug;7(2):147–159. [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Lewshenia R., Möller G., Wehle B., Falk R. E. Lymphocytic responses to streptococcal antigens in glomerulonephritic patients. Science. 1970 May 29;168(3935):1105–1108. doi: 10.1126/science.168.3935.1105. [DOI] [PubMed] [Google Scholar]


